Abstract
Food and Drug Administration (FDA) recently approved co-formulated celecoxib and tramadol for the treatment of acute pain in adults. Three spectrophotometric methods were efficiently applied to estimate the co-formulated Celecoxib and Tramadol in their tablets; second derivative 2D-spectrophotometry technique (method I), induced dual-wavelength technique (method II) and dual-wavelength resolution technique (method III). The proposed methods were successfully validated following the International Council for Harmonisation (ICH) guidelines and statistically assessed based on the correlation coefficients, relative standard deviations as well as detection and quantitation limits. The obtained results revealed non-significant differences compared to the reported results as revealed by the variance ratio F test and Student t test. Moreover, the applied techniques were further assessed concerning their greenness based on the analytical eco-scale method revealing an excellent green scale with a final score of 95. The proposed spectrophotometric techniques could be applied for the routine analysis and quality control of the studied drugs in their dosage form.
Similar content being viewed by others
Introduction
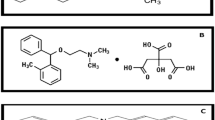
Celecoxib (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benzene sulfonamide) (Fig. 1) is a COX-2 selective NSAID, approved for the treatment of patients suffering from rheumatism and osteoarthritis1. It is considered an alternative drug to curtail the upper gastrointestinal toxicity of non-selective NSAIDs and can be prescribed in rheumatoid arthritis or for osteoarthritic patients as a first-line NSAID2. Other studies suggest its potential use against numerous cancers, whether in combination with other drugs3 or via modification of its chemical structure and creation of different analogs4. Several reported methods of analysis for Celecoxib are found in the literature whether alone or with other drugs, including spectrophotometric methods5,6,7, HPLC8,9,10,11, TLC12,13,14, and electrochemical methods15,16,17.
Tramadol (trans‐2‐ [(dimethylamino) methyl] ‐1‐ (3‐methoxyphenyl) cyclohexanol) (Fig. 1) is a centrally acting analgesic drug utilized for the treatment of moderate to severe pain. Caution must be exercised upon the usage of tramadol because of its potential for substantial abuse and adverse side effects18. Recently, Tramadol was suggested as being beneficial in the treatment of COVID-1919. Numerous methods were reported for the analysis of Tramadol either alone or with different drugs, viz. HPLC20,21,22,23,24,25, HPTLC26, Gas Chromatography27,28, spectrophotometric methods29,30,31, electrochemical methods32,33,34,35, and capillary electrophoresis36,37.
Pharmaceutical cocrystals are a novel class of engineered solid forms and are one of the approaches for ameliorating the physicochemical and biopharmaceutical properties of drugs with no change in their chemical structures38,39. The mixture of interest was first identified as a novel active pharmaceutical ingredient (API-API) co-crystal formed by an intrinsic 1:1 molecular ratio of racemic-tramadol.HCl and celecoxib40 were found to produce antinociceptive effects in a postoperative pain model in rats41. Some clinical trials were applied and found that the mixture may provide an appropriate addition to pain therapy because of its unique structure and improved pharmacokinetics in addition to its higher risk–benefit ratio42,43,44. Furthermore, the co-formulation approach offers several advantages, such as low medication errors and augmented convenience for patients, albeit the disadvantages of increased complexity in drug development, characterization, and quality control45. An oral tablet dosage form each containing 56 mg of celecoxib as well as 44 mg of tramadol was later approved in October 202146. The preparation is indicated for the management of acute pain in adults in cases in which other alternative treatments have proven inadequate or not tolerated by patients47.
To the best of our knowledge, only one spectrophotometric method for the assessment of the aforementioned mixture was reported so far48. In addition, the complete overlap of spectra of both drugs poses a challenge for the simultaneous estimation of both drugs. This paper presents three spectrophotometric methods; 2nd derivative, induced dual-wavelength, and dual-wavelength resolution techniques for the simultaneous assay of celecoxib and tramadol in their commercial product. The proposed method is successfully applied for the simultaneous estimation of the studied drugs in dosage form with better LOD and LOQ for tramadol. In addition, using the 2nd derivative technique requires minimal mathematical manipulation than the others. Moreover, the IDW technique is considered relatively new.
An expanded attentiveness to the implementation of green chemistry principles in analytical techniques has grown vastly49. For assessing the greenness of analytical techniques, several methods are employed viz. the Analytical Eco-Scale Metric that considers various features of the analytical technique to calculate the procedure's green index50. The assessment of our applied techniques using the Analytical Eco-Scale Metric was therefore employed for the determination of their greenness indices.
