Abstract
Patients with Takotsubo syndrome (TTS) admitted to the intensive care unit (ICU) always confront a higher risk of in-hospital death than those hospitalized in the cardiology unit. The prognosis of the latter was analyzed by a large number of studies. However, there was no utility model to predict the risk of in-hospital death for patients with TTS in the ICU. This study aimed to establish a model predicting in-hospital death in patients with TTS admitted to ICU. We retrospectively included ICU patients with TTS from the MIMIC-IV database. The outcome of the nomogram was in-hospital death. Least Absolute Shrinkage Selection Operator (LASSO) analysis selected predictors preliminarily. The model was developed by multivariable logistic regression analysis. Calibration, decision curve analysis (DCA), and receiver operating characteristic (ROC) measured the performance of the nomogram on the accuracy, clinical utility, and discrimination, respectively. Eventually, 368 ICU patients with TTS were enrolled in this research. The in-hospital mortality was 13.04%. LASSO regression and multivariate logistic regression analysis verified risk factors significantly associated with in-hospital mortality. They were potassium, prothrombin time (PT), age, myocardial infarction, white cell count (WBC), hematocrit, anion gap, and sequential organ failure assessment (SOFA) score. This nomogram excellently discriminated against patients with a risk of in-hospital death. The area under curve (AUC) was 0.779 (95%CI: 0.732–0.826) in training set and 0.775 (95%CI: 0.711–0.839) in test set. The calibration plot and DCA showed good clinical benefits for this nomogram. We developed a nomogram that predicts the probability of in-hospital death for ICU patients with TTS. This nomogram was able to discriminate patients with a high risk of in-hospital death and performed clinical utility.
Similar content being viewed by others
Introduction
Takotsubo syndrome is a clinical syndrome, viewed as “stimulation cardiomyopathy,” “broken heart syndrome,” or “transient apical ballooning”. It is characterized an acute and transient left ventricular dysfunction. TTS is always induced by physical or emotional stimulation, however, up to 40% of TTS patients present without any trigger event1. TTS is easily misdiagnosed as an acute coronary syndrome (ACS), approximately 1–3% of all patients who present symptoms consistent with acute coronary syndrome and undergo coronary angiography have been identified as TTS2. Despite numerous studies reporting TTS as a reversible myocardial insufficiency, almost half of the patients with TTS occur cardiovascular complications. Furthermore, the inpatient mortality due to cardiogenic shock, myocardial rupture, or life-threatening arrhythmias is comparable to MI (4–5%)3,4,5,6. Several serious complications may increase mortality, which is comparable to that of patients with ACS7. Recently, the reported mortality rate is higher than the previous8. One research based on the data from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry) published that the 30-day mortality rate in TTS was higher than non-STEMI (NSTEMI)9.
Compared with non-ICU patients with TTS, ICU patients encounter a higher risk of in-hospital death. One study reported that the mortality occurrence of ICU patients with TTS was much higher than the mortality of patients with TTS admitted to the cardiology unit (30.8% vs. 4.1%)10. ICU patients always present multiple organ failures and unstable physical conditions. They have much more risk factors threatening life in the short term than non-ICU patients. Reduction of in-hospital death in ICU patients with TTS plays a vital role in prognosis. However, previous studies mostly involved the investigation of clinical outcomes in non-ICU patients with TTS. Prevention of in-hospital death in ICU patients with TTS hasn’t been addressed and emphasized. For this kind of population, a predictive model effectively identifies how great in-hospital death exposure individuals faced. Clinicians can take action to decrease the risk according to the predicted outcome. Therefore, a prediction model is necessary to develop for decreasing the rate of in-hospital death in ICU patients with TTS.
Nowadays, the clinical prediction model is a good tool that provides valuable guidance for clinical decision-making. Nomogram as a visually predictive model calculates the risk scores for individuals. It is convenient for clinicians to estimate the clinical outcomes of patients. Nomogram has been widely applied in the evaluation of prognosis in critically ill patients these years11,12,13,14,15. Therefore, we aimed to develop a nomogram that indicates the probability of in-hospital death for ICU patients with TTS. Clinicians will early discriminate against patients with a higher risk of death by this nomogram and make decisions on treatment as well as intensive care.
