Abstract
Greater graft-failure-risk of female-to-male liver transplantation (LT) is thought to be due to acute decrease in hepatic-estrogen-signaling. Our previous research found evidence that female hepatic-estrogen-signaling decreases after 40 years or with macrosteatosis. Thus, we hypothesized that inferiority of female-to-male LT changes according to donor-age and macrosteatosis. We stratified 780 recipients of grafts from living-donors into four subgroups by donor-age and macrosteatosis and compared graft-failure-risk between female-to-male LT and other LTs within each subgroup using Cox model. In recipients with ≤ 40 years non-macrosteatotic donors, graft-failure-risk was significantly greater in female-to-male LT than others (HR 2.03 [1.18–3.49], P = 0.011). Within the subgroup of recipients without hepatocellular carcinoma, the inferiority of female-to-male LT became greater (HR 4.75 [2.02–11.21], P < 0.001). Despite good graft quality, 1y-graft-failure-probability was 37.9% (23.1%–57.9%) in female-to-male LT within this subgroup while such exceptionally high probability was not shown in any other subgroups even with worse graft quality. When donor was > 40 years or macrosteatotic, graft-failure-risk was not significantly different between female-to-male LT and others (P > 0.60). These results were in agreement with the estrogen receptor immunohistochemistry evaluation of donor liver. In conclusion, we found that the inferiority of female-to-male LT was only found when donor was ≤ 40 years and non-macrosteatotic. Abrogation of the inferiority when donor was > 40 years or macrosteatotic suggests the presence of dominant contributors for post-transplant graft-failure other than graft quality/quantity and supports the role of hepatic-estrogen-signaling mismatch on graft-failure after female-to-male LT.
Similar content being viewed by others
Introduction
The sex difference in graft failure risk is well-known in liver transplantation (LT). That is, post-transplant graft failure is more prevalent in male recipients with female donors, so-called female-to-male LT, compared to other donor-recipient sex combinations. Since the landmark study by Kahn et al.1, clinical studies have consistently demonstrated the inferiority of female-to-male LT2,3,4,5,6,7. Recent research further validated the inferiority of female-to-male LT in living donor LT8,9,10. Our previous research further demonstrated that the inferiority of female-to-male LT is not attributed to anatomical size mismatch between females and males (e.g. relatively small graft size)11.
Although the exact mechanisms underlying the sex difference is not fully understood, hepatic estrogen signaling mismatch is considered to play an important role12,13,14,15. The liver is one of the target organs of estrogen, and hepatic estrogen signaling plays an important role in mitigating injury and enhancing recovery of the liver10, 12, 16,17,18. Hepatic estrogen signaling increases in relation to the increase in systemic estrogen secretion or hepatic estrogen receptor content19. In this regard, previous experimental and clinical studies have demonstrated that the tolerance of various liver injuries is greater in females with greater systemic estrogen secretion and hepatic estrogen receptor content compared to males18, 20, 21. However, abrupt decrease in hepatic estrogen receptor occurs in the transplanted female liver after exposure to the male hormonal milieu14, and consequent decrease in hepatic estrogen signaling and impairment of hepatic protection capacity has been implicated in the greater graft failure risk6,7,8.
In our recent study of healthy living liver donors, we found that the tolerance of hepatic ischemia–reperfusion injury is greater in females than in males only when the age is ≤ 40 years (in relation to the decrease in female systemic estrogen secretion after 40 years, which is the age of poor ovarian reserve)22,23,24,25 and the liver is without macrosteatosis (in relation to the decrease in female hepatic estrogen receptor expression with macrosteatosis)16, 18, 26, 27. These findings suggested that female’s superior hepatic protection capacity decreases in relation to the decrease in hepatic estrogen signaling with poor ovarian reserve or macrosteatosis; thus, we deduced that the degree of acute post-transplant defeminization of the graft after female-to-male LT decreases in relation to donor poor ovarian reserve and macrosteatosis. In this study, we aimed to evaluate whether the inferiority of female-to-male LT changes by donor age of 40 years or graft macrosteatosis.