Theoretical background
Method I: induced dual wavelength method (IDW)
This method is intended for application to a binary mixture (X and Y) having completely overlapped zero-order absorption spectra at two wavelengths λ1 and λ2 respectively, in which the absorbance of the interfering substances at the selected wavelengths are not equal (so that absorbance difference at the said wavelengths is not equal to zero), rendering the conventional dual-wavelength method inapplicable51. The technique is relatively new and was utilized a few times to resolve some challenging components in mixtures5,51,52,53,54,55,56,57. Briefly, the equations describing this situation are as follows:
where
A1 is the mixture absorbance at λ1 which is chosen as the λmax of X.
A2 is the mixture absorbance at λ2 which is any other wavelength.
An equality factor is calculated for component Y to eliminate its effect at the two wavelengths as follows:
Substitution in Eq. (1) gives:
Then multiplying Eq. (2) by the equality factor FY gives:
Subtraction of Eq. (4) from Eq. (3) gives:
The inspection of Eq. (5) indicates the sole dependence of the absorbance difference of the mixture on the CX with no interference from CY. Thus, the concentration of component X can be computed from the following regression equation:
Via plotting the absorbance difference values of the zero-order spectra of pure X at the selected wavelengths (∆A = A1 − FYA2) versus the corresponding concentrations of X, the corresponding regression equation can be obtained.
Method II: dual wavelength resolution technique (DWRT)
This method is applied when the IDW method is inapplicable to determine the concentration of the other component Y51. After the calculation of the concentration of the 1st component X using the IDW technique, the zero-order spectrum of component X is acquired by multiplication of the calculated concentration by the normalized absorptivity curve of component Y. The normalized absorptivity curve of component X is obtained by the division of the whole spectrum of X by its corresponding concentration giving a spectrum indicating the absorptivity of the analyte of interest (aX) against all the measured wavelengths. The next step is obtaining the spectrum of component Y by subtracting the calculated spectrum of X from the spectrum of the corresponding mixture. Due to the problem of obtaining indefinite peaks in the zero-order spectrum of Y, the first derivative spectrum was obtained as a result and the corresponding regression equation was computed.
Method III: 2nd derivative technique
The 2nd derivative spectrophotometry was successfully applied for the simultaneous estimation of both drugs without a prior separation step. This method has the advantage of the evaluation of complex mixtures without chemical treatment. However, difficulties may sometimes arise as the signal-to-noise ratio can be reduced, in addition to the complex profiles obtained58.
Experimental
Apparatus
A Shimadzu ultraviolet–visible (UV–Vis) 1601 recording Spectrophotometer (P/N 206-67001, Japan) over the range of 190–500 nm was utilized for all measurements. The path length of the used cuvettes was 1 cm. Spectral mathematical manipulation was performed using UVProbe 2.42 software.
Pure standards
Tramadol HCl was obtained from Fluka BioChemika, Buchs, Switzerland, with labeled purity of 99.0%.
Celecoxib with labeled purity of 99.44% was obtained from Amoun Pharmaceutical Co. (Cairo, Egypt).
Excipients used in the prepared tablets include talc, starch, magnesium stearate, avicel PH 112 FMC, and gelatin. All excipients were obtained from Sigma Co. for Pharmaceutical Industries, Quesna, Egypt.
Solvents and chemicals
Methanol, ethanol, and acetonitrile (HPLC grade) were purchased from Fisher Scientific (UK).
Sodium hydroxide and HCl were purchased from El Nasr Pharmaceutical Chemicals Company (ADWIC, Cairo, Egypt).
Standard solutions
Stock solutions of each celecoxib and tramadol were prepared separately using methanol to reach the concentration of 100 μg/mL. Further working solutions were freshly prepared using distilled water in 10 mL volumetric flasks as needed.
Procedure
Spectral characteristics
Working solutions of celecoxib and tramadol were prepared separately by transferring appropriate volumes from corresponding stock solutions in 10 mL volumetric flasks. The volumes were completed to the mark using distilled water as a solvent. The blank used was distilled water and methanol in the ratio 1:1. The zero-order spectra of each compound were measured within the range 190–500 nm and stored in the computer.
Application of second derivative spectrophotometry
Series of different concentrations (2, 2.5, 3, 5, 7, 9, 12, 17, and 20 μg/mL) of celecoxib were prepared by transferring different volumes from stock solution into 10 mL volumetric flasks and performing dilution with distilled water to the mark. Similarly, a series of different concentrations (5, 7, 12, 15, 17, 25, 30, 35, 40, 45, and 50 μg/mL) of tramadol HCl were prepared using distilled water for dilution. The absorption spectra of each concentration were measured against a blank consisting of distilled water and methanol in the ratio of 1:1 and then were stored in the computer. Then, each spectrum was smoothed (∆λ = 4 nm) and the second derivative of each spectrum was obtained (∆λ = 4 nm, scaling factor = 100). The chosen wavelengths were 269.45 nm and 295.58 nm for tramadol and celecoxib, respectively. The calibration curve was constructed by plotting the amplitudes at the chosen wavelengths versus the corresponding concentrations and the regression equation was subsequently obtained.
