Abstract
It is unclear whether outcome measures used in degenerative lumbar spinal stenosis (DLSS) have been validated for this condition. Cross-sectional analysis of studies for DLSS included in systematic reviews (SA) and meta-analyses (MA) indexed in the Cochrane Library. We extracted all outcome measures for pain and disability. We assessed whether the studies provided external references for the validity of the outcome measures and the quality of the validation studies. Out of 20 SA/MA, 95 primary studies used 242 outcome measures for pain and/or disability. Most commonly used were the VAS (n = 69), the Oswestry Disability Index (n = 53) and the Zurich Claudication Questionnaire (n = 22). Although validation references were provided in 45 (47.3%) primary studies, only 14 validation studies for 9 measures (disability n = 7, pain and disability combined n = 2) were specifically validated in a DLSS population. The quality of the validation studies was mainly poor. The Zurich Claudication Questionnaire was the only disease specific tool with adequate validation for assessing treatment response in DLSS. To compare results from clinical studies, outcome measures need to be validated in a disease specific population. The quality of validation studies need to be improved and the validity in studies adequately cited.
Similar content being viewed by others
Introduction
Degenerative lumbar spinal stenosis (DLSS) is defined by diminished space for the neural and vascular elements in the central canal of the lumbar spine secondary to degenerative changes of the facet joints, ligaments, vertebrae, and intervertebral discs1,2. DLSS is a common disease in elderly patients and typically presents with neurogenic claudication symptoms including pain in the buttocks and lower extremities provoked by walking or extended standing and relieved by rest and bending forward3. The treatment options range from nonsurgical approaches such as analgesics, physiotherapy, and epidural corticosteroid injections to surgical methods.
In the past, a multitude of studies assessed the effects of these treatment options for DLSS. In order to be able to establish firm and stringent evidence-based clinical guidelines on the cost-effective use of treatment interventions, results based on clinical trials need to be compared. This is particularly important in systematic reviews and meta-analyses where conclusions are based on the available studies4. However, many trials use different outcome measures which complicate the comparison of trial results. Further, studies may use measures that were not validated in the DLSS population and therefore, may not identify clinically relevant changes or differences in this patient population. Indeed, one study showed that depending on the outcome measure that was used and the cut-off values for clinically important improvement, the conclusion of a study may be strongly influenced5. To date, no study has systematically assessed the outcome measures used in clinical studies for DLSS and their validation specifically for DLSS.
We performed a cross-sectional analysis of treatment studies for DLSS included in systematic reviews and meta-analyses published between 2006 and April 2021. After extracting the outcome measures for the domains of pain and disability, we assessed whether these instruments were validated specifically for DLSS and critically appraised the quality of the validation studies.
Methods
Study design and eligibility criteria
Cross-sectional analysis of outcome measures for pain and disability in treatment studies for DLSS. We included randomized controlled studies (RCT) and observational studies (OS) which were previously included in systematic reviews (SR) or meta-analyses (MA) and were published in the Cochrane library. This approach allowed us to include a complete set of studies for each treatment intervention that was previously assessed for their methodological validity. Spinal stenosis caused by other conditions than degenerative origin (e.g. traumatic, congenital, spondylolisthesis) and other study designs were excluded. This study is not a systematic review, however, reporting will be based, if applicable, on the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-Analysis Protocols (PRISMA statement)6 and the Statement for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE statement)7.
Search strategy
We searched for SR and MA assessing surgical and non-surgical treatments for DLSS published in the Cochrane library from its inception (1996) to April 2021. An update search which did not identify additional SR or MA was conducted on June 21, 2022.
Search terms included “lumbar spinal stenosis” in the title, abstract, or keywords and MeSH term “spinal stenosis”.
Selection process
Two reviewers (DR and MW) independently screened the titles and abstracts for eligible SR and MA according to the pre-defined inclusion criteria. Subsequently, two reviewers (DR and FB) extracted all RCT and OS from the included SR respectively MA into an Endnote database for the analysis. The full text of all RCT and OS were then reviewed for inclusion by DR and confirmed by FB. In case of inconclusive or uncertain eligibility or discrepancies, studies were discussed between the two reviewers and resolved by consensus or by a third party (MW).
If necessary, authors of protocols for systematic reviews and meta-analysis were contacted for further information.
Data extraction process
The following information was systematically extracted by one reviewer (DR): Author, publication year, study design, treatment intervention, outcome measures for pain and disability, references for validation studies. A second reviewer confirmed the extracted information (FB). Subsequently, all cited validation studies were retrieved and read in full text.
Quality of validation study
Two reviewers (DR and MW) analyzed the methodological quality of the validation process using the COnsensus-based Standards for the selection of health status Measurement Instruments (COSMIN, https://www.cosmin.nl/tools/checklists-assessing-methodological-study-qualities, assessed on December 2, 2022) checklist8. The COSMIN checklist was developed to assess the methodological quality of studies on measurement properties of health-related patient reported outcomes. We extracted information on eight domains: the content validity, internal consistency, construct validity, criterion validity, reliability, responsiveness, flooring/ceiling effect, and interpretability.
Content validity Was there a clear description of the measurement aim, the target population, widely accepted or appropriate methods and concepts were used, the item selection, and the investigators / experts involved in item selection are reported. Number of patients adequate (very good ≥ 50, adequate 30–49).
Internal consistency Scale or subscale is unidimensional. Were factor analyses performed in an adequate sample (≥ 100 patients very good, adequate 50–99) and Cronbach’s alpha(s) calculated per dimension (Cronbach’s alpha(s) 0.70–0.95)?
Criterion validity Was a correlation with the gold standard assessed (at least ≥ 0.70)? Number of patients adequate (≥ 50 very good, 30–49 adequate).
Construct validity Were pre-specified hypotheses defined and the results in ≥ 75% in correspondence with these hypotheses (target sample size for this (sub)group analysis ≥ 50 patients)?
Reliability Two independent measurements in similar conditions. Was a test–retest intraclass correlation coefficients (ICC)) or weighted Kappa calculated (at least ≥ 0.70, sample size ≥ 50 patients)?