Patients and methods
Patients and data sources
We screened the records of 813 recipients who underwent a first adult-to-adult living donor LT between December 2005 and April 2016 (representing the 500th–1700th LT) in our hospital. We excluded 32 recipients of grafts consisting of a partial liver other than the right lobe (extended right lobe, n = 16; left lobe, n = 12; extended left lobe, n = 3; and left lateral segment, n = 1). We further excluded one recipient who underwent an auxiliary partial orthotopic LT. The remaining 780 recipients were included in the analysis. All data analyzed in the study were derived from our institution’s electronic medical records and LT database (prospectively collected). All recipients were followed until September 2017 or the last medical follow-up, for a maximum of 5 years. The Institutional Review Board of Samsung Medical Center approved this retrospective cohort study (SMC 2020-03-015-002) and waived the requirement for written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Liver donation and transplant criteria
The sex of the donor and recipient was not considered in selection of the donor. Acceptance criteria for liver donation were age of ≤ 65 y, body mass index < 35 kg/m2, macrosteatosis degree ≤ 30%28,29,30, and donor residual liver volume ≥ 30%. Individuals with any type of hepatitis, including steatohepatitis, or liver fibrosis were excluded from donation.
Histologic macrosteatosis evaluation
All living donors underwent a wedge liver biopsy before liver resection, and frozen biopsy sections were stained with hematoxylin–eosin and Oil Red O. In general, the size of the biopsy section was 3 μm in thickness, 15 mm in length, and 5 mm in width, containing 15–20 portal fields. Permanent biopsy sections were prepared with tissues being fixed in 4% neutral buffered formalin solution, embedded in paraffin, and stained with hematoxylin–eosin. The pathology report was recorded based on the review process of both frozen and permanent biopsy sections (5–8 serial sections in general), describing the average percentage of macrovesicular fatty droplets occupying the surface area of the parenchyma. For the purpose of the study, macrosteatosis was defined if ≥ 5% hepatocytes contain macrovesicular fat droplets based on a non-alcoholic fatty liver disease activity score system31, 32 as well as the study reporting that hepatic estrogen receptor content is lower in females with > 5% macrosteatosis than in females with ≤ 5% macrosteatosis16.
Estrogen receptor evaluation by immunohistochemistry
To examine the mechanisms underlying the specificity of female-to-male LT and its interaction with graft macrosteatosis and donor age, the data of our previous study on immunohistochemistry for estrogen receptor performed for the liver tissues biopsied during the donor hepatectomy were analyzed while 457 patients with available tissues were overlapped between the two studies. The liver tissue was stored in an in-house bio-bank using a a Bond-max autoimmunostainer (Leica Biosystem, Melbourne, Australia) with Bond™ Polymer refined detection, DS9800 (Vision Biosystems, Melbourne, Australia). After deparaffinization of formalin-fixed and paraffin-embedded tissue sections in xylene for a total of 15 min, antigen retrieval was performed at 97 °C for 20 min in ER2 buffer. Briefly, antigen retrieval was performed at 97 °C for 20 min in ER2 buffer. After blocking endogenous peroxidase activity with 3% hydrogen peroxidase for 10 min, slides were incubated with mouse monoclonal estrogen receptor antibody (NCL-L-ER-6F11, Novocastra, Newcastle, United Kingdom) and rabbit monoclonal AR-V7 specific antibody (ab198394, Abcam, San Francisco, CA, USA) for 15 min at room temperature, at a dilution of 1:200. Normal breast tissue was used as a positive control. For ER status evaluation, the Allred score, which is the sum of two scores (percentage of positive hepatocytes showing nuclear staining, range 0–3; intensity of immunoreactivity, range 0–5), was calculated18, 33. ER positive expression was defined when the Allred score is greater than or equal to 4 based on previous reserach34.