Application of induced dual wavelength (IDW) technique
Series of concentrations (6, 8, 9, 10, 11.5, 13, 14, 15, 17, 18, and 20 μg/mL) were prepared as previously described in accordance with the ratio of celecoxib in dosage forms (5.5 tramadol: 7 celecoxib). The absorbance at wavelengths 250 nm and 271 nm was recorded. The equality factor required to diminish the effect of Tramadol in the mixture was calculated by measuring the absorbance of the corresponding tramadol HCl concentrations at the same wavelengths and dividing the first absorbance reading (250 nm) by the second one (271 nm). Afterward, the absorbance of celecoxib at 271 nm was multiplied by this equality factor, and the result was subtracted from the absorbance at 250 nm to obtain ∆A. The calibration curve was constructed by plotting ∆A against the corresponding concentration and the regression equation was computed.
Application of dual wavelength resolution technique (DWR)
Firstly, the normalized absorptivity curve for celecoxib was calculated by dividing the absorption spectrum of the drug by its corresponding concentration. A number of 11 normalized absorptivity curves was obtained and the average absorptivity curve was calculated. Secondly, the calculated absorption spectrum of celecoxib was obtained (after calculation of the concentration using the previous method) by multiplication of the calculated concentration of celecoxib by its average normalized absorptivity curve. After that, the corresponding absorption spectrum of tramadol was obtained by subtracting the calculated spectrum of celecoxib from the whole spectrum of the mixture. Finally, the obtained spectrum was smoothed (∆λ = 4 nm) and the first derivative was obtained (∆λ = 4 nm, scaling factor = 100). The absorbance of tramadol was then measured at 281.68 nm. The calibration curve was constructed by plotting the amplitudes at the chosen wavelengths against the corresponding concentrations and the regression equation was obtained.
Preparation of synthetic mixtures
A number of 11 mixtures was prepared with concentration ratios in the range of 5–15 μg/mL and 6–20 μg/mL for Tramadol and Celecoxib, respectively for the construction of calibration curves for both the Induced dual wavelength (IDW) and Dual wavelength resolution (DWRT) techniques. For the 2nd derivative technique, Celecoxib concentrations of 2, 2.5, 3, 5, 7, 9, 12, 17, and 20 μg/mL and Tramadol HCl concentrations of 5, 7, 12, 15, 17, 25, 30, 35, 40, 45and 50 μg/mL were used for the construction of calibration curves.
Laboratory-prepared tablets
Since the dosage form is not yet available in the Egyptian market, the laboratory-prepared tablet was used. Formula per tablet was prepared by weighing 56 mg of celecoxib, 44 mg of tramadol HCl, talc, starch, magnesium stearate, gelatin, and Avicel pH 112 FMC. The ingredients were triturated in a porcelain mortar and then transferred to a 100 mL volumetric flask. Around 60 mL of methanol was added. After sonication for 30 min, the volume was completed to 100 mL with the same solvent. The solution was subsequently double-filtered using Whatmann no. 1 filter paper. Aliquot volumes were transferred to 10 mL measuring flasks and completed to the mark with distilled water to reach the following concentration ratios (7:9, 9:11.5, and 10:13 tramadol to celecoxib, respectively).
Evaluation of the method greenness
The method greenness was assessed based on the analytical Eco-scale approach which utilizes attributing penalty points to parameters that don’t abide by the ideal green analysis. The analytical eco-scale was obtained using the following equation49:
Results and discussion
The completely overlapped spectra of the two drugs represented a challenge for their estimation (Fig. 2), hampering the use of other conventional methods such as the ratio spectra, ratio derivative, etc.
The IDW technique was successfully applied to resolve celecoxib in the mixture of interest (Fig. 3).
The DWRT was used for the estimation of tramadol as the IDW technique was inapplicable (Fig. 4).
The 2nd derivative technique was successfully applied for estimation of both drugs in their binary mixtures (Figs. 5, 6).
Optimization of experimental conditions
Effect of various solvents
Different solvents such as methanol, ethanol, and acetonitrile in addition to 0.1N HCl and 0.1N NaOH were used. No significant difference was found from spectra obtained in water, so distilled water was the solvent of choice due to its availability, cheap cost, and environmental safety.
