Abstract
Division of labor is a hallmark characteristic of social insect colonies. While it is understood that worker differentiation is regulated through either the queen or her brood, the understanding of the physiology behind task regulation varies within social species. Studies in eusocial insects have shown that juvenile hormone (JH) is associated with division of labor and the onset of foraging tasks. Although, outside of a few key species, this interaction has yet to be elucidated in the red imported fire ant, Solenopsis invicta. In this study, we evaluated the role of a JH analog, S-hydroprene in worker task transition in Solenopsis invicta. S-hydroprene was applied to nurses to observe behavioral changes. S-hyroprene application to nurses did not affect phototaxis, but there was a shift in behavior from internal, nest-based behaviors to external, foraging-based behaviors. These results show that JH may be implicated in worker task transition in S. invicta and may function similarly as it does in other eusocial insects.
Similar content being viewed by others
Introduction
Division of labor occurs when individuals within a colony perform certain tasks associated with growth and maintenance of the whole colony. It is considered a hallmark characteristic of social insect structure1. Distinctions of task allocation can sometimes be indicated by polymorphic individuals that have morphological characters distinct to a specific caste. Social insects can also be monomorphic where the differences between individuals performing different tasks can be physiologically based and associated with nutrition, colony needs and age polyethism determine the task that is performed by workers2,3. Understanding the physiological basis of division of labor can provide insight on the evolutionary trajectory of eusocial colony structure.
In insects, juvenile hormone (JH) is typically associated with metamorphosis, development and reproduction4. However, there has been much research among both solitary and social insects, especially that of insects within the order Hymenoptera, that show its role in behavioral transition and task determination in insects4,5,6,7,8. In the honey bee Apis mellifera, high JH titers are associated with foraging behavior while low titers are associated with nursing behavior8,9. In some ant species such as Pogonomyrmex californicus, Myrmicaria eumenodies, and Harpegnathos saltator, JH titers were significantly higher in ants displaying extranidal behavior such as foraging and nest defense10,11, and topical applications of JH or JH-analogs induced foraging-like physiological changes12.
The red imported fire ant, Solenopsis invicta, provides a good model to further study the hormonal basis of work task transition, specifically the correlation of JH on task transition. The worker caste is comprised of sterile females which perform all tasks necessary for colony survival, growth, and maintenance13. For S. invicta, the most well-known aspect of JH is its function in queen development and behavior, often reflecting the reproductive status of the individual queens and their social form14,15,16. However, the role of JH in S. invicta workers remains understudied. Previously, the effect of the application of the JH analog S-hydroprene on the expression of genes such as vitellogenin and hexamerin, which are possibly co-opted for task allocation in workers, was evaluated. The application of JH analog to nurses caused shifts in expression of hexamerins to be similar to that of a forager, indicating a possible role in the shift from nurses to foragers17,18. However, these studies did not evaluate if the application also resulted in changes in the behavior of the ants.
In this study, we provide a behavioral assay to evaluate the prolonged effect of the application of the JH analog, S-hydroprene, on worker behavior, specifically in medium nurses. Nurses were treated with the analog or shams for a 7-day period, and the effect of the application was observed on phototaxis preferred placement in a microcolony (nest-like or foraging-like).
Materials and methods
Fire ant colony maintenance
Colonies were obtained from the Dale Watts Cross Country Course behind the Urban Entomology building on the Texas A&M campus (30° 37′ 12.7″ N 96° 22′ 09.6″ W) from January to March 2020. The colonies were kept in plastic trays coated with Fluon (Insect-a-slip, BioQuip products, CA, USA) and maintained at 27 ± 2 °C in a 12:12 h light/dark photoperiod. The colonies were fed daily with a 20% honey water solution and crickets, Acheta domestica, as well as being provided with water ad libitum.
Medium nurse ants were selected based on their head measurements as described in previous studies19. Medium workers were used in this study because they exhibit more behavioral flexibility compared to minor and majors, as well as more stability over time in the task they perform20. Ants in the nest directly interacting with the brood were considered nurses.
JH-analog applications
Medium sized nurses were collected from the colonies and treated with 1 µl of S-hydroprene (diluted at 25 ng/µl dissolved in acetone), 1 µl of acetone, or sham-treated on the dorsal portion of the abdomen of the insect. For sham treatments, the method of JH analog application was mimicked on ants, however the pipette was empty. Following treatment, each ant was housed individually within a glass test tube stoppered with cotton and provided honey water and water during this time. Over a 7-day period, these ants were dosed again every other day at the same time for a total of 4 doses and returned to their test tube after each dose. In total, the experiment was performed 5 times: each replicate was performed using insects collected from a different colony (there were 9 or 10 ants per treatment in each replicate due to mortality). The rate of mortality after JH analog or acetone application was 6% for each treatment.