Operative management
All study donors underwent computed tomography angiography and magnetic resonance cholangiopancreatography to determine the anatomy of the hepatic vascular and biliary system. Expert liver radiologists and transplant surgeons carefully discussed anatomical considerations and related surgical issues in a joint meeting prior to transplantation. All grafts consisted of segment 5 through 8, excluding the middle hepatic vein trunk. Graft implantation was performed using the piggyback technique. After the portal vein anastomosis was complete, the graft was reperfused by consecutively unclamping the hepatic vein and portal vein. Subsequently, hepatic artery anastomosis was followed by biliary anastomosis. Transfusion of allogeneic blood was strictly controlled based on a restrictive and prophylactic strategy in which each blood component was transfused separately according to its respective indication35. Blood salvage and auto-transfusion were routinely used to reduce recipient exposure to allogeneic red blood cells36, 37. Immunosuppression and hepatitis B virus prophylaxis were performed according to the standardized protocol, as described previously38.
Statistical analysis
The primary outcome was post-transplant graft failure (death or retransplantation). The secondary outcome was post-transplant overall death. Survival analysis was performed using the Cox model, and the results were described using hazard ratios (HR) with 95% confidence interval. Backward stepwise selection was performed for multivariable analysis with p < 0.05 for inclusion and p > 0.10 for removal. Subgroup analysis was performed within the recipients who did not have hepatocellular carcinoma (HCC) to remove the bias from HCC-related graft failure because our recent research demonstrated that the risk of post-transplant HCC recurrence is greater in recipients of grafts from male donors38. Furthermore, for graft failure occurred during the early postoperative period (90 days), early allograft failure simplified estimation (EASE) score, which is the indicator for quantifying the early post-operative failure risk, was calculated compared between two groups39, 40. Regarding estrogen receptor analysis, the multivariable Cox model was used to test whether estrogen content of donor liver is associated with graft failure risk while the independent variables which were significant during the multivariable analysis for graft failure were included. Because the largest part of graft failure occurred during the early post-transplant period, we performed the analysis for the first 6 months post-transplantation. The continuous variables were described as median with interquartile range and analyzed using the Mann–Whitney U-test. The categorical variables were expressed as frequency (%) and analyzed using χ2 test or Fisher's exact test. All reported p-values were two-sided, and p < 0.05 was considered significant. Data were analyzed using SPSS 19.0 (SPSS Inc, Chicago, IL, US).
Results
The indications for transplantation in the 780 recipients were as follows: HCC arising from viral hepatitis (n = 401; hepatitis B, n = 367; hepatitis C, n = 32; and hepatitis B and C, n = 2), alcoholic hepatitis (n = 16), or unknown origin (n = 18); liver failure secondary to viral hepatitis (n = 216; hepatitis A, n = 7; hepatitis B, n = 184; hepatitis C, n = 21; hepatitis B and C, n = 3; and hepatitis A and B, n = 1), alcoholic hepatitis (n = 51), biliary obstruction (n = 14), metabolic disease (n = 5), or unknown origin (n = 17); autoimmune hepatitis (n = 16); toxic hepatitis (n = 13); Budd-Chiari syndrome (n = 7); and liver tumors other than HCC (n = 6). There were 207 male recipients of grafts from female donors. Other 573 recipients consisted of 59 female recipients with female donors, 104 female recipients with male donors, and 410 male recipients with male donors. Clinical data of male recipients with female donors and other recipients are described in Table 1 (≤ 40 years non-macrosteatotic donors and ≤ 40 years macrosteatotic donors), Table 2 (> 40 years non-macrosteatotic donors and > 40 years macrosteatotic donors). There were no extremely small grafts with < 0.6% graft-to-recipient weight ratio.