Analytical performance and method validation
The methods were validated in accordance with the ICH guidelines59. The data indicated that the methods were of acceptable accuracy, precision, and specificity over the specified linearity range.
Linearity and range
The linear range as well as the correlation coefficient are calculated and the obtained results are summarized in Table 1. The linearity was evaluated by the least squares method indicating acceptable accuracy in accordance with ICH guidelines59.
Limits of detection (LOD) and limits of quantification (LOQ)
The Limit of Detection (LOD) and limit of quantification (LOQ) for each method were mathematically computed following ICH guidelines59 and the data obtained are abridged in Table 1. The LOD was calculated from the following equation:
While the LOQ was calculated from the following equation:
where σ is the standard deviation of the response and s is the slope of the calibration curve estimated from the regression line. The ICH guidelines mandate the signal-to-noise ratio to be equal to 3:1 and 10:1 in the case of LOD and LOQ, respectively59.
Precision and accuracy
For the estimation of accuracy, the results of the proposed methods were compared to those obtained from previously reported methods following the guidelines of ICH using three replicate results for three different concentrations within the linear range59. For tramadol HCl, it was determined in methanol through its zero order spectra at λmax = 275 nm60, whereas for celecoxib, it was determined in 0.1N NaOH as solvent through its 1st order spectra at λmax = 269 nm61. These methods were also applied to the dosage forms and no significant difference was found as per the values of t test and F values62 (Table 2).
Intra and Inter day precision were studied by applying the proposed methods for the determination of three concentrations within the studied ranges on three successive times or three successive days in accordance with the ICH guidelines59. (Table 3).
Robustness
Distilled water was used for simplicity throughout the determination. No significant change was found when using different pH such as 0.1N NaOH or 0.1N HCl, so no buffer was used during the procedure. The robustness of the experimental procedure is thus confirmed as it was proven reliable regarding deliberate variations in parameters in agreement with ICH guidelines59.
Selectivity
Using the 2nd derivative technique, celecoxib was determined at λmax = 295.58 nm with no interference from tramadol HCl. Likewise, tramadol was determined at λmax = 269.45 nm with no interference from celecoxib, indicating acceptable Selectivity. Furthermore, Using the IDW technique, the selectivity was enhanced and the interference from tramadol HCl was completely diminished by the equality factor, leading to the successful determination of celecoxib. Additionally, tramadol was successfully determined using DWRT with no interference from celecoxib. The excipients added to the laboratory-prepared tablets showed no interference with the applied techniques. Based on these findings, these methods provide good selectivity for the simultaneous determination of both drugs in a synthetic mixture and prepared tablet in the presence of excipients according to the requirements of the ICH59.
Application on laboratory prepared tablets
Table 4 shows the data of the proposed methods applied to the laboratory-prepared tablet. The results indicate that the proposed methods have acceptable accuracy and precision with respect to both drugs under investigation. In addition, no significant interference from the excipients in the prepared tablets was found, indicating acceptable selectivity for both drugs in the laboratory-prepared tablets.
Evaluation of the method greenness according to the analytical eco-scale approach
The concept of green chemistry aims at reducing both using and generating toxic hazardous materials. However, certain difficulties might arise upon evaluating the analytical methodologies such as the number of analytes to be determined, the techniques to be used as well as some important parameters needed to be considered such as LOD. In addition, the least green phase in the analytical process is sample preparation according to previously reported data49. Table 5 estimates the final score to be 95, indicating excellent green analysis techniques.
Conclusion
The goal of this study was to implement three Eco-friendly spectrophotometric methods for the assessment of the synthetic mixtures in addition to laboratory-prepared tablets of the recently approved drug combination (celecoxib/tramadol HCl). The applied methods including; the second derivative 2D-spectrophotometry technique (method I), induced dual-wavelength technique (method II), and dual-wavelength resolution technique (method III) revealed acceptable accuracy, good linearity, reproducibility as well as precision and can be applied for the routine analysis and quality control of the co-formulated mixture. Further, the methods also have the additional advantages of speed and simplicity, in addition to environmental safety as they don’t require the use of hazardous solvents or sophisticated instruments. Statistical analysis has been accomplished illustrating non-significant differences compared to the previously reported data. Furthermore, the greenness index of the applied methods was measured via Eco-scale metric revealing an excellent green index of the methods employed.
The proposed study poses simple, rapid, and reliable methods for the analytical assessment of the newly approved mixture besides being Eco-friendly.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Lipton, R. B., Munjal, S., Tepper, S. J., Iaconangelo, C. & Serrano, D. A multicenter, randomized, double-blind, placebo-controlled study of the efficacy, tolerability, and safety of celecoxib oral solution (ELYXYB) in acute treatment of episodic migraine with or without aura. J. Pain Res. 14, 2529–2542. https://doi.org/10.2147/jpr.s322292 (2021).