Methods
Data source
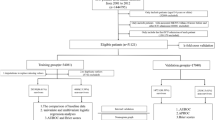
In this study, we retrospectively extracted data from the MIMIC-IV database (version 1.0). The MIMIC-IV database extensively contained all medical records of patients admitted to ICU or the emergency department between 2008–2019 in the Beth Israel Deaconess Medical Center (BIDMC)16. The latest version of the MIMIC-IV database is version 1.0. One of the authors (C.J, certification ID: 8,979,131) in this study gained permission to document the database after online training at the National Institutes of Health (NIH). This study relied exclusively on publicly available and anonymous data. As a result, individuals’ permission wasn’t required. To ensure patients' privacy, all methods were carried out by relevant guidelines.
Patients selection
We enrolled patients who aged more than 18 years old. They were hospitalized in ICU and diagnosed with TTS by the International Classification of Diseases version 9 (“42,983”) and version 10 diagnosis codes (“I5181”) in the MIMIC-IV database. We excluded patients according to the following criteria: (I) No survival outcome data; (II) Being pregnant and the postpartum condition; (III) Incomplete or unobtainable documented or other vital medical records.
Clinical and laboratory data
All data analyzed in this study included general information, vital signs, and laboratory tests. The time points of vital signs and laboratory parameters used in this analysis were the first document after hospital admission within 24 h. The general information included age, height, weight, and comorbidities such as diabetes, hypertension, chronic lung disease, myocardial infarction, and heart failure. Vital signs contained mean blood pressure (MBP), diastolic blood pressure (DBP), systolic blood pressure (SBP), body temperature (T), respiratory rate (RR), heart rate (HR), pulse oximetry-derived oxygen saturation (SpO2). Blood laboratory tests consisted of hemoglobin, hematocrit, creatinine, anion gap, lactate, blood urea nitrogen (BUN), pH, white blood cell count, platelet count, chloride, glucose, prothrombin time (PT), serum potassium, serum sodium, and serum calcium. Therapies were also recorded containing the use of vasoactive drugs (norepinephrine), and continuous renal replacement therapy (CRRT) during the hospitalization. The sequential organ failure assessment (SOFA) score17 of every patient also be calculated after hospital admission within 24 h. In this study, the endpoint was in-hospital mortality viewed as survival status at hospital discharge.
Statistical analysis
The whole dataset was randomly divided into a training set and a test set with a proportion of 7:3. Mean ± standard deviation (SD) documented the normal distribution of continuous variables. Medians with upper and lower quartiles described the abnormal distribution of continuous variables. Continuous variables were tested by T-test or Wilcoxon rank-sum test and categorical variables were analyzed by chi-square test or Fisher's exact test for group comparisons. Firstly, LASSO regression preliminarily screened predictors based on the whole study database. Secondly, these predictors were further analyzed by multivariable regression. Recognized predictors were covered to establish a nomogram based on the training set. The scores for each predictor were calculated based on coefficients of logistic regression variables in the model. A discriminate ability of the nomogram was evaluated by ROC. The AUC of the ROC of more than 0.7 demonstrated a nomogram with discriminate capacity. The calibration curve analyzed by Hosmer–Lemeshow test assessed the fitting degree of nomogram. The DCA evaluated the clinical utility by quantifying net benefits against a range of threshold probabilities. Nomogram was validated in the test set. These results were presented by odds ratio (OR) with 95% confidence intervals (CIs). All tests were two-tailed tests and P ≤ 0.05 was considered statistically significant. We used STATA 15.1 (Stata Corporation, College Station, Texas, USA) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) for statistical analysis.
Ethics approval and consent to participate
The establishment of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA), and consent was obtained for the original data collection. Therefore, the ethical approval statement and the need for informed consent were waived for this manuscript.