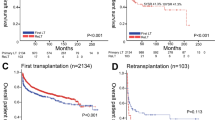
The median follow-up time was 35 months with interquartile range of 20 to 60 months. Within the subgroup of recipients with ≤ 40 years non-macrosteatotic donors (Fig. 1A), graft failure risk was significantly greater in female-to-male LT than in other LTs (HR 2.39 [1.48–3.85], p < 0.001). The probability of graft failure after 1, 2, and 5 years post-transplantation was 25.4% (16.8%–37.2%), 31.2% (21.7%–43.4%), and 40.1% (29.36%–53.1%), respectively, in female-to-male LT and 8.5% (5.6%–12.8%), 14.9% (10.9%–20.1%), and 20.9% (16.0%–27.0%), respectively, in other LTs. In particular, there were no recipients with < 0.6% graft-to-recipient weight ratio. As shown in Table 3, the results of multivariable analysis demonstrated that female-to-male donation is an independent risk factor for graft failure within the subgroup of recipients with ≤ 40 years non-macrosteatotic donors (HR 2.03 [1.18–3.49], p = 0.011 and HR 1.88 [1.16–3.04], p = 0.011, respectively). Consistent effect sizes indicated the insignificant confounding effects from covariables and reliability of the dominant impact of donor/recipient sex. As shown in Fig. 1BCD, graft failure risk is not significantly different between female-to-male LT and other LTs when the donor was > 40 years or macrosteatotic (p > 0.60). In graft failure case occurred during the early postoperative period (90 days), EASE score was significantly lower in female-to-male LT than other LTs [Median (IQR) −2.2 (−3.2, 0.175) vs. −1.5 (−2.9, 1), p = 0.036], indicating that graft quality was not a factor confoundg the result. In line with graft failure, death risk was also significantly greater in female-to-male LT than in other LTs only within the subgroup of recipients with ≤ 40 years non-macrosteatotic donors (HR 2.35 [1.35–4.09], p = 0.003), whereas it was comparable when the donor was > 40 years or macrosteatotic (p > 0.70), as shown in Supplementary Fig. 1. Supplementary Table 1 shows the most probable cause of death in female-to-male LTs and other LTs, respectively, within the recipients with ≤ 40 years non-macrosteatotic donors.
Figure 2 shows the opposite effect of better graft quality (≤ 40 years and non-macrosteatosis) on graft failure risk in female-to-male LT and other LTs. In agreement with the general consensus, graft failure risk tended to be lower with better graft quality (≤ 40 years non-macrosteatotic donors vs. > 40 years or macrosteatotic donors) in other LTs than female-to-male LT (HR 0.77 [0.54–1.10], p = 0.155). In contrast, graft failure risk was insignificantly greater with better graft quality in female-to-male LT (HR 1.60 [0.97–2.64], p = 0.065).
Within the subgroup of non-HCC recipients, the gap in graft failure risk between female-to-male LT and other LTs when donors were ≤ 40 years and non-macrosteatotic became greater (HR 4.75 [2.02–11.21], p < 0.001) compared to the results for the whole cohort (Supplementary Fig. 2A). The probability of graft failure after 1, 2, and 5 years post-transplantation was 37.9% (23.1%–57.9%) in female-to-male LT (all graft failures occurred within 1 year) and 5.9% (2.7%–12.6%), 8.9% (4.7%–16.3%), and 9.9% (5.5%–17.7%), respectively, in other LTs. The causes of 11 graft failures after female-to-male LT were hepatic artery complication (n = 4), primary non-function (n = 2), biliary complication (n = 1), viral recurrence (n = 1), graft rejection (n = 1), and infectious complication (n = 2). When donors were > 40 years or macrosteatotic, there were no significant differences between female-to-male LT and other LTs (Supplementary Fig. 2BCD) in line with the results for the whole cohort. Supplementary Table 2 shows the most probable cause of graft failure in female-to-male LT and other LTs, respectively, within the non-HCC recipients with ≤ 40 years non-macrosteatotic donors.
As shown in Fig. 3 and Table 4, in the subgroup of patients who underwent female-to-male LT, the risk of graft failure within 6 months after transplantation was significantly greater in patients who received a graft positive at estrogen receptor (HR 5.54 [1.36–22.61], p = 0.017). In contrast, in the subgroup of patients who underwent other LTs than female-to-male LT, the risk of graft failure within 6 months was not significantly different (HR 0.76 [0.31–1.87], p = 0.543).