Nurmohamed, M. et al. Cardiovascular safety of celecoxib in rheumatoid arthritis and osteoarthritis patients: A systematic review and meta-analysis. PLoS ONE 16, e0261239. https://doi.org/10.1371/journal.pone.0261239 (2021).
Srivastava, S. et al. Piperine and celecoxib synergistically inhibit colon cancer cell proliferation via modulating Wnt/β-catenin signaling pathway. Phytomedicine 84, 153484. https://doi.org/10.1016/j.phymed.2021.153484 (2021).
Sobolewski, C. & Legrand, N. Celecoxib analogues for cancer treatment: An update on OSU-03012 and 2,5-dimethyl-celecoxib. Biomolecules 11, 1049. https://doi.org/10.3390/biom11071049 (2021).
Attala, K. & Elsonbaty, A. Smart UV spectrophotometric methods based on simple mathematical filtration for the simultaneous determination of celecoxib and ramipril in their pharmaceutical mixtures with amlodipine: A comparative statistical study. Spectrochim. Acta Part A 244, 118853. https://doi.org/10.1016/j.saa.2020.118853 (2021).
Sharkawi, M. M. Z., Mohamed, N. R., El-Saadi, M. T. & Amin, N. H. Five spectrophotometric methods for simultaneous determination of amlodipine besylate and celecoxib in presence of its toxic impurity. Spectrochim. Acta Part A 263, 120137. https://doi.org/10.1016/j.saa.2021.120137 (2021).
Attala, K., Eissa, M. S., Hasan, M. A., El-Henawee, M. M. & Abd El-Hay, S. S. An enhanced first derivative synchronous spectrofluorimetric method for determination of the newly co-formulated drugs, amlodipine and celecoxib in pharmaceutical preparation and human plasma. Spectrochim. Acta Part A 240, 118533. https://doi.org/10.1016/j.saa.2020.118533 (2020).
Gadge, M. & Jagtap, V. G. Stability indicating HPLC method for development and validation of simultaneous estimation of amlodipine and celecoxib from bulk and marked formulation. Int. J. Res. Publ. Rev., 133–140 (2020).
Abdel Hamid, M. A., Mabrouk, M. M. & Michael, M. A. A fast and green reversed-phase HPLC method with fluorescence detection for simultaneous determination of amlodipine and celecoxib in their newly approved fixed-dose combination tablets. J. Sep. Sci. 43, 3197–3205. https://doi.org/10.1002/jssc.202000345 (2020).
Attimarad, M., Venugopala, K. N., SreeHarsha, N., Aldhubiab, B. E. & Nair, A. B. Validation of rapid RP-HPLC method for concurrent quantification of amlodipine and celecoxib in pure and formulation using an experimental design. Microchem. J. 152, 104365. https://doi.org/10.1016/j.microc.2019.104365 (2020).
Srinivasu, M. K., Sreenivas Rao, D. & Reddy, G. O. Determination of celecoxib, a COX-2 inhibitor, in pharmaceutical dosage forms by MEKC. J. Pharm. Biomed. Anal. 28, 493–500. https://doi.org/10.1016/S0731-7085(01)00670-7 (2002).
Rizk, M., Toubar, S., Ramzy, E. & Helmy, M. Sensitive and validated TLC densitometry method coupled with fluorescence detection for quantitative determination of the newly co-formulated drugs, celecoxib and amlodipine besylate in tablet dosage form. Acta Chromatogr. 34, 150–161. https://doi.org/10.1556/1326.2021.00890 (2021).
Rizk, M. M. S., Toubar, S. S., Gadallah, E. R. A. A. & Helmy, M. I. M. A Rapid and reliable thin-layer chromatographic method for the simultaneous estimation of celecoxib and diacerein in their binary mixture using nanosilica gel plate. JPC J. Planar Chromatogr. Mod. TLC 33, 511–522. https://doi.org/10.1007/s00764-020-00064-7 (2020).
Attala, K., Eissa, M. S., El-Henawee, M. M. & Abd El-Hay, S. S. Application of quality by design approach for HPTLC simultaneous determination of amlodipine and celecoxib in presence of process-related impurity. Microchem. J. 162, 105857. https://doi.org/10.1016/j.microc.2020.105857 (2021).
Parsaee, Z., Karachi, N., Abrishamifar, S. M., Kahkha, M. R. R. & Razavi, R. Silver-choline chloride modified graphene oxide: Novel nano-bioelectrochemical sensor for celecoxib detection and CCD-RSM model. Ultrason. Sonochem. 45, 106–115. https://doi.org/10.1016/j.ultsonch.2018.03.009 (2018).