Responsiveness Proposed criterion can be considered as a reasonable gold standard. Was the ability to detect a clinical important change over time assessed (AUC ≥ 0.70 or Gyatt’s responsiveness ratio > 1.96)? Number of patients adequate (very good ≥ 50, adequate 30–49)?
Floor or ceiling effects: Was a floor or ceiling effect assessed and not detected (sample size ≥ 50 patients)?
Interpretability Was the degree to which one can assign qualitative meaning to quantitative scores assessed (anchor-based method recommended, to determine the minimal clinical difference; sample size ≥ 50 patients)?
Two reviewers (DR and MW) independently assessed each domain and rated the domain as fulfilled (+ , defined as very good or adequately addressed), not fulfilled (-, doubtful or inadequate), not applicable (NA), and nor reported (NR). Disagreement between the reviewers were discussed and resolved by consensus. In case no consensus could be reached, the study was discussed with a third reviewer (FB). All disagreements were resolved by consensus. Finally, a quality score was calculated ranging from 0 (no domain was fulfilled) to 8 points (all domains were fulfilled).
Outcome of interest
Primary outcome were outcome measures in the domains of pain and disability.
Data synthesis
We summarized categorical variables with number and percentage and continuous data with mean and standard deviation. All analyses were conducted with the statistical software R (version 3.6.1).
Results
Study selection
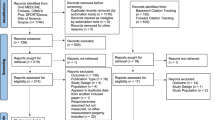
The literature search in the Cochrane library retrieved 31 eligible references. Twenty references met our inclusion criteria and were included in the study (systematic reviews n = 15, meta-analysis n = 3, combined systematic review and meta-analysis n = 2). Subsequently, a total of 256 primary studies were extracted for full-text assessment. For details see Table 1.
After full text screening, 95 primary studies were included in the final analysis. One hundred and forty-two studies did not fulfill our inclusion criteria and were excluded. The main reason for exclusion were duplicates (n = 94). The study selection process is depicted in Fig. 1.
Characteristics of the included primary studies
The characteristics of the included primary studies are summarized in Table 2. Most of the studies were randomized controlled trials (n = 50, 48.5%) and prospective cohort studies (n = 34, 35.8%). Almost three quarters (73%) of the primary studies involved at least one surgical intervention. Studies were published between 1983 and 2016.
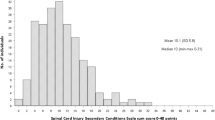
The primary studies included a total of 7′878 participants with a median age of 63.5 ± 7.1 years (range 44–76.2 years). The median follow-up duration was 78.1 ± 81.3 weeks (range 1–480 weeks).
Table 3 summarizes the outcome measures used in the primary studies. In total, 242 outcome measures were identified. In the domain of pain four outcome measures were detected. The Visual Analogue Scale (VAS, n = 69, 90%) respectively Numeric Rating Scale (NSR, n = 9, 9%) were most commonly used. In the domain of disability, a total of 12 outcome parameters were identified. The Oswestry Disability Index (ODI, n = 53, 47%) and various tests assessing walking tolerance (n = 34, 29%) were mostly used (walking ability9,10,11, pain free walking12, walking distance13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37, walking test38, walking time39, walking < 15 minutes40, walking tolerance 41).
In the domain of pain and disability combined, the Zurich Claudication Questionnaire (ZCQ, n = 22, 47%) and the SF-36 (n = 15, 32%) were frequently applied.
Outcome measures and reference studies
In total, 45 primary studies (47.3%) provided a reference for at least one outcome measure. In the domain of pain references were provided for the VAS (n = 5) and the NRS (n = 2), respectively. In the domain of disability, the ODI (n = 22) and the Roland Morris Disability Questionnaire (RMQ, n = 8) were most frequently referenced. In the domain of pain and disability combined the ZCQ (n = 14) was commonly referenced.
For nine outcome measures (disability n = 7, pain and disability combined n = 2) a total of 14 validation studies specifically for a DLSS population were found. For the ZCQ (n = 4)42,43,44,45 and the ODI (n = 3)43,46,47 more than one validation study was identified. For details see Table 4.
Quality assessment of the validation studies
None of the validation studies assessed all predefined domains of the COSMIN checklist8 (Table 4). Twelve of the included 14 studies reached a quality score of 3/8 or less, indicating low methodological quality. None of the validation studies reached the score maximum (range 2/8–7/8). The two studies by Stucki et al.44,45 assessing the validation of the ZCQ in DLSS population, achieved the highest scores (6/8 respectively 7/8).
The Beaujon scoring system (BSS) and various tests assessing walking tolerance were tested in a DLSS population. However, the methodology of the validation study was not in agreement with the methodological items proposed for measurements of health-related patient reported outcomes8.
Discussion
Main findings
The results of this cross-sectional analysis indicate the reporting of outcome measures in randomized clinical trials and observational studies in DLSS is insufficient. Less than half of the included primary studies provided a reference for at least one outcome measure in the domain of pain, disability, or combined pain and disability. A total of 14 validation studies for nine outcome measures were found. The quality assessment of the validation studies revealed low quality for the majority of the studies. Within the DLSS population three validation studies were found for the ODI and four validation studies for the ZCQ, respectively. However, all three validation studies for ODI scored unsatisfactory in the quality assessment. Based on this study, the ZCQ represents the only disease specific tool with adequate validation for assessing treatment response in DLSS.