Results
The characteristics of study patients
There were 368 eligible patients (85 males and 283 females) in this study. Their average age was 66.29 ± 16.09 years old. 48 patients (16 males and 32 females) died during the hospitalization. The incidence of in-hospital death was 13.04%. Non-survivors tend to be older, and with higher values of anion gap, WBC count, and SOFA score (as shown in Table 1). In the male group, the in-hospital mortality was 18.82% (16/85) and in the female group, the in-hospital mortality was 11.31% (32/283), there was no significant difference between the male group and female group (P = 0.105). Male patients tend to have a worse renal function, as shown in Supplementary Table 1. In addition, Kaplan-Meir survival analysis showed no significant difference in in-hospital mortality between males and females (as shown in Supplementary Fig. 1). The whole sample was randomly divided into a training set and a test set with a proportion of 7:3, and there was no significant difference in variables between the training set and the validation set (as shown in Table 2).
Predictors and nomogram for in-hospital mortality
We performed lasso regression analysis to preliminarily select predictors (as shown in Fig. 1). Screened variables had a large regression coefficient meaning great influence on the outcomes. They were age, myocardial infarction history, gender, potassium, PT, WBC, hematocrit, anion gap, and SOFA score. Finally, age, myocardial infarction history, WBC, anion gap, PT, and SOFA score were demonstrated as independent risk factors for in-hospital mortality of ICU patients with TTS (as shown in Table 3). We included the above independent risk factors in the nomogram (as shown in Fig. 2).
Texture feature selection using the least absolute shrinkage and selection operator (LASSO) binary logistic regression model. (a) Each curve in the figure represents the change trajectory of each independent variable coefficient. The ordinate is the value of the coefficient, the lower abscissa is log(λ), and the upper abscissa is the number of non-zero coefficients in the model at this time. (b) tenfold cross-cross validation fitting and then selecting the model, and at the same time have a more accurate estimate of the performance of the model. For each λ value, around the mean value of the target parameter shown by the red dot, we can obtain a confidence interval for the target parameter. The two dashed lines indicate two special λ values:c (cvfit$lambda.min, cvfit$lambda.1se). The mean squared error was plotted vs. log(λ).
The nomogram for predicting the risk of in-hospital mortality in takotsubo syndrome patients. The top row of the ‘Points’ represents a scale for each risk factors, and points of each predictor were acquired by drawing a straight line upwards from the corresponding value to the “Points” line. Then, the points received from each predictor are summed, and the number is located on the “Total Points” axis. To conclude the patient’s sort of probability for in-hospital mortality, draw a straight line down to the corresponding “Risk of death” axis. resp_rate: respiratory rate. For example, a 74-years-old male without myocardial infarction history, his clinical data of admission was as followed: PT: 30 s, anion gap: 24 mEq/L, WBC: 10*10^9, hematocrit: 35% and SOFA score were 4 points. The corresponding score of each predictor were 6.8 points, 2.5 points, 1.0 points, 6.2 points, 1.3 points, 6.7 points, and 0.8 points respectively. Then his total score was about 24.5 points, and the risk of in-hospital mortality is 45.5%.
Evaluation and validation of the nomogram
This prediction model showed a good discriminate ability (AUC: 0.779, 95% CI: 0.732–0.826) (Fig. 3A). We validated the prediction model in the test set. A similar ROC result was found (AUC: 0.775, 95% CI: 0.711–0.839) (Fig. 3B). Additionally, we performed calibration curve analysis to test the fitting degree of the nomogram. The calibration curve plot indicated a concordance between predicted probabilities and observed death rates both in the test and training sets (as shown in Fig. 3C and Fig. 3D). We evaluated the clinical utility of the prediction model by decision curve analysis. DCA curve demonstrated a clinical utility in model practice (as shown in Fig. 3E and F).