Arkan, E., Karimi, Z., Shamsipur, M. & Saber, R. Electrochemical determination of celecoxib on a graphene based carbon ionic liquid electrode modified with gold nanoparticles and its application to pharmaceutical analysis. Anal. Sci. 29, 855–860 (2013).
Ghoneim, M. Adsorptive stripping voltammetric determination of the anti-inflammatory drug celecoxib in pharmaceutical formulation and human serum. Talanta 60, 911–921. https://doi.org/10.1016/s0039-9140(03)00151-6 (2003).
Nakhaee, S. et al. A review on tramadol toxicity: Mechanism of action, clinical presentation, and treatment. Forensic Toxicol. 39, 293–310. https://doi.org/10.1007/s11419-020-00569-0 (2021).
El-Ashmawy, N. E. et al. The plausible mechanisms of tramadol for treatment of COVID-19. Med. Hypotheses 146, 110468. https://doi.org/10.1016/j.mehy.2020.110468 (2021).
Ibrahim, A. E., Hashem, H., Elhenawee, M. & Saleh, H. Core–shell particles and monolithic columns; tools for simultaneous LC analysis of avanafil, sildenafil, apomorphine, trazodone, yohimbine, tramadol and dapoxetine in pharmaceutical dosage forms, counterfeit products and human plasma. RSC Adv. 10, 1379–1387. https://doi.org/10.1039/c9ra08717f (2020).
Pereira, F. J., Rodríguez-Cordero, A., López, R., Robles, L. C. & Aller, A. J. Development and validation of an RP-HPLC-PDA method for determination of paracetamol, caffeine and tramadol hydrochloride in pharmaceutical formulations. Pharmaceuticals 14, 466. https://doi.org/10.3390/ph14050466 (2021).
Hamdy, M. M. A. & Abdel Moneim, M. M. HPLC-fluorescence detection for assay of tramadol binary mixtures with ibuprofen or chlorzoxazone in tablets and plasma: Analytical Eco-Scale and GAPI tools for green assessment. Acta Chromatogr. 34, 185–196. https://doi.org/10.1556/1326.2021.00901 (2021).
Yu, H., Hong, S., Jeong, C.-H., Bae, J.-W. & Lee, S. Development of a linear dual column HPLC–MS/MS method and clinical genetic evaluation for tramadol and its phase I and II metabolites in oral fluid. Arch. Pharmacal. Res. 41, 288–298. https://doi.org/10.1007/s12272-017-0993-z (2018).
Abdelshakour, M. A., Salam, R. A. A., Hadad, G. M., Abo-ElMatty, D. M. & Hameed, E. A. A. HPLC-UV and UPLC-MS/MS methods for the simultaneous analysis of sildenafil, vardenafil, and tadalafil and their counterfeits dapoxetine, paroxetine, citalopram, tramadol, and yohimbine in aphrodisiac products. RSC Adv. 11, 8055–8064 (2021).
Belal, F. et al. Direct injection microemulsion HPLC method for simultaneous determination of morphine, tramadol and lornoxicam in biological fluids using monolithic column. Curr. Pharm. Anal. 16, 1148–1156. https://doi.org/10.2174/1573412915666190617172144 (2020).
Dhumal, B. R., Bhusari, K. P., Patra, A., Thareja, S. & Jain, N. S. Stability indicating high performance thin layer chromatographic method for the determination of tramadol hydrochloride in pharmaceutical formulation. J. Liq. Chromatogr. Relat. Technol. 38, 1088–1093. https://doi.org/10.1080/10826076.2015.1020167 (2015).
Naguib, I. A., Ali, N. A., Elroby, F. A., Ghobashy, M. R. E. & Abdallah, F. F. US FDA-validated green GC–MS method for analysis of gabapentin, tramadol and/or amitriptyline mixtures in biological fluids. Bioanalysis 12, 1521–1533. https://doi.org/10.4155/bio-2020-0217 (2020).
Yilmaz, B. & Erdem, A. F. Simultaneous determination of tramadol and its metabolite in human urine by the gas chromatography–mass spectrometry method. J. Chromatogr. Sci. 53, 1037–1043 (2015).
Akula, G., Sapavatu, S. N., Jadi, R. K., Battineni, J. K. & Boggula, N. Analytical method development and validation for the estimation of tramadol in bulk and its formulations by UV-spectroscopy. J. Adv. Sci. Res. 12, 77–83 (2021).