Results in light with the literature
The findings of our study are in agreement with a systematic review and meta-analysis on outcome measures for neurogenic claudication48. The authors evaluated 15 separate walking outcome measures and concluded that walking outcome measures for patients with neurogenic claudication are lacking. The development of a measurement instrument involves testing validity and reliability with a defined target population49. Choosing a measurement instrument wisely can be challenging given the growing number of choices available. Meaningful use of a measurement instrument depends not only on the validity of the instrument itself, but also on the context in which it is used50. Web-based systems such as PROMIS have been developed from efforts to optimize and simplify the process of selecting an appropriate measurement instrument51. The stated goal is to provide well-constructed, generalizable, and clinically relevant endpoints for studies52. These systems facilitate the completion of questionnaires for subjects, as otherwise there would be a considerable administrative burden. In 2006, the North America Spine Society (NASS) Compendium for the Assessment and Research of Spinal Disorders recommended the Quebec Back Pain Disability Scale, the Roland Morris Disability Questionnaire, and the Waddell Nonorganic Signs for lumbar pain as measurement tools53. In contrast to lumbar back pain, there are currently no specific recommendations for the use of measurement tools in DLSS54. However, measurement tools that are valid for patients with nonspecific back pain do not necessarily measure the relevant endpoints for patients with DLSS. The latter have a different clinical presentation with typical claudication symptoms. Consequently, depending on the conception and design of a questionnaire, clinical outcomes may vary significantly5. The variance of measured symptoms can vary widely, as shown in a recently published study55. The comparison of measurement instruments in patients with DLSS showed that there was a variability of 40–70% depending on cut-off and measurement instrument. In a recently published study56, the ZCQ was the most responsive tool to assess symptoms and function in DLSS supporting the findings of the current systematic analysis. The use of non-validated, nonspecific measurement instruments in studies has an impact on future clinical decisions. The extent of this variation was relevant enough to lead to completely different interpretations of a study. Kimberlin et al.57 argue that although any outcome of a measurement instrument is only an approximation of the actual truth, the use of non-validated measurement instruments has the same effect on study quality as a poor study design or an insufficient number of patients. Our study shows that many of the measurement tools used have not been validated in DLSS patients and it is therefore unclear whether they represent what is relevant to patients.
The issue of inclusion of a magnitude of different outcomes in trials of the same intervention is not novel. For example, in their systematic review from 2017 Mayo-Wilson et al.58 identified variation in outcomes across reports of RCTs the effect of gabapentin for treating neuropathic pain and quetiapine for bipolar depression, respectively. The authors found that the RCTs included hundreds of outcomes and concluded that researchers may cherry-pick what they report from multiple source of RCT information. This results in challenges for interpreting clinical trials and obstacles in comparing clinical trials in meta-analyses.
The development of a measurement instrument involves testing validity and reliability with a defined target population49. Choosing a measurement instrument wisely can be challenging given the growing number of choices available. In recent years, various efforts have been made to systematically assess the validity of measurement instruments59. Meaningful use of a measurement instrument depends not only on the validity of the instrument itself, but also on the context in which it is used50 Web-based systems such as PROMIS have been developed from efforts to optimize and simplify the process of selecting an appropriate measurement instrument60 The stated goal is to provide well-constructed, generalizable, and clinically relevant endpoints for studies.
Strength and limitations
To the best of our knowledge this is the first cross-sectional analysis of outcome measures used in randomized clinical trials and observational studies in DLSS. In addition, we conducted a validity check of the outcomes applying existing guidelines for conducting systematic literature reviews51.
As we focused on systematic reviews and meta-analyses, it is potentially possible that individual studies may not be identified in our analysis. However, we are confident that our methodology included the most relevant papers. The main limitation of this study is that this approach did not capture all validation studies conducted to date. To include an overview of all validation studies ever conducted in patients with DLSS would require a systematic review. By using complete sets of studies included in SR and MA, we assessed the quality of reporting of validation studies and the quality of the validation studies themselves. Therefore, we did not aim to provide a complete overview for all validation studies conducted in DLSS. Thus, when included in this systematic literature review, a study underwent two selection processes.
Implications for clinical research
In order to assess the effectiveness of treatment studies in patients with DLSS, valid and comparable measurement instruments are central. Our study shows that many different and partly unvalidated instruments are used. In addition, there is a lack of information on the minimal clinically important change of the respective measurement instruments. Researchers should systematically conduct high quality validation studies for the measurement instruments in DLSS patients. In addition, the patients’ perspective should be included in the selection of measurement instruments. Further validation studies of measurement instruments specific for DLSS patients with at least 50 patients and considering the quality criteria of Terwee et al.61 will help to quantify the symptoms relevant for DLSS patients and thus have a direct impact on the validity of future RCTs and OS.
Implications for clinical practice
Increasingly, patient-centered measurement instruments are recommended or required for measuring treatment outcome. Our study shows that the selection of adequately validated measurement instruments for DLSS patients is important and that many measurement instruments are not validated in this patient population. In particular, reliable and valid questionnaires specific to DLSS are helpful for everyday clinical practice, as clinical progress can be monitored and responses are less influenced by the treating individuals. For monitoring treatment response in DLSS, we believe that ZCQ provides the most differentiated results. In particular, this questionnaire has the advantage of combining the assessment of pain, satisfaction and disability at the same time.
Conclusion
Reporting of the validity of outcome measures was poor and only in validation in one outcome measure was adequate. In order to be able to compare results from clinical studies, outcome measures need to be validated in a disease specific population and external validation studies should be indicated adequately. For monitoring treatment response in DLSS, the use of the ZCQ is recommended.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arbit, E. & Pannullo, S. Lumbar stenosis: A clinical review. Clin. Orthop. Relat. Res. 384, 137–143 (2001).
Kreiner, D. S. et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J. 13, 734–743. https://doi.org/10.1016/j.spinee.2012.11.059 (2013).
Boden, S. D., Davis, D. O., Dina, T. S., Patronas, N. J. & Wiesel, S. W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J. Bone Joint Surg. Am. 72, 403–408 (1990).
Johnston, B. C. et al. Patient-reported outcomes in meta-analyses–Part 1: Assessing risk of bias and combining outcomes. Health Qual. Life Outcomes 11, 109. https://doi.org/10.1186/1477-7525-11-109 (2013).
Wertli, M. M. et al. A comparison between different outcome measures based on “meaningful important differences” in patients with lumbar spinal stenosis. Eur. Spine J. 26, 450–461. https://doi.org/10.1007/s00586-016-4587-0 (2017).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Br. Med. J. 339, 2700. https://doi.org/10.1136/bmj.b2700 (2009).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Terwee, C. B. et al. Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system for the COSMIN checklist. Qual. Life Res. 21, 651–657. https://doi.org/10.1007/s11136-011-9960-1 (2012).
Malmivaara, A. et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine 32, 1–8. https://doi.org/10.1097/01.brs.0000251014.81875.6d (2007).