(A) Receiver operating characteristic (ROC) curve of the nomogram in the training set. The area under the curve (AUC) of ROC was (AUC: 0.779, 95% CI: 0.732–0.826). (B) Receiver operating characteristic (ROC) curve of the nomogram in the test set. The area under the curve (AUC) of ROC was (AUC: 0.775, 95% CI: 0.711–0.839). (C) Calibration curve of the nomogram in the training set. The x-axis represents the predicted probability of in-hospital mortality of TTS patients. The y-axis represents the actual in-hospital mortality of TTS patients. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line represents the performance of the nomogram, of which a closer fit to the diagonal dotted line represents a better prediction. The figure shows that the prediction model have a good predictive ability. (D) Calibration curve of the nomogram in the test set. The x-axis represents the predicted probability of in-hospital mortality of TTS patients. The y-axis represents the actual in-hospital mortality of TTS patients. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line represents the performance of the nomogram, of which a closer fit to the diagonal dotted line represents a better prediction. The figure shows that the prediction model has a good predictive ability. (E) Decision curve analysis of the nomogram for TTS patients (training set). The DCA curve of the nomogram for TTS patients. Solid line: The patient does not apply the nomogram, and the net income is zero; Gray line: All patients used the nomogram. The further the red solid line was from the dotted line, the greater the clinical application value. (F) Validation of the DCA curve of the nomogram for TTS patients (test set). Solid line: The patient does not apply the nomogram, and the net income is zero; Gray line: All patients used the nomogram. The further the red solid line was from the dotted line, the greater the clinical application value.
Discussion
In recent years, various studies have analyzed the prognosis of patients with TTS18,19,20,21,22, however, they mostly focused on the long-term prognosis. There was still limited research on the short-term prognosis, especially for ICU patients with TTS. These patients have a higher rate of mortality than non-ICU patients with TTS10. This study retrospectively investigated ICU patients with TTS. The incidence of in-hospital mortality was 13.04%. The rate was higher than previously reported mortality of non-ICU patients (2–5%)3,4,5,6. Compared with non-ICU patients, a majority of ICU patients are associated with multiple organ failures, hemodynamic instability, and severe complications. They often suffer from TTS triggered by physical stress. These risk factors lead them to be more serious and complicated. The probability of in-hospital death always is higher than in non-ICU populations. In this nomogram, age, myocardial infarction history, PT, WBC, hematocrit, anion gap, and SOFA score were covered as predictors for in-hospital mortality of TTS patients. This nomogram presented a good predictive ability.
Age and SOFA score were viewed as risk factors for in-hospital death in ICU patients with TTS. The mean age of non-survivors tended to be older than survivors (72.09 ± 13.30 vs 65.42 ± 16.31, P = 0.007). A previous report divided participants according to age. They showed the generation of 50–64 years (OR = 0.75, P = 0.05) as a predictor for in-hospital mortality19. Different from this report, this nomogram found a risk of death in patients aging of 19–96 years to a different extent. Each additional 11 years significantly increases the risk by 9%. Patients (> 68 years) are exposed in death risk of more than 50%. SOFA score evaluated the severity of illness by describing the time course of multiple organ dysfunction23. Previous studies supposed SOFA score as a predictor of clinical outcome24. In this study, the SOFA score of non-survivors was statistically higher than survivors (7.27 vs 5.78, P = 0.016). This nomogram indicates a risk of in-hospital mortality when the SOFA score is more than five.
Santoro et al. stratified the risk of in-hospital complications in patients with TTS by a GEIST score25. The GEIST score doesn’t consist of laboratory values. However, we discovered an association between in-hospital death with PT, WBC, and anion gap. PT is used to estimate the tissue factor and coagulation pathways. A prolonged PT indicates a defect of the coagulation factor or the presence of coagulation factor inhibitors26. Patients with PT prolongation always have a higher risk of hemorrhage. Previous studies discovered that aneurysmal subarachnoid hemorrhage (aSAH) can trigger TTS. TTS patients with aSAH have a higher risk of in-hospital mortality.27,28. When patients with TTS occur a prolongation of PT, clinicians should evaluate the risk of hemorrhage and monitor the adverse reaction created by anticoagulant. Anion gap is applied to evaluate the acid–base equilibrium. Anion gap elevation results from acute or chronic acid–base disorders, such as lactic acidosis, ketoacidosis, toxic alcohol poisoning, salicylate intoxication, and acute or chronic kidney disease29. Some cases reported acidosis as one of the trigger reasons for TTS, especially ketoacidosis30. Acidosis physiologically increases stress hormones such as catecholamines, and cortisol and deleteriously affect myocyte. In this nomogram, the in-hospital mortality risk is less than 5% in patients with an anion gap of 8–16 mmol/L. When the anion gap is more than 18 mmoL/L, every 2 mmoL/L in elevation of anion gap concentration increases mortality risk by 10–20%. White cell count is an important value in the prediction of TTS severity, they participate in process of oxidative stress. These three values haven’t been discussed in previous reports, we first found their impact on in-hospital death for ICU patients with TTS.