Discussion
Continuing from our previous work, demonstrating the greater tolerance of hepatic ischemia-reperfusion injury in females than in males only with age ≤ 40 years and no macrosteatosis18, the inferiority of female-to-male LT in graft failure, which is thought to result from the acute decrease in hepatic estrogen signaling and loss of hepatic protection capacity6,7,8, 14, was found only when the donor was ≤ 40 years and with no macrosteatosis. An exceptionally high graft failure risk of female-to-male LT, despite good graft quality and lower EASE score value, suggests the presence of an estrogen-related dominant contributor for graft failure that outweighs the effects of graft quality. Such high graft failure risk was not observed in female-to-male LT with > 40 years donors (decreased systemic estrogen secretion in the lack of ovarian reserve [infertility] or macrosteatotic donors (decreased hepatic estrogen receptor expression) despite worse graft quality12,13,14,15, 18, supporting the role of hepatic sex hormonal mismatch in the inferiority of female-to-male LT. When donor was > 40 years or macrosteatotic, the inferiority of female-to-male LT was abrogated and graft failure risk was comparable between female-to-male LT and other LTs, suggesting that anatomical size mismatch (e.g. small graft size or insufficient graft-to-recipient weight ratio) is not the reason of the inferiority of female-to-male LT, being in agreement with our recent research11. Overall, our data suggest the presence of dominant contributors for graft failure other than graft quality/quantity and supports the role of hepatic estrogen signaling mismatch in greater graft failure after female-to-male LT. Also, our data suggest the risk of female-to-male LT can be changed by modification of donor/recipient hepatic estrogen signaling.
Post-transplant liver graft failure has been known to be more prevalent in male recipients of female donors compared to other donor-recipient sex combinations and hepatic estrogen signaling is considered to play an important role in this sex difference12,13,14,15. Hepatic estrogen signaling is positively correlated with hepatic protection capacity10, 12, 16,17,18. A previous study in mice demonstrated that 100% of female mice survived after 70% hepatic inflow occlusion for 45 min, whereas all male mice died within 5 d with greater initial hepatocyte injury10. In the same study, ovariectomy or estrogen antagonist administration increased hepatic ischemia–reperfusion injury, reduced regenerative capacity, and increased mortality, whereas administration of 17β-estradiol improved recovery10. In rats, translocation of estrogen receptors from the cytosol to nucleus and activation of cell signaling occurred after liver injury to stimulate the healing process41,42,43. Our recent study of healthy living liver donors has also demonstrated that the tolerance of hepatic ischemia–reperfusion injury following intermittent hepatic inflow occlusion was greater in females than in males18. Thus, it has been hypothesized that acute decrease in hepatic estrogen signaling (including decreased hepatic estrogen receptor content) of the transplanted female liver after the exposure to male hormonal milieu impairs the recovery of female grafts6,7,8. The abrogation of the inferiority of female-to-male LT when the donor was > 40 years22,23,24,25 or with macrosteatosis16, 26, 27 supports the relationship because experiencing some degree of defeminization with lower hepatic estrogen signaling prior to transplantation may mitigate the impact of male hormonal milieu after transplantation.