Tafazal, S., Ng, L., Chaudhary, N. & Sell, P. Corticosteroids in peri-radicular infiltration for radicular pain: A randomised double blind controlled trial. One year results and subgroup analysis. Eur. Spine J. 18, 1220–1225. https://doi.org/10.1007/s00586-009-1000-2 (2009).
Tafazal, S. I., Ng, L. & Sell, P. Randomised placebo-controlled trial on the effectiveness of nasal salmon calcitonin in the treatment of lumbar spinal stenosis. Eur. Spine J. 16, 207–212. https://doi.org/10.1007/s00586-006-0154-4 (2007).
Richter, A., Halm, H. F., Hauck, M. & Quante, M. Two-year follow-up after decompressive surgery with and without implantation of an interspinous device for lumbar spinal stenosis: A prospective controlled study. J. Spinal Disord. Tech. 27, 336–341. https://doi.org/10.1097/BSD.0b013e31825f7203 (2014).
Amundsen, T. et al. Lumbar spinal stenosis: Conservative or surgical management?: A prospective 10-year study. Spine 25, 1424–1435. https://doi.org/10.1097/00007632-200006010-00016 (2000).
Beyer, F. et al. Percutaneous interspinous spacer versus open decompression: A 2-year follow-up of clinical outcome and quality of life. Eur. Spine J. 22, 2015–2021. https://doi.org/10.1007/s00586-013-2790-9 (2013).
Celik, S. E., Celik, S., Goksu, K., Kara, A. & Ince, I. Microdecompressive laminatomy with a 5-year follow-up period for severe lumbar spinal stenosis. J. Spinal Disord. Tech. 23, 229–235. https://doi.org/10.1097/BSD.0b013e3181a3d889 (2010).
Comer, C. M. et al. The effectiveness of walking stick use for neurogenic claudication: Results from a randomized trial and the effects on walking tolerance and posture. Arch. Phys. Med. Rehabil. 91, 15–19. https://doi.org/10.1016/j.apmr.2009.08.149 (2010).
Eskola, A. et al. Calcitonin treatment in lumbar spinal stenosis: A randomized, placebo-controlled, double-blind, cross-over study with one-year follow-up. Calcif. Tissue Int. 50, 400–403. https://doi.org/10.1007/BF00296769 (1992).
Fukusaki, M., Kobayashi, I., Hara, T. & Sumikawa, K. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin. J. Pain 14, 148–151. https://doi.org/10.1097/00002508-199806000-00010 (1998).
Goren, A., Yildiz, N., Topuz, O., Findikoglu, G. & Ardic, F. Efficacy of exercise and ultrasound in patients with lumbar spinal stenosis: A prospective randomized controlled trial. Clin. Rehabil. 24, 623–631. https://doi.org/10.1177/0269215510367539 (2010).
Grob, D., Humke, T. & Dvorak, J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J. Bone Joint Surg. Am. 77, 1036–1041. https://doi.org/10.2106/00004623-199507000-00009 (1995).
Gurelik, M. et al. unilateral laminotomy for decompression of lumbar stenosis is effective and safe: A prospective randomized comparative study. J. Neurol. Sci. Turk. 29, 744–753 (2012).
Holinka, J., Krepler, P., Matzner, M. & Grohs, J. G. Stabilising effect of dynamic interspinous spacers in degenerative low-grade lumbar instability. Int. Orthop. 35, 395–400. https://doi.org/10.1007/s00264-010-1017-5 (2011).
Koc, Z., Ozcakir, S., Sivrioglu, K., Gurbet, A. & Kucukoglu, S. Effectiveness of physical therapy and epidural steroid injections in lumbar spinal stenosis. Spine 34, 985–989. https://doi.org/10.1097/BRS.0b013e31819c0a6b (2009).
Levendoglu, F., Oguz, H., Polat, E. & Bodur, S. The Effect of Corset on Walking Time in Lumbar Spinal Stenosis. Turkiye Klinikleri J. Med. Sci. 29, 1172–1177 (2009).
Ng, L., Chaudhary, N. & Sell, P. The efficacy of corticosteroids in periradicular infiltration for chronic radicular pain—A randomized, double-blind, controlled trial. Spine 30, 857–862. https://doi.org/10.1097/01.brs.0000158878.93445.a0 (2005).
Paker, N., Türkmen, C., Bugdayci, D., Tekdös, D. & Erbil, M. Comparison of conservative and surgical treatment in lumbar spinal stenosis. Turk. Neurosurg. 15, 182–184 (2005).
Podichetty, V. K., Segal, A. M., Lieber, M. & Mazanec, D. J. Effectiveness of salmon calcitonin nasal spray in the treatment of lumbar canal stenosis: A double-blind, randomized, placebo-controlled, parallel group trial. Spine 29, 2343–2349. https://doi.org/10.1097/01.brs.0000143807.78082.7f (2004).
Prateepavanich, P., Thanapipatsiri, S., Santisatisakul, P., Somshevita, P. & Charoensak, T. The effectiveness of lumbosacral corset in symptomatic degenerative lumbar spinal stenosis. J. Med. Assoc. Thai 84, 572–576 (2001).
Richter, A., Schutz, C., Hauck, M. & Halm, H. Does an interspinous device (Coflex) improve the outcome of decompressive surgery in lumbar spinal stenosis? One-year follow up of a prospective case control study of 60 patients. Eur. Spine. J. 19, 283–289. https://doi.org/10.1007/s00586-009-1229-9 (2010).
Sahin, F., Yilmaz, F., Kotevoglu, N. & Kuran, B. The efficacy of physical therapy and physical therapy plus calcitonin in the treatment of lumbar spinal stenosis. Yonsei Med. J. 50, 683–688. https://doi.org/10.3349/ymj.2009.50.5.683 (2009).
Slatis, P. et al. Long-term results of surgery for lumbar spinal stenosis: A randomised controlled trial. Eur. Spine J. 20, 1174–1181. https://doi.org/10.1007/s00586-010-1652-y (2011).