In this study, higher hematocrit and history of infarction history reduce the risk of in-hospital mortality. Hematocrit is one of laboratory tests used to diagnose anemia. Patients with lower hematocrit levels tend to be anemia. Some evidence supported the relationship of anemia with poor outcomes of TTS31,32. Some analyses applied hematocrit to predict in-hospital death in patients with heart failure. They demonstrated a mortality incidence of more than 50% in heart failure patients with a hematocrit of less than 30%33. The normal hematocrit levels are 40%-50% in males and 37–48% in females. This nomogram for the first time predicted a higher mortality risk of more than 16% in TTS patients (hematocrit < 37%), compared with patients (hematocrit 37–52%). There is limited evidence on the role of myocardial infarction history on in-hospital death of TTS. In this study, survivors combined with myocardial infarction were higher than non-survivors (31.88% vs 18.75%, P = 0.093). The protective role of myocardial infarction history on in-hospital outcomes for TTS patients deserved to investigate in the following research.
Nowadays, it is controversy about whether gender is a risk factor for in-hospital death of TTS. Although female was identified as a risk factor for TTS, researchers haven’t reached a consensus on the predicted effect of female on in-hospital death. This nomogram didn’t include gender as a predictor. We analyzed the baseline characteristics of participants by stratification of gender. We didn’t discover a significant difference between males and females in baseline characteristics, except for BUN, creatinine, and potassium. This finding didn’t support the predicted value of gender on in-hospital mortality. Therefore, gender can’t be a predictor for in-hospital death in ICU patients with TTS.
Study limitation
Several limitations must be acknowledged. Firstly, we aimed to establish a rapid and simple nomogram for predicting in-hospital mortality in TTS patients. The situation of TTS patients was very complex and many factors are related to the prognosis of their hospitalization. We only included the clinical data of the patients within 24 h after hospital admission and did not consider the intervention measures at the onset or after hospitalization. Secondly, this study did not further analyze the causes of TTS, such as physical triggers or psychological triggers. Future studies can conduct a subgroup analysis of specific causes of cardiac arrest to provide more evidence. Thirdly, some records weren’t included, such as mechanical ventilation, echocardiography parameters, mechanical hemodynamic support record, and intraaortic balloon pumps. Because these records are incomplete. The results of this study were only suitable for ICU patients with TTS.
Conclusion
We have established a risk prediction model by admission characteristics of ICU patients with TTS. This model provides some evidence to identify patients with a high risk of in-hospital death.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- TTS:
-
Takotsubo syndrome
- ICD:
-
International Classification of Diseases
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- CI:
-
Confidence intervals
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- MBP:
-
Mean blood pressure
- SPO2 :
-
Pulse oximetry derived oxygen saturation
- BUN:
-
Blood urea nitrogen
- PT:
-
Prothrombin time
- WBC:
-
White blood cell
- CRRT:
-
Continuous renal replacement therapy
- SOFA:
-
Sequential organ failure assessment
- ICU:
-
Intensive care unit
- HOS:
-
Hospital
- LOS:
-
Length of stay
References
Ali, M. et al. Advancements in the diagnostic workup, prognostic evaluation, and treatment of takotsubo syndrome. Heart Fail Rev 25(5), 757–771 (2020).
Akashi, Y. J., Nef, H. M. & Lyon, A. R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 12(7), 387–397 (2015).
Cacciotti, L. et al. Observational study on Takotsubo-like cardiomyopathy: Clinical features, diagnosis, prognosis and follow-up. BMJ Open 2(5), e001165 (2012).