The current study suggests that the insignificance of female-to-male LT in few previous articles were attributable to the lack of consideration of hepatic estrogen signaling or pretransplant liver graft defeminization according to reproductive aging and macrosteatosis44,45,46, along with the significant multicollinearity between donor sex and donor characteristics like weight and hight as indicated previously11. Also, biasing effects of HCC-related graft failure might have contributed because the impact of donor gender on HCC-unrelated graft failure and HCC-related graft failure is different based on our and other data38. In other words, analyzing the interaction of donor/recipient sex with donor age and macrosteatosis, instead of simply incorporating those variables in multivariable model, on HCC-related/unrelated graft failure may help improve research quality and derive more consistent results regarding the impact of female-to-male donation. This is important because there is a possibility that donor selection process may ultimately improve post-transplant clinical outcome if donor sex is appropriately taken into account while the greater graft failure risk in female-to-male LT has been being reported repeatedly. This is particularly relevant in living donor LT because multiple donation candidates can be obtained in some cases44. On the other hand, our data suggested that female-to-male LT is not inferior in all cases; thus, variables interacting with donor sex (e.g. donor age and macrosteatosis in the current study) needs to be further elucidated to minimize the risk subgroup. Also, our data suggest that pre/post-transplant hepatic estrogen conditioning to prevent acute defeminization of the graft helps maintain the hepatic protection capacity and prevent graft failure. More scientific data are warranted to incorporate donor sex and hepatic estrogen signaling into LT process.
In our analysis, we observed that the significant difference in graft failure between female-to-male LT and other LTs was only found with non-macrosteatotic donors ≤ 40 years. The absence of a correlation between EASE score and female-to-male sex match further supports that the physiopathologic mechanism underlying the inferiority of the female-to-male LT is not dependent on graft quality. An unexpected finding was a significant protective effect of recurrent ascites in the subgroup ‘female-to-male LT'. Despite the initial counterintuitive nature of this finding, potential confounding factors and complex underlying mechanisms need to be considered. The presence of recurrent ascites may trigger closer monitoring, early interventions, and more intensive management strategies, leading to improved graft outcomes. These interventions may include adjustments in immunosuppressive regimens, fluid balance optimization, and close surveillance for complications. An interesting finding was the association between female-to-male LT and sepsis-induced graft failure. Because the number of event was too small with the lack of statistical power, the current study cannot draw a conclusion for this finding warranting further investigation.
Despite the retrospective design, our study presented several strengths for generation of robust data. First, this study included a homogeneous living donor liver transplant population. All recipients received right hemi-liver graft without variation. Most recipients underwent elective surgery and were in stable condition without acute deterioration. Accordingly, thorough perioperative anesthetic and surgical care could be performed strictly based on the institutional standardized protocols. Second, only three patients were lost to follow-up, and graft failure was defined as a clear objective outcome; thus, the data regarding the time-to-graft failure were highly reliable. Another advantage of this study was the routine use of Oil Red O staining to examine liver parenchyma. Conventional hematoxylin and eosin staining alone tends to underestimate the degree of steatosis45.
This study has several limitations as well. First, due to the retrospective nature of the study design, we were unable to exclude the possibility of bias from unmeasured variables, although we performed adjustment of all established contributors by subgroup analysis and multivariable analysis. Second, mechanisms underlying the sex differences in post-transplant graft failure as well as its change according to donor age and macrosteatosis have not yet been confirmed although the involvement of hepatic estrogen signaling can be assumed based on results of previous and the current studies. Third, in contrast to living donors, deceased donors are frequently attended with multiorgan dysfunction, unstable hemodynamics, vasoactive drug use, lack of sufficient hepatic inflow, and prolonged graft ischemia time46. Thus, it is unclear that the effects of donor age and macrosteatosis are observed in the same way in deceased donor LT because more various factors, aside from donor age and macrosteatosis, may affect hepatic estrogen signaling.
In the current study, we found that the greater graft failure risk of female-to-male LT was abrogated when the donor was > 40 years or with macrosteatosis, which is known to be related to the decrease in hepatic estrogen signaling. Therefore, our findings shed light on the importance of hepatic estrogen signaling mismatch between donor and recipient to post-transplant graft failure and suggest that the strategies to avoid abrupt decrease in hepatic estrogen signaling prevent graft failure in female-to-male LT.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EASE:
-
Early allograft failure simplified estimation
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hazard ratio
- LT:
-
Liver transplantation
References
Kahn, D. et al. Gender of donor influences outcome after orthotopic liver transplantation in adults. Dig. Dis. Sci. 38, 1485–1488 (1993).