Phototaxis experiment
To determine if the topical application of the JH-analog altered worker phototaxis, after the 7-day treatment the workers were placed individually into a 4-inch petri dish half covered with black paint applied on the outside top and bottom of the petri dish. The paint was applied to the outside to ensure that the workers never interacted with the paint (Fig. 1)21. Workers were placed gently in the center of the petri dish for each bioassay. Workers were given 5 min to habituate to the conditions within the petri dish before recording. After 5 min, the workers were recorded with a camera, observing, and tracking the time spent in the light portion of the petri dish for 10 min. After 10 min, the workers were removed and placed within a microcolony with the other workers from the same treatment group (there were 9 or 10 ants per treatment in each replicate due to mortality).
Microcolony bioassay
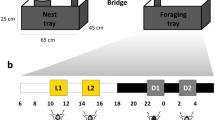
To discern if there were behavioral changes associated with the JH-analog treatments, workers were grouped into microcolonies based on their treatment groups (because of mortality there were 9 or 10 ants per microcolony for a total of 47 ants per treatment). The experiment was performed 5 times. Each microcolony contained crickets, 20% honey water, and 0.015 g of brood placed on a damp cotton pad. Brood was taken from the same colony as the workers and had an equal distribution of eggs, larvae, and pupae. The food and the brood were placed on opposite ends of the microcolony. Microcolonies were defined as a 12 × 6 cm plastic container coated with fluon to prevent escape, with an ample supply of protein and carbohydrate food sources on one end and a moist cotton to prevent desiccation and a spot to house brood on the other. The bottom of the microcolonies were covered with a non-stick lab bench protector paper. Once the workers were placed in the microcolony, they were allowed to acclimate to the new conditions for 24 h. After 24 h, cameras were set up to record the microcolonies for 8 h, observing the presence of workers within the microcolony every 30 min to determine whether the ants were on the brood side or the food side. Foraging- and nest-like conditions were determined by the position of the ants on top of cotton with carbohydrate and protein sources on the foraging side and the moist cotton with brood on the on the nest side of the microcolony to control random wandering and difficulty determining the location of workers on the border between each side (Fig. 2).
Statistical analysis
Data was collected and displayed as means with standard error bars. Data normality distribution was determined by a Chi-squared goodness-of-fit test. The results of the phototaxis and microcolony bioassays were analyzed using a Kruskal–Wallis Ranked Sums test with a Wilcoxon Each Pair post hoc analysis when necessary. JMP Pro 16 was used for all statistical tests, considering p-values of less than 0.05 as significant.
Results
Phototaxis experiment
The amount of time medium workers spent in the light when given a choice after 5 successive applications of the S-hydroprene, acetone, or sham treatment over a 7-day period was recorded. There was no significant difference between any of the three treatments (χ2 = 3.7547, df = 2, p = 0.1530) (Fig. 3).
Time spent in light by treated workers (n = 47/treatment with 9 or 10 ants per treatment in each replicate due to mortality) during a 10-min observation period to determine differences in phototaxis after 5 treatments with S-hydroprene, acetone, or sham over a 7-day period (p = 0.1530). The bars represent the standard error of the mean.
Microcolony bioassay
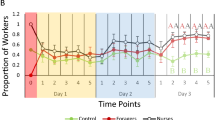
The average number of workers found in nest and foraging conditions over an 8-h observation period were calculated. Overall, there was a significant relationship between treatment and the position of the workers throughout the foraging bioassay (Nest Conditions: χ2 = 67.6263, df = 2, p < 0.0001; Foraging Conditions: χ2 = 46.0139, df = 2, p < 0.0001). There were significantly more workers found in nest conditions in groups treated with acetone or sham compared to S-hydroprene treated groups (S-Hydroprene versus Acetone: Z = − 6.12284, p < 0001; S-Hydroprene versus Sham: Z = − 6.77520, p < 0.001) (Fig. 4). There was a significantly higher number of workers found within nest conditions in the sham treatment compared to workers treated with acetone (Acetone versus Sham: Z = 4.62265, p < 0.0001) (Fig. 4). Additionally, there were significantly more workers found in foraging conditions in groups treated with S-hydroprene compared to both acetone groups and workers left untreated (S-Hydroprene versus Acetone: Z = 6.07514, p < 0001; S-Hydroprene versus Sham: Z = 5.095633, p < 0.001) (Fig. 5).