Glavanović, S., Glavanović, M. & Tomišić, V. Simultaneous quantitative determination of paracetamol and tramadol in tablet formulation using UV spectrophotometry and chemometric methods. Spectrochim. Acta Part A 157, 258–264. https://doi.org/10.1016/j.saa.2015.12.020 (2016).
Tolba, M. M. & Salim, M. M. Inclusive study for segregation of two commonly used anticancer drugs with tramadol: Applying a green fluorimetric strategy to pharmaceutical dosage forms and human plasma. Microchem. J. 162, 105859. https://doi.org/10.1016/j.microc.2020.105859 (2021).
Aflatoonian, M. R. et al. A screen-printed electrode modified with graphene/Co3O4 nanocomposite for electrochemical detection of tramadol. Front. Chem. 8, 532308. https://doi.org/10.3389/fchem.2020.562308 (2020).
Dehdashti, A. & Babaei, A. Designing and characterization of a novel sensing platform based on Pt doped NiO/MWCNTs nanocomposite for enhanced electrochemical determination of epinephrine and tramadol simultaneously. J. Electroanal. Chem. 862, 113949. https://doi.org/10.1016/j.jelechem.2020.113949 (2020).
Bagherinasab, Z., Beitollahi, H., Yousefi, M., Bagherzadeh, M. & Hekmati, M. Rapid sol gel synthesis of BaFe12O19 nanoparticles: An excellent catalytic application in the electrochemical detection of tramadol in the presence of acetaminophen. Microchem. J. 156, 104803. https://doi.org/10.1016/j.microc.2020.104803 (2020).
Jahromi, Z., Mirzaei, E., Savardashtaki, A., Afzali, M. & Afzali, Z. A rapid and selective electrochemical sensor based on electrospun carbon nanofibers for tramadol detection. Microchem. J. 157, 104942. https://doi.org/10.1016/j.microc.2020.104942 (2020).
Naghdi, E. & Fakhari, A. R. Simultaneous chiral separation of tramadol and methadone in tablets, human urine, and plasma by capillary electrophoresis using maltodextrin as the chiral selector. Chirality 30, 1161–1168. https://doi.org/10.1002/chir.23008 (2018).
Zhang, C., Liu, T., Zhang, X. & Liu, E. Determination of tramadol in human serum by capillary electrophoresis with the end-column electrochemiluminescence detection. Open J. Biophys. https://doi.org/10.4236/ojbiphy.2013.32015 (2013).
Schultheiss, N. & Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 9, 2950–2967. https://doi.org/10.1021/cg900129f (2009).
Good, D. J. & Rodríguez-Hornedo, N. Cocrystal eutectic constants and prediction of solubility behavior. Cryst. Growth Des. 10, 1028–1032. https://doi.org/10.1021/cg901232h (2010).
Almansa, C. et al. Co-crystal of tramadol hydrochloride-celecoxib (ctc): A novel API–API co-crystal for the treatment of pain. Cryst. Growth Des. 17, 1884–1892. https://doi.org/10.1021/acs.cgd.6b01848 (2017).
Merlos, M. et al. Administration of a co-crystal of tramadol and celecoxib in a 1:1 molecular ratio produces synergistic antinociceptive effects in a postoperative pain model in rats. Eur. J. Pharmacol. 833, 370–378. https://doi.org/10.1016/j.ejphar.2018.06.022 (2018).
Videla, S. et al. Single-dose pharmacokinetics of co-crystal of tramadol–celecoxib: Results of a four-way randomized open-label phase I clinical trial in healthy subjects. Br. J. Clin. Pharmacol. 83, 2718–2728. https://doi.org/10.1111/bcp.13395 (2017).
Gascon, N. et al. Co-crystal of tramadol-celecoxib: Preclinical and clinical evaluation of a novel analgesic. Expert Opin. Investig. Drugs 28, 399–409. https://doi.org/10.1080/13543784.2019.1612557 (2019).
López-Cedrún, J. et al. Co-crystal of tramadol-celecoxib in patients with moderate to severe acute post-surgical oral pain: A dose-finding, randomised, double-blind, placebo- and active-controlled, multicentre. Phase II Trial. Drugs in R&D 18, 137–148. https://doi.org/10.1007/s40268-018-0235-y (2018).
Kim, J. et al. Analytical characterization of coformulated antibodies as combination therapy. In mAbs vol. 12, doi:https://doi.org/10.1080/19420862.2020.1738691 (2020).
FDA, <https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process> (
Hemant Kumar, T., Manjunath, S. Y. & Nagamani, P. Development and validation of RP-HPLC method for estimation of amlodipine besylate and celecoxib in pharmaceutical formulation. J. Drug Deliv. Therap. 10, 31–36. https://doi.org/10.22270/jddt.v10i6.4521 (2020).