Dias, A. et al. Clinical features of Takotsubo cardiomyopathy—A single-center experience. Cardiology 126(2), 126–130 (2013).
Weihs, V. et al. Stress-induced cardiomyopathy (Tako-Tsubosyndrome) in Austria. Eur. Heart J. Acute Cardiovasc. Care 2(2), 137–146 (2013).
Núñez Gil, I. J. et al. Characterization of Tako-tsubo cardiomyopathy in Spain: Results from the RETAKO National Registry. Rev. Esp. Cardiol. (Engl. Ed.) 68(6), 505–512 (2015).
Templin, C. et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 373(10), 929–938 (2015).
Stiermaier, T. et al. Long-term excess mortality in takotsubo cardiomyopathy: Predictors, causes and clinical consequences. Eur. J. Heart Fail. 18(6), 650–656 (2016).
Redfors, B. et al. Short- and long-term clinical outcomes for patients With Takotsubo syndrome and patienswith myocardial infarction: A report from the Swedish coronary angiography and angioplasty registry. J. Am. Heart Assoc. 10(17), e017290 (2021).
Doyen, D. et al. Incidence, clinical features and outcome of Takotsubo syndrome in the intensive care unit. Arch Cardiovasc Dis. 113(3), 176–188 (2020).
Bai, Z. H. et al. A nomogram to predict the 28-day mortality of critically Ill patients with acute kidney injury and treated with continuous renal replacement therapy. Am. J. Med. Sci. 361(5), 607–615 (2021).
Yang, S., Su, T., Huang, L., Feng, L. H. & Liao, T. A novel risk-predicted nomogram for sepsis associated-acute kidney injury among critically ill patients. BMC Nephrol. 22(1), 173 (2021).
Zhou, Y. et al. Development and validation a nomogram for predicting the risk of severe COVID-19: A multi-center study in Sichuan, China. PLoS ONE 15(5), e0233328 (2020).
Liu, J., Tao, L., Gao, Z., Jiang, R. & Liu, M. Development and validation of a prediction model for early identification of critically ill elderly COVID-19 patients. Aging (Albany NY) 12(19), 18822–18832 (2020).
Jiang, X. et al. Prognostic nomogram for acute pancreatitis patients: An analysis of publicly electronic healthcare records in intensive care unit. J. Crit. Care 50, 213–220 (2019).
Johnson, A., Bulgarelli, L., Pollard, T., Horng, S., Celi, L. A., Mark, R. MIMIC-IV (version 1.0). PhysioNet. (2021). https://doi.org/10.13026/s6n6-xd98.
Raith, E. P. et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 317(3), 290–300 (2017).
Caravaca Perez, P. et al. Serum potassium dynamics during acute heart failure hospitalization. Clin. Res. Cardiol. https://doi.org/10.1007/s00392-020-01753-3 (2020).
Yerasi, C. et al. Predictors of 90-day readmission and in-hospital mortality in Takotsubo cardiomyopathy: An analysis of 28,079 index admissions. Cardiovasc. Revasc. Med. 20(11), 973–979. https://doi.org/10.1016/j.carrev.2019.08.004 (2019).
Sobue, Y. et al. Physically triggered Takotsubo cardiomyopathy hasa higher in-hospital mortality rate. Int. J. Cardiol. 15(235), 87–93. https://doi.org/10.1016/j.ijcard.2017.02.090 (2017) (Epub 2017 Feb 23).
Murakami, T. et al. Gender differences in patients with Takotsubo cardiomyopathy: Multi-center registry from Tokyo CCU network. PLoS ONE 10(8), e0136655. https://doi.org/10.1371/journal.pone.0136655 (2015).
Pelliccia, F. et al. Long-term prognosis and outcome predictors in Takotsubo syndrome: A systematic review and meta-regression study. JACC Heart Fail. 7(2), 143–154. https://doi.org/10.1016/j.jchf.2018.10.009 (2019) (Epub 2019 Jan 2).