Average number of workers present in nest conditions observed over an 8-h period at 30-min snapshots (n = 47/treatment with 9 or 10 ants per treatment in each replicate due to mortality). Bars represent standard error of average number of workers found in nest conditions. Letters denote significance at p < 0.0001. The bars represent the standard error of the mean.
Average number of workers present in foraging conditions observed over an 8-h period at 30-min snapshots (n = 47/treatment with 9 or 10 ants per treatment in each replicate due to mortality). Bars represent standard error of average number of workers found in foraging conditions. Letters denote significance at p < 0.0001. The bars represent the standard error of the mean.
Discussion
In insects, juvenile hormone titers are important in the development and reproduction of both solitary and social insects4,22. The results from our study show that the JH analog can cause a behavioral shift in S. invicta workers. JH has been shown to influence worker behavior and task in many other insect species, however the extent of this influence has yet to be fully understood.
Our results show that the direct application of the JH analog does not influence worker phototaxis or preference to light (Fig. 3). In some ant species, phototaxis can be used as a proxy for foraging. For example, in the study of the effect of JH in the behavior of Acromyrmex octospinosus workers, an increased attraction towards light was tied to behaviors relating to leaving the nest, typically that of foraging23. However, S. invicta workers are more influenced by temperature than light when it comes to foraging24,25. Therefore, the results of the phototaxis assay paired with that of the behavioral assay in the microcolonies indicate that light plays a small role if any in influencing foraging in S. invicta, as more workers were found within foraging conditions when treated with S-hydroprene compared to sham or acetone treatments (Fig. 5).
Several factors such as age or nutritional status of the worker could affect its propensity to switch from nursing to foraging. Indeed, it is known that age in eusocial insects like Bombus impatiens26, A. mellifera3 and even in S. invicta27 plays a role in the behavior the insect performs. In this study, the age of the workers was not controlled, instead, nurse ants interacting with the brood were randomly selected to perform the experiment. Despite this, our results show that the effect of the application of a JH analog on S. invicta worker behavior and task was consistent with those found in other ant, wasp, bee, and termite species when the insects were treated with a JH analog7,8,10,11,28. Future experiments could assess whether these other factors influence the effect of the juvenile hormone analog on the behavior of fire ant workers.
Overall, this study supports the notion that the role of JH might be conserved in S. invicta as a major regulator for division of labor.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Wilson, E. O. The insect societies. The insect societies. (1971).
Wilson, E. O. Division of labor in fire ants based on physical castes (Hymenoptera: Formicidae: Solenopsis). J. Kansas Entomol. Soc. 51, 615–636 (1978).
Seeley, T. D. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav. Ecol. Sociobiol. 4, 287–293 (1982).
Nijhout, H. F. & Wheeler, D. E. Juvenile hormone and the physiological basis of insect polymorphisms. Q. Rev. Biol. 57, 109–133 (1982).
Amsalem, E., Malka, O., Grozinger, C. & Hefetz, A. Exploring the role of juvenile hormone and vitellogenin in reproduction and social behavior in bumble bees. BMC Evol. Biol. 14, 45 (2014).
Azevedo, D. O., de Paula, S. O., Zanuncio, J. C., Martinez, L. C. & Serrão, J. E. Juvenile hormone downregulates vitellogenin production in Ectatomma tuberculatum (Hymenoptera: Formicidae) sterile workers. J. Exp. Biol. 219, 103–108 (2016).
Cornette, R., Gotoh, H., Koshikawa, S. & Miura, T. Juvenile hormone titers and caste differentiation in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Termopsidae). J. Insect Physiol. 54(6), 922–930 (2008).
Sullivan, J. P., Jassim, O., Fahrbach, S. E. & Robinson, G. E. Juvenile hormone paces behavioral development in the adult worker honey bee. Horm. Behav. 37(1), 1–14 (2000).
Robinson, G. E. & Vargo, E. L. Juvenile hormone in adult eusocial Hymenoptera: gonadotropin and behavioral pacemaker. Arch. Insect Biochem. Physiol. 35, 559–583 (1997).
Dolezal, A. G., Brent, C. S., Gadau, J., Hölldobler, B. & Amdam, G. V. Endocrine physiology of the division of labour in Pogonomyrmex californicus founding queens. Anim. Behav. 77, 1005–1010 (2009).
Penick, C. A., Liebig, J. & Brent, C. S. Reproduction, dominance, and caste: endocrine profiles of queens and workers of the ant Harpegnathos saltator. J. Comp. Physiol. A. 197, 1063–1071 (2011).