Abdelazim, A. H. et al. Quantitative spectrophotometric analysis of celecoxib and tramadol in their multimodal analgesia combination tablets. J. AOAC Int. 105, 1479–1483. https://doi.org/10.1093/jaoacint/qsac049 (2022).
Gałuszka, A., Migaszewski, Z. M., Konieczka, P. & Namieśnik, J. Analytical eco-scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 37, 61–72. https://doi.org/10.1016/j.trac.2012.03.013 (2012).
Sajid, M. & Płotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 238, 123046. https://doi.org/10.1016/j.talanta.2021.123046 (2022).
Lotfy, H. M., Saleh, S. S., Hassan, N. Y. & Salem, H. Novel two wavelength spectrophotometric methods for simultaneous determination of binary mixtures with severely overlapping spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 136, 1786–1796. https://doi.org/10.1016/j.saa.2014.10.084 (2015).
El-Henawee, M. M., Abd El-Hay, S. S., Attala, K. & Eissa, M. S. Smart UV spectrophotometric methods based on simple mathematical filtration and classical methods for the simultaneous determination of tamsulosin and solifenacin: A comparative study of efficacy and spectral resolution. Spectrochim. Acta Part A 247, 119151. https://doi.org/10.1016/j.saa.2020.119151 (2021).
Abdel Razeq, S. A., Abdel Aziz, S. E. & Ahmed, N. S. Stability-indicating UPLC, TLC-densitometric and UV-spectrophotometric methods for alcaftadine determination. J. Chromatogr. B 1177, 122804. https://doi.org/10.1016/j.jchromb.2021.122804 (2021).
Alhaj Sakur, A. & Kayali, Z. Development of four UV-spectrometric techniques for concurrent estimation of aspirin and sildenafil citrate in their binary mixture and pharmaceutical formulations. Bull. Pharm. Sci. Assiut 45, 761–773 (2022).
Patel, E. S., Hinge, M. A. & Patel, A. A. Development and validation of UV spectrophotometric methods for simultaneous estimation of trimetazidine hydrochloride and metoprolol succinate in tablet dosage form. J. Pharm. Sci. (2016).
Demerdash, A. O. E., Razeq, S. A. A., Fouad, M. M. & Sanabary, H. F. E. Densitometric and UV-spectrophotometric methods for simultaneous determination of spiramycin adipate in binary mixture with oxytetracycline-HCl or tetracycline-HCl. Int. Res. J. Pure Appl. Chem. 17, 1–21. https://doi.org/10.9734/irjpac/2018/44345 (2018).
Abdel Razeq, S. A., Khalil, I. A. & Mohammed, S. A. Stability-indicating chromatographic and UV spectrophotometric methods for determination of colchicine. J. Iran. Chem. Soc. 17, 2415–2427. https://doi.org/10.1007/s13738-020-01937-8 (2020).
Gupta, D. et al. Simultaneous spectrophotometric determination of drug components from their dosage formulations. Spectrochim. Acta Part A 270, 120819. https://doi.org/10.1016/j.saa.2021.120819 (2022).
ICH guideline Q2(R2) on validation of analytical procedures. In International Conference on Harmonization, Geneva (2022).
Küçük, A. & Kadıoğlu, Y. Determination of tramadol hydrochloride in ampoule dosage forms by using UV spectrophotometric and HPLC-DAD methods in methanol and water media. Il Farmaco 60, 163–169. https://doi.org/10.1016/j.farmac.2004.12.002 (2005).
Bebawy, L., Moustafa, A. & Abo-Talib, N. Stability-indicating methods for the determination of doxazosin mezylate and celecoxib. J. Pharm. Biomed. Anal. 27, 779–793 (2002).
James N Miller & Miller, J. C. in Statistics and Chemometrics for Analytical Chemistry. Sixth edn, (Pearson Education Limited, 2010).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
H.S.R.: Formal analysis, Data curation, Writing—original draft, Conceptualization, Visualization, Investigation, Validation. G.M.H.: Conceptualization, Visualization, Validation, Writing—review & editing. R.A.A.: Conceptualization, Visualization, Validation, Writing—review & editing. F.B.: Conceptualization, Visualization, Validation, Writing—review & editing. M.M.S.: Conceptualization, Methodology, Software, Visualization, Validation, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramadan, H.S., Salam, R.A.A., Hadad, G.M. et al. Eco-friendly simultaneous multi-spectrophotometric estimation of the newly approved drug combination of celecoxib and tramadol hydrochloride tablets in its dosage form. Sci Rep 13, 11716 (2023). https://doi.org/10.1038/s41598-023-38702-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38702-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.