Marino, I. R. et al. Effect of donor age and sex on the outcome of liver transplantation. Hepatology 22, 1754–1762 (1995).
Brooks, B. K. et al. Influence of donor and recipient gender on the outcome of liver transplantation. Transplantation 62, 1784–1787 (1996).
Smith, C. M., Davies, D. B. & McBride, M. A. Liver transplantation in the United States: A report from the organ procurement and transplantation network. Clin. Transpl. 1, 19–30 (2000).
Rustgi, V. K. et al. Role of gender and race mismatch and graft failure in patients undergoing liver transplantation. Liver Transpl. 8, 514–518 (2002).
Croome, K. P. et al. Female donor to male recipient gender discordance results in inferior graft survival: A prospective study of 1,042 liver transplants. J. Hepatobiliary Pancreat. Sci. 21, 269–274 (2014).
Francavilla, R. et al. Gender matching and outcome after pediatric liver transplantation. Transplantation 66, 602–605 (1998).
Yoshizumi, T. et al. Risk factors that increase mortality after living donor liver transplantation. Transplantation 93, 93–98 (2012).
Grande, L. et al. Impact of donor gender on graft survival after liver transplantation. Transplant. Proc. 29, 3373–3374 (1997).
Harada, H. et al. Effects of gender on reduced-size liver ischemia and reperfusion injury. J. Appl. Physiol. 91, 2816–2822 (2001).
Lee, K. W. et al. Higher risk of posttransplant liver graft failure in male recipients of female donor grafts might not be due to anastomotic size disparity. Transplantation 102, 1115–1123 (2018).
Eagon, P. K. et al. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin. Liver Dis. 5, 59–69 (1985).
Kahn, D. et al. Estrogen and androgen receptors in the liver after orthotopic liver transplantation. Transplant. Proc. 21, 409–410 (1989).
Kahn, D. et al. Orthotopic liver transplantation and the cytosolic estrogen-androgen receptor status of the liver: The influence of the sex of the donor. Hepatology 10, 861–866 (1989).
Soric, S. et al. Impact of female sex hormones on liver tissue lactic acidosis during ischemia. Transplantation 84, 763–770 (2007).
Erkan, G. et al. Presence and extent of estrogen receptor-alpha expression in patients with simple steatosis and NASH. Pathol. Res. Pract. 209, 429–432 (2013).
Chen, K. L. & Madak-Erdogan, Z. Estrogens and female liver health. Steroids 133, 38–43 (2018).
Han, S. et al. Sex difference in the tolerance of hepatic ischemia-reperfusion injury and hepatic estrogen receptor expression according to age and macrosteatosis in healthy living liver donors. Transplantation 106, 337 (2021).
Nilsson, S. et al. Mechanisms of estrogen action. Physiol. Rev. 81, 1535–1565 (2001).
Chow, J. D. et al. A selective estrogen receptor alpha agonist ameliorates hepatic steatosis in the male aromatase knockout mouse. J. Endocrinol. 210, 323–334 (2011).
Shimizu, I. et al. Female hepatology: Favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J. Gastroenterol. 13, 4295–4305 (2007).
Burger, H. G. et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J. Clin. Endocrinol. Metab. 84, 4025–4030 (1999).
Randolph, J. F. Jr. et al. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: Effects of ethnicity and age. J. Clin. Endocrinol. Metab. 89, 1555–1561 (2004).
Pal, L. et al. Characterizing the reproductive hormone milieu in infertile women with diminished ovarian reserve. Fertil. Steril. 93, 1074–1079 (2010).
Wallace, W. H. & Kelsey, T. W. Human ovarian reserve from conception to the menopause. PLoS ONE 5, e8772 (2010).
Hart-Unger, S. et al. Hormone signaling and fatty liver in females: Analysis of estrogen receptor alpha mutant mice. Int. J. Obes. 41, 945–954 (2017).
Chambliss, K. L. et al. Nonnuclear estrogen receptor activation improves hepatic steatosis in female mice. Endocrinology 157, 3731–3741 (2016).