Lengyel, F., Westerlund, S. & Kaib, M. Juvenile hormone III influences task-specific cuticular hydrocarbon profile changes in the ant Myrmicaria eumenoides. J. Chem. Ecol. 33, 167–181 (2007).
Khila, A. & Abouheif, E. Reproductive constraint is a developmental mechanism that maintains social harmony in advanced ant societies. PNAS 105, 17884–17889 (2008).
Brent, C. S. & Vargo, E. L. Changes in juvenile hormone biosynthetic rate and whole body content in maturing virgin queens of Solenopsis invicta. J. Insect Physiol. 49, 967–974 (2003).
Calkins, T. L. et al. Brain gene expression analyses in virgin and mated queens of fire ants reveal mating-independent and socially regulated changes. Ecol. Evol. 8, 4312–4327. https://doi.org/10.1002/ece3.3976 (2018).
Vargo, E. L. Reproductive development and ontogeny of queen pheromone production in the fire ant Solenopsis invicta. Physiol. Entomol. 24, 370–376. https://doi.org/10.1046/j.1365-3032.1999.00153.x (1999).
Hawkings, C., Calkins, T. L., Pietrantonio, P. V. & Tamborindeguy, C. Caste-based differential transcriptional expression of hexamerins in response to a juvenile hormone analog in the red imported fire ant (Solenopsis invicta). PLoS ONE 14, e0216800 (2019).
Hawkings, C. & Tamborindeguy, C. Expression analysis of vitellogenins in the workers of the red imported fire ant (Solenopsis invicta). PeerJ 6, e4875 (2018).
Tschinkel, W. R. The fire ants. (Harvard University Press, 2006).
Cassill, D. L. & Tschinkel, W. R. Task selection by workers of the fire ant Solenopsis invicta. Behav. Ecol. Sociobiol. 45, 301–310 (1999).
Norman, V. C. & Hughes, W. O. Behavioural effects of juvenile hormone and their influence on division of labour in leaf-cutting ant societies. J. Exp. Biol. 219, 8–11 (2016).
Wyatt, G. R. & Davey, K. G. in Advances in insect physiology Vol. 26 1–155 (Elsevier, 1996).
Norman, V. C. & Hughes, W. O. Behavioural effects of juvenile hormone and their influence on division of labour in leaf-cutting ant societies. J. Exp. Biol. 219(1), 8–11 (2016).
Drees, B. M., Summerlin, B. & Vinson, S. B. Foraging activity and temperature relationship for the red imported fire ant. Southwest. Entomol. 32, 149–155 (2007).
Vogt, J. T., Smith, W. A., Grantham, R. A. & Wright, R. E. Effects of temperature and season on foraging activity of red imported fire ants (Hymenoptera: Formicidae) in Oklahoma. Environ. Entomol. 32, 447–451 (2004).
Tobback, J., Mommaerts, V., Vandersmissen, H. P., Smagghe, G. & Huybrechts, R. Age-and task-dependent foraging gene expression in the bumblebee Bombus terrestris. Arch. Insect Biochem. Physiol. 1, 40–42 (2011).
Mirenda, J. T. & Vinson, S. B. Division of labour and specification of castes in the red imported fire ant Solenopsis invicta Buren. Anim. Behav. 29, 410–420 (1981).
Giray, T., Giovanetti, M. & West-Eberhard, M. J. Juvenile hormone, reproduction, and worker behavior in the neotropical social wasp Polistes canadensis. Proc. Natl. Acad. Sci. 102, 3330–3335 (2005).
Acknowledgements
We would like to thank the Dr. Roger E. Gold Endowed Graduate Student Scholarship, the J.H. Benedict Sr. Memorial Graduate Student Scholarship, and the Pest Management Foundation Scholarship from the National Pest Management Association for additional funding that was put towards the projects performed in this manuscript. We would also like to thank the Department of Entomology at Texas A&M University for the additional funding sources and opportunities that have been provided to further aid in our research. Jesse Starkey is a PhD candidate in the Entomology Graduate Program at Texas A&M University.
Author information
Authors and Affiliations
Contributions
All authors designed the experiments; J.S. analyzed the data, wrote the main manuscript and prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Starkey, J., Hawkings, C. & Tamborindeguy, C. Influence of juvenile hormone analog on behavior in the red imported fire ant, Solenopsis invicta. Sci Rep 13, 14726 (2023). https://doi.org/10.1038/s41598-023-41540-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41540-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.