Slätis, P. et al. Randomised study to compare surgery or conservative treatment for lumbar spinal stenosis. 6-years follow-up. Suomen Ortopedia Ja Traumatologia 29, 250–253 (2006).
Sobottke, R. et al. Clinical outcomes and quality of life 1 year after open microsurgical decompression or implantation of an interspinous stand-alone spacer. Minim. Invasive Neurosurg. 53, 179–183. https://doi.org/10.1055/s-0030-1263108 (2010).
Waikakul, W. & Waikakul, S. Methylcobalamin as an adjuvant medication in conservative treatment of lumbar spinal stenosis. J. Med. Assoc. Thai 83, 825–831 (2000).
Whitman, J. M. et al. A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis: A randomized clinical trial. Spine 31, 2541–2549. https://doi.org/10.1097/01.brs.0000241136.98159.8c (2006).
Yaksi, A., Ozgonenel, L. & Ozgonenel, B. The efficiency of gabapentin therapy in patients with lumbar spinal stenosis. Spine 32, 939–942. https://doi.org/10.1097/01.brs.0000261029.29170.e6 (2007).
Yasar, B. et al. Functional and clinical evaluation for the surgical treatment of degenerative stenosis of the lumbar spinal canal. J. Neurosurg. Spine 11, 347–352. https://doi.org/10.3171/2009.3.SPINE08692 (2009).
Moojen, W. A. et al. Interspinous process device versus standard conventional surgical decompression for lumbar spinal stenosis: Randomized controlled trial. Br. Med. J. 347, f6415. https://doi.org/10.1136/bmj.f6415 (2013).
Mekhail, N., Vallejo, R., Coleman, M. H. & Benyamin, R. M. Long-term results of percutaneous lumbar decompression mild ((R)) for spinal stenosis. Pain Pract. 12, 184–193. https://doi.org/10.1111/j.1533-2500.2011.00481.x (2012).
Lingreen, R. & Grider, J. S. Retrospective review of patient self-reported improvement and post-procedure findings for mild (minimally invasive lumbar decompression). Pain Physician 13, 555–560 (2010).
Fu, Y. S., Zeng, B. F. & Xu, J. G. Long-term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine 33, 514–518. https://doi.org/10.1097/BRS.0b013e3181657dde (2008).
Comer, C. M., Conaghan, P. G. & Tennant, A. Internal construct validity of the Swiss Spinal Stenosis questionnaire: Rasch analysis of a disease-specific outcome measure for lumbar spinal stenosis. Spine 36, 1969–1976. https://doi.org/10.1097/BRS.0b013e3181fc9daf (2011).
Pratt, R. K., Fairbank, J. C. & Virr, A. The reliability of the shuttle walking test, the Swiss spinal stenosis questionnaire, the oxford spinal stenosis score, and the oswestry disability index in the assessment of patients with lumbar spinal stenosis. Spine 27, 84–91. https://doi.org/10.1097/00007632-200201010-00020 (2002).
Stucki, G. et al. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine 21, 796–803. https://doi.org/10.1097/00007632-199604010-00004 (1996).
Stucki, G., Liang, M. H., Fossel, A. H. & Katz, J. N. Relative responsiveness of condition-specific and generic health status measures in degenerative lumbar spinal stenosis. J. Clin. Epidemiol. 48, 1369–1378. https://doi.org/10.1016/0895-4356(95)00054-2 (1995).
Fairbank, J. C. & Pynsent, P. B. The Oswestry disability index. Spine 25, 2940–2952. https://doi.org/10.1097/00007632-200011150-00017 (2000).
Fritz, J. M., Erhard, R. E., Delitto, A., Welch, W. C. & Nowakowski, P. E. Preliminary results of the use of a two-stage treadmill test as a clinical diagnostic tool in the differential diagnosis of lumbar spinal stenosis. J. Spinal Disord. 10, 410–416 (1997).
Anderson, D. B. et al. Measurement properties of walking outcome measures for neurogenic claudication: A systematic review and meta analysis. Spine J. 19, 1378–1396. https://doi.org/10.1016/j.spinee.2019.04.004 (2019).
Deyo, R. A., Diehr, P. & Patrick, D. L. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin. Trials 12, 142S-158S. https://doi.org/10.1016/s0197-2456(05)80019-4 (1991).
Sechrest, L. Validity of measures is no simple matter. Health Serv. Res. 40, 1584–1604. https://doi.org/10.1111/j.1475-6773.2005.00443.x (2005).
Salameh, J. P. et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. Br. Med. J. 370, m2632. https://doi.org/10.1136/bmj.m2632 (2020).
Cook, K. F. et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J. Clin. Epidemiol. 73, 89–102. https://doi.org/10.1016/j.jclinepi.2015.08.038 (2016).
Muller, U., Roder, C. & Greenough, C. G. Back related outcome assessment instruments. Eur. Spine J. 15(Suppl 1), S25-31. https://doi.org/10.1007/s00586-005-1054-8 (2006).
Pincus, T. et al. A review and proposal for a core set of factors for prospective cohorts in low back pain: A consensus statement. Arthritis Rheum 59, 14–24. https://doi.org/10.1002/art.23251 (2008).
Howe, J. & Frymoyer, J. W. The effects of questionnaire design on the determination of end results in lumbar spinal surgery. Spine 10, 804–805. https://doi.org/10.1097/00007632-198511000-00004 (1985).
Fujimori, T., Ikegami, D., Sugiura, T. & Sakaura, H. Responsiveness of the Zurich claudication questionnaire, the Oswestry disability index, the Japanese orthopaedic association back pain evaluation questionnaire, the 8-item short form health survey, and the Euroqol 5 dimensions 5 level in the assessment of patients with lumbar spinal stenosis. Eur. Spine J. 31, 1399–1412. https://doi.org/10.1007/s00586-022-07236-5 (2022).
Kimberlin, C. L. & Winterstein, A. G. Validity and reliability of measurement instruments used in research. Am. J. Health Syst. Pharm. 65, 2276–2284. https://doi.org/10.2146/ajhp070364 (2008).
Mayo-Wilson, E. et al. Multiple outcomes and analyses in clinical trials create challenges for interpretation and research synthesis. J. Clin. Epidemiol. 86, 39–50. https://doi.org/10.1016/j.jclinepi.2017.05.007 (2017).