Han, S. et al. Comparison of the tolerance of hepatic ischemia/reperfusion injury in living donors: Macrosteatosis versus microsteatosis. Liver Transpl. 20, 775–783 (2014).
Han, S. et al. Microsteatosis may not interact with macrosteatosis in living donor liver transplantation. J. Hepatol. 62, 556–562 (2015).
Han, S. et al. Effect of pure microsteatosis on transplant outcomes after living donor liver transplantation: a matched case-control study. Liver Transpl. 20, 473–482 (2014).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Tannapfel, A. et al. Histopathological diagnosis of non-alcoholic and alcoholic fatty liver disease. Virchows Arch. 458, 511–523 (2011).
Harvey, J. M. et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 17, 1474–1481 (1999).
Shousha, S. Oestrogen receptor status of breast carcinoma: Allred/H score conversion table. Histopathology 53, 346–347 (2008).
Kwon, J. H. et al. Decrease in the risk of posttransplant hepatocellular carcinoma recurrence after the conversion to prestorage leukoreduction for transfused red blood cells. Transplantation 105, 577–585 (2021).
Han, S. et al. Safety of the use of blood salvage and autotransfusion during liver transplantation for hepatocellular carcinoma. Ann. Surg. 264, 339–343 (2016).
Kwon, J. H. et al. Blood salvage and autotransfusion with single leukoreduction does not increase the risk of tumor recurrence after liver transplantation for advanced hepatocellular carcinoma. Ann. Surg. 276, e842–e850 (2022).
Han, S. et al. Risk of post-transplant hepatocellular carcinoma recurrence is higher in recipients of livers from male than female living donors. Ann. Surg. 268, 1043–1050 (2018).
Avolio, A. W. et al. Availability of a web and smartphone application to stratify the risk of of early allograft failure requiring liver retransplantation. Hepatol. Commun. 6, 247–248 (2022).
Avolio, A. W. et al. Development and validation of a comprehensive model to estimate early allograft failure among patients requiring early liver retransplant. JAMA Surg. 155, e204095 (2020).
Francavilla, A. et al. Regenerating rat liver: Correlations between estrogen receptor localization and deoxyribonucleic acid synthesis. Gastroenterology 86, 552–557 (1984).
Fisher, B. et al. Relation of estrogen and its receptor to rat liver growth and regeneration. Cancer Res. 44, 2410–2415 (1984).
Francavilla, A. et al. Sex hormone-related functions in regenerating male rat liver. Gastroenterology 91, 1263–1270 (1986).
Han, S. et al. Response to comment on “Risk of posttransplant hepatocellular carcinoma recurrence is higher in recipients of livers from male than female living donors”. Ann. Surg. 269, e71 (2019).
McCormack, L. & Clavien, P. A. Understanding the meaning of fat in the liver. Liver Transpl. 11, 137–139 (2005).
Kalisvaart, M. et al. Comparison of postoperative outcomes between donation after circulatory death and donation after brain death liver transplantation using the comprehensive complication index. Ann. Surg. 266, 772–778 (2017).
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), Funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number : HI22C189500) and also supported by a grant provided by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and Information and Communication Technology (NRF-2021R1F1A106299613).
Author information
Authors and Affiliations
Contributions
S.H. and J.H.K. designed the study, collected data, analyzed data, and wrote the manuscript; K.W.L. and S.L. collected data, interpreted data, and gave critical revisions; G.S.C., J.M.K., and S.Y.H. contributed to conception and data acquisition/interpretation; J.S.K., M.S.K., and G.S.K. interpreted data, gave critical revisions with writing of article; J.W.J. designed the study, interpreted data, and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, S., Kwon, J.H., Lee, K.W. et al. Abrogation of greater graft failure risk of female-to-male liver transplantation with donors older than 40 years or graft macrosteatosis greater than 5%. Sci Rep 13, 12914 (2023). https://doi.org/10.1038/s41598-023-38113-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38113-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.