Roedl, K. et al. Epidemiology of intensive care unit cardiac arrest: Characteristics, comorbidities, and post-cardiac arrest organ failure—A prospective observational study. Resuscitation 156, 92–98. https://doi.org/10.1016/j.resuscitation.2020.09.003 (2020) (Epub 2020 Sep 10).
de Grooth, H. J., Geenen, I. L., Girbes, A. R., Vincent, J. L. & Parienti, J. J. Oudemans-van Straaten HMSOFA and mortality endpoints in randomized controlled trials: A systematic review and meta-regression analysis. Crit. Care 21(1), 38. https://doi.org/10.1186/s13054-017-1609-1 (2017).
Santoro, F. et al. Assessment of the German and Italian stresscardiomyopathy score for risk stratification for in-hospital complications in patients with Takotsubo syndrome. JAMA Cardiol. 4(9), 892–899. https://doi.org/10.1001/jamacardio.2019.2597 (2019).
Dorgalaleh, A., Favaloro, E. J., Bahraini, M. & Rad, F. Standardization of prothrombin time/international normalized ratio (PT/INR). Int. J. Lab Hematol. 43(1), 21–28. https://doi.org/10.1111/ijlh.13349 (2021) (Epub 2020 Sep 26).
Wagner, S. et al. Aneurysmal subarachnoid hemorrhage as a trigger for Takotsubo syndrome: A comprehensive review. Rev. Cardiovasc. Med. 22(4), 1241–1251. https://doi.org/10.31083/j.rcm2204132 (2021).
Elgendy, A. Y., Elgendy, I. Y., Mansoor, H. & Mahmoud, A. N. Clinical presentations and outcomes of Takotsubo syndrome in the setting of subarachnoid hemorrhage: A systematic review and meta-analysis. Eur. Heart J. Acute Cardiovasc. Care 7(3), 236–245. https://doi.org/10.1177/2048872616679792 (2018) (Epub 2016 Nov 16).
Ayala-Lopez, N. & Harb, R. Interpreting anion gap values in adult and pediatric patients: Examining the reference interval. J. Appl. Lab Med. 5(1), 126–135. https://doi.org/10.1373/jalm.2019.029496 (2020).
Mhanna, M., Beran, A., Srour, O., Ghazaleh, S. & Elzanaty, A. A case of Takotsubo cardiomyopathy triggered by diabetic ketoacidosis and hypothermia. Cureus. 12(10), e10842. https://doi.org/10.7759/cureus.10842 (2020).
Lu, X. et al. Prognostic factors of Takotsubo cardiomyopathy: A systematic review. ESC Heart Fail. 8(5), 3663–3689. https://doi.org/10.1002/ehf2.13531 (2021) (Epub 2021 Aug 9).
Lu, X., Li, P., Teng, C., Cai, P. & Wang, B. Anemia is associated with poor clinical outcomes in hospitalized patients with Takotsubo cardiomyopathy. Angiology 72(9), 842–849. https://doi.org/10.1177/0003319721999492 (2021) (Epub 2021 Mar 9).
McClellan, W. M., Flanders, W. D., Langston, R. D., Jurkovitz, C. & Presley, R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J. Am. Soc. Nephrol. 13, 1928–1936 (2002).
Acknowledgements
We acknowledge MIMIC database for providing their platforms and contributors for uploading their meaningful datasets. A preprint has previously been published.
Funding
Zhejiang traditional Chinese medicine science and technology plan project (2019ZQ047); Wu Jieping Medical Foundation (320.6750.2020-04-44), General Research Project of Education Department of Zhejiang Province (Y202248708). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.Q. and M.Z.W. conceived and designed the research; C.J., W.Y.M., and M.Z.W. collected the data and conducted the research; C.J., S.X.Y., and W.Y.M. analyzed and interpreted the data; C.J. wrote the initial paper; M.Z.W. revised the paper; C.J. had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, J., Wang, Y., Shou, X. et al. Development and validation of a prognostic nomogram for Takotsubo syndrome patients in the intensive care units: a retrospective cohort study. Sci Rep 13, 477 (2023). https://doi.org/10.1038/s41598-022-27224-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27224-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.