Quality, A. f. H. R. a. https://www.ahrq.gov/data/resources/index.html.
Patel, A. A. et al. Validation of patient reported outcomes measurement information system (PROMIS) computer adaptive tests (CATs) in the surgical treatment of lumbar spinal stenosis. Spine 43, 1521–1528. https://doi.org/10.1097/BRS.0000000000002648 (2018).
Terwee, C. B. et al. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 60, 34–42. https://doi.org/10.1016/j.jclinepi.2006.03.012 (2007).
Ammendolia, C. et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD010712 (2013).
Ammendolia, C. et al. What interventions improve walking ability in neurogenic claudication with lumbar spinal stenosis? A systematic review. Eur. Spine J. 23, 1282–1301. https://doi.org/10.1007/s00586-014-3262-6 (2014).
Chou, D., Lau, D., Hermsmeyer, J. & Norvell, D. Efficacy of interspinous device versus surgical decompression in the treatment of lumbar spinal stenosis: A modified network analysis. Evid. Based Spine Care J. 2, 45–56. https://doi.org/10.1055/s-0030-1267086 (2011).
Helm, S., Benyamin, R. M., Chopra, P., Deer, T. R. & Justiz, R. Percutaneous adhesiolysis in the management of chronic low back pain in post lumbar surgery syndrome and spinal stenosis: A systematic review. Pain Physician 15, E435–E462 (2012).
Hong, P. W., Liu, Y. & Li, H. D. Comparison of the efficacy and safety between interspinous process distraction device and open decompression surgery in treating lumbar spinal stenosis: A meta analysis. J. Invest. Surg. 28, 40–49. https://doi.org/10.3109/08941939.2014.932474 (2015).
Iversen, M. D., Choudhary, V. R. & Patel, S. C. Therapeutic exercise and manual therapy for persons with lumbar spinal stenosis. Int. J. Clin. Rheumatol. 5, 425–437 (2010).
Jarrett, M. S., Orlando, J. F. & Grimmer-Somers, K. The effectiveness of land based exercise compared to decompressive surgery in the management of lumbar spinal-canal stenosis: A systematic review. BMC Musculoskel. Dis. https://doi.org/10.1186/1471-2474-13-30 (2012).
Kim, K. H. et al. Acupuncture for lumbar spinal stenosis: A systematic review and meta-analysis. Complement. Ther. Med. 21, 535–556. https://doi.org/10.1016/j.ctim.2013.08.007 (2013).
Kovacs, F. M., Urrutia, G. & Alarcon, J. D. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: A systematic review of randomized controlled trials. Spine 36, E1335-1351. https://doi.org/10.1097/BRS.0b013e31820c97b1 (2011).
Kreiner, D. S., MacVicar, J., Duszynski, B. & Nampiaparampil, D. E. The mild (R) procedure: A systematic review of the current literature. Pain Med. 15, 196–205. https://doi.org/10.1111/pme.12305 (2014).
Macedo, L. G. et al. Physical therapy interventions for degenerative lumbar spinal stenosis: A systematic review. Phys. Ther. 93, 1646–1660. https://doi.org/10.2522/ptj.20120379 (2013).
Machado, G. C. et al. Surgical options for lumbar spinal stenosis. Cochrane Database Syst. Rev. 11, CD012421. https://doi.org/10.1002/14651858.CD012421 (2016).
May, S. & Comer, C. Is surgery more effective than non-surgical treatment for spinal stenosis, and which non-surgical treatment is more effective? A systematic review. Physiotherapy 99, 12–20. https://doi.org/10.1016/j.physio.2011.12.004 (2013).
McGregor, A. H. et al. Rehabilitation following surgery for lumbar spinal stenosis a cochrane review. Spine 39, 1044–1054. https://doi.org/10.1097/Brs.0000000000000355 (2014).
Moojen, W. A., Arts, M. P., Bartels, R. H. M. A., Jacobs, W. C. H. & Peul, W. C. Effectiveness of interspinous implant surgery in patients with intermittent neurogenic claudication: A systematic review and meta-analysis. Eur. Spine J. 20, 1596–1606. https://doi.org/10.1007/s00586-011-1873-8 (2011).
Overdevest, G. M. et al. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD010036.pub2 (2015).
Podichetty, V. K., Varley, E. S. & Lieberman, I. Calcitonin treatment in lumbar spinal stenosis a meta-analysis. Spine 36, E357–E364. https://doi.org/10.1097/BRS.0b013e318201b834 (2011).
Reiman, M. P., Harris, J. Y. & Cleland, J. A. Manual therapy interventions for patients with lumbar spinal stenosis: A systematic review. N. Z. J. Physiother. 37, 17–28 (2009).
Wu, A. M. et al. Interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: A systematic review and meta-analysis. PLoS One 9, e97142. https://doi.org/10.1371/journal.pone.0097142 (2014).
Zaina, F., Tomkins-Lane, C., Carragee, E. & Negrini, S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD010264.pub2 (2016).
Forsth, P. et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N. Engl. J. Med. 374, 1413–1423. https://doi.org/10.1056/NEJMoa1513721 (2016).
Komp, M. et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: A prospective, randomized, controlled study. Pain Physician 18, 61–70 (2015).
Lonne, G. et al. Minimally invasive decompression versus x-stop in lumbar spinal stenosis: A randomized controlled multicenter study. Spine 40, 77–85. https://doi.org/10.1097/BRS.0000000000000691 (2015).
Mobbs, R. J., Li, J., Sivabalan, P., Raley, D. & Rao, P. J. Outcomes after decompressive laminectomy for lumbar spinal stenosis: Comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: Clinical article. J. Neurosurg. Spine 21, 179–186. https://doi.org/10.3171/2014.4.SPINE13420 (2014).
Chopko, B. W. Long-term results of percutaneous lumbar decompression for LSS: Two-year outcomes. Clin. J. Pain 29, 939–943. https://doi.org/10.1097/AJP.0b013e31827fb803 (2013).
Davis, R. J., Errico, T. J., Bae, H. & Auerbach, J. D. Decompression and coflex interlaminar stabilization compared with decompression and instrumented spinal fusion for spinal stenosis and low-grade degenerative spondylolisthesis: Two-year results from the prospective, randomized, multicenter, food and drug administration investigational device exemption trial. Spine 38, 1529–1539. https://doi.org/10.1097/BRS.0b013e31829a6d0a (2013).
Durkin, B. et al. Report from a quality assurance program on patients undergoing the MILD procedure. Pain Med. 14, 650–656. https://doi.org/10.1111/pme.12079 (2013).
Liu, X., Yuan, S. & Tian, Y. Modified unilateral laminotomy for bilateral decompression for lumbar spinal stenosis: Technical note. Spine 38, 732–737. https://doi.org/10.1097/BRS.0b013e31828fc84c (2013).
Rajasekaran, S., Thomas, A., Kanna, R. M. & Prasad Shetty, A. Lumbar spinous process splitting decompression provides equivalent outcomes to conventional midline decompression in degenerative lumbar canal stenosis: A prospective, randomized controlled study of 51 patients. Spine 38, 1737–1743. https://doi.org/10.1097/BRS.0b013e3182a056c1 (2013).
Stromqvist, B. H. et al. X-stop versus decompressive surgery for lumbar neurogenic intermittent claudication: Randomized controlled trial with 2-year follow-up. Spine 38, 1436–1442. https://doi.org/10.1097/BRS.0b013e31828ba413 (2013).
Wang, J. J., Bowden, K., Pang, G. & Cipta, A. Decrease in health care resource utilization with MILD. Pain Med. 14, 657–661. https://doi.org/10.1111/pme.12117 (2013).
Basu, S. Mild procedure: Single-site prospective IRB study. Clin. J. Pain 28, 254–258. https://doi.org/10.1097/AJP.0b013e31822bb344 (2012).
Brown, L. L. A double-blind, randomized, prospective study of epidural steroid injection vs. the mild (R) procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract. 12, 333–341. https://doi.org/10.1111/j.1533-2500.2011.00518.x (2012).
Deer, T. R., Kim, C. K., Bowman, R. G. 2nd., Ranson, M. T. & Yee, B. S. Study of percutaneous lumbar decompression and treatment algorithm for patients suffering from neurogenic claudication. Pain Physician 15, 451–460 (2012).
Kim, H. J. et al. Posterior interspinous fusion device for one-level fusion in degenerative lumbar spine disease: Comparison with pedicle screw fixation—preliminary report of at least one year follow up. J. Korean Neurosurg. Soc. 52, 359–364. https://doi.org/10.3340/jkns.2012.52.4.359 (2012).
Mekhail, N., Costandi, S., Abraham, B. & Samuel, S. W. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract. 12, 417–425. https://doi.org/10.1111/j.1533-2500.2012.00565.x (2012).
Wilkinson, J. S. & Fourney, D. R. Failure of percutaneous remodeling of the ligamentum flavum and lamina for neurogenic claudication. Neurosurgery 71, 86–92. https://doi.org/10.1227/NEU.0b013e31825356f5 (2012).
Wong, W. H. mild Interlaminar decompression for the treatment of lumbar spinal stenosis: Procedure description and case series with 1-year follow-up. Clin. J. Pain 28, 534–538. https://doi.org/10.1097/AJP.0b013e31823aaa9d (2012).
Aalto, T. J. et al. Postoperative rehabilitation does not improve functional outcome in lumbar spinal stenosis: A prospective study with 2-year postoperative follow-up. Eur. Spine J. 20, 1331–1340. https://doi.org/10.1007/s00586-011-1781-y (2011).
Chopko, B. W. A novel method for treatment of lumbar spinal stenosis in high-risk surgical candidates: Pilot study experience with percutaneous remodeling of ligamentum flavum and lamina. J. Neurosurg. Spine 14, 46–50. https://doi.org/10.3171/2010.9.SPINE091012 (2011).
McGregor, A. H., Dore, C. J., Morris, T. P., Morris, S. & Jamrozik, K. ISSLS prize winner: Function after spinal treatment, exercise, and rehabilitation (FASTER): A factorial randomized trial to determine whether the functional outcome of spinal surgery can be improved. Spine 36, 1711–1720. https://doi.org/10.1097/BRS.0b013e318214e3e6 (2011).
Postacchini, R., Ferrari, E., Cinotti, G., Menchetti, P. P. & Postacchini, F. Aperius interspinous implant versus open surgical decompression in lumbar spinal stenosis. Spine J. 11, 933–939. https://doi.org/10.1016/j.spinee.2011.08.419 (2011).
Watanabe, K. et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: A randomized controlled study. J. Neurosurg. Spine 14, 51–58. https://doi.org/10.3171/2010.9.SPINE09933 (2011).
Azzazi, A. & Elhawary, Y. Dynamic stabilization using X-stop versus transpedicular screw fixation in the treatment of lumbar canal stenosis; comparative study of the clinical outcome. Neurosurg. Quar. 20, 165–169 (2010).
Chopko, B. & Caraway, D. L. MiDAS I (mild decompression alternative to open surgery): A preliminary report of a prospective, multi-center clinical study. Pain Physician 13, 369–378 (2010).
Galarza, M., Fabrizi, A. P., Maina, R., Gazzeri, R. & Martinez-Lage, J. F. Degenerative lumbar spinal stenosis with neurogenic intermittent claudication and treatment with the Aperius PercLID system: A preliminary report. Neurosurg. Focus 28, E3. https://doi.org/10.3171/2010.3.FOCUS1034 (2010).
Ryu, S. & Kim, I. S. Interspinous implant with unilateral laminotomy for bilateral decompression of degenerative lumbar spinal stenosis in elderly patients. J. Korean Neurosurg. Soc. 47, 823–829 (2009).
Weinstein, J. N. et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the spine patient outcomes research trial. Spine 35, 1329–1338. https://doi.org/10.1097/BRS.0b013e3181e0f04d (2010).
Kuchta, J., Sobottke, R., Eysel, P. & Simons, P. Two-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosis. Eur. Spine J. 18, 823–829. https://doi.org/10.1007/s00586-009-0967-z (2009).
Lee, J. H., An, J. H. & Lee, S. H. Comparison of the effectiveness of interlaminar and bilateral transforaminal epidural steroid injections in treatment of patients with lumbosacral disc herniation and spinal stenosis. Clin. J. Pain 25, 206–210. https://doi.org/10.1097/AJP.0b013e3181878f9e (2009).
Manchikanti, L. et al. The preliminary results of a comparative effectiveness evaluation of adhesiolysis and caudal epidural injections in managing chronic low back pain secondary to spinal stenosis: A randomized, equivalence controlled trial. Pain Physician 12, E341-354 (2009).
Manchikanti, L., Singh, V., Cash, K. A., Pampati, V. & Datta, S. A comparative effectiveness evaluation of percutaneous adhesiolysis and epidural steroid injections in managing lumbar post surgery syndrome: A randomized, equivalence controlled trial. Pain Physician 12, E355-368 (2009).
Matsudaira, K. et al. The efficacy of prostaglandin E1 derivative in patients with lumbar spinal stenosis. Spine 34, 115–120. https://doi.org/10.1097/BRS.0b013e31818f924d (2009).
Park, S. C. et al. Minimum 2-year follow-up result of degenerative spinal stenosis treated with interspinous u (coflex). J. Korean Neurosurg. Soc. 46, 292–299. https://doi.org/10.3340/jkns.2009.46.4.292 (2009).
Yagi, M., Okada, E., Ninomiya, K. & Kihara, M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J. Neurosurg. Spine 10, 293–299. https://doi.org/10.3171/2009.1.SPINE08288 (2009).
Bhadra, A. K., Raman, A. S., Tucker, S. & Noordeen, H. H. Interspinous implant in lumbar spinal stenosis: A prospective cohort. Eur. J. Orthop. Surg. Tr. 18, 489–493. https://doi.org/10.1007/s00590-008-0340-7 (2008).
Brussee, P., Hauth, J., Donk, R. D., Verbeek, A. L. & Bartels, R. H. Self-rated evaluation of outcome of the implantation of interspinous process distraction (X-Stop) for neurogenic claudication. Eur. Spine J. 17, 200–203. https://doi.org/10.1007/s00586-007-0540-6 (2008).
Yano, S. et al. A new ceramic interspinous process spacer for lumbar spinal canal stenosis. Neurosurgery 63, 108–113. https://doi.org/10.1227/01.neu.0000335024.98863.19 (2008).
Athiviraham, A. & Yen, D. Is spinal stenosis better treated surgically or nonsurgically?. Clin. Orthop. Relat. Res. 458, 90–93. https://doi.org/10.1097/BLO.0b013e31803799a9 (2007).
Cavusoglu, H. et al. Efficacy of unilateral laminectomy for bilateral decompression in lumbar spinal stenosis. Turk. Neurosurg. 17, 100–108 (2007).
Cho, D. Y., Lin, H. L., Lee, W. Y. & Lee, H. C. Split-spinous process laminotomy and discectomy for degenerative lumbar spinal stenosis: A preliminary report. J. Neurosurg. Spine 6, 229–239. https://doi.org/10.3171/spi.2007.6.3.229 (2007).
Kim, K. A., McDonald, M., Pik, J. H., Khoueir, P. & Wang, M. Y. Dynamic intraspinous spacer technology for posterior stabilization: Case-control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg. Focus 22, E7 (2007).
Kong, D. S., Kim, E. S. & Eoh, W. One-year outcome evaluation after interspinous implantation for degenerative spinal stenosis with segmental instability. J. Korean Med. Sci. 22, 330–335. https://doi.org/10.3346/jkms.2007.22.2.330 (2007).
Mannion, A. F., Denzler, R., Dvorak, J., Muntener, M. & Grob, D. A randomised controlled trial of post-operative rehabilitation after surgical decompression of the lumbar spine. Eur. Spine J. 16, 1101–1117. https://doi.org/10.1007/s00586-007-0399-6 (2007).
Pua, Y. H., Cai, C. C. & Lim, K. C. Treadmill walking with body weight support is no more effective than cycling when added to an exercise program for lumbar spinal stenosis: A randomised controlled trial. Aust. J. Physiother. 53, 83–89. https://doi.org/10.1016/s0004-9514(07)70040-5 (2007).
Siddiqui, M., Smith, F. W. & Wardlaw, D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine 32, 1345–1348. https://doi.org/10.1097/BRS.0b013e31805b7694 (2007).
Anderson, P. A., Tribus, C. B. & Kitchel, S. H. Treatment of neurogenic claudication by interspinous decompression: Application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J. Neurosurg. Spine 4, 463–471. https://doi.org/10.3171/spi.2006.4.6.463 (2006).
Hsu, K. Y. et al. Quality of life of lumbar stenosis-treated patients in whom the X STOP interspinous device was implanted. J. Neurosurg. Spine 5, 500–507. https://doi.org/10.3171/spi.2006.5.6.500 (2006).
Kondrashov, D. G., Hannibal, M., Hsu, K. Y. & Zucherman, J. F. Interspinous process decompression with the X-STOP device for lumbar spinal stenosis: A 4-year follow-up study. J. Spinal Disord. Tech. 19, 323–327. https://doi.org/10.1097/01.bsd.0000211294.67508.3b (2006).
Murphy, D. R., Hurwitz, E. L., Gregory, A. A. & Clary, R. A non-surgical approach to the management of lumbar spinal stenosis: A prospective observational cohort study. BMC Musculoskelet. Disord. 7, 16. https://doi.org/10.1186/1471-2474-7-16 (2006).
Veihelmann, A. et al. Epidural neuroplasty versus physiotherapy to relieve pain in patients with sciatica: A prospective randomized blinded clinical trial. J. Orthop. Sci. 11, 365–369. https://doi.org/10.1007/s00776-006-1032-y (2006).
Atlas, S. J., Keller, R. B., Wu, Y. A., Deyo, R. A. & Singer, D. E. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine 30, 936–943. https://doi.org/10.1097/01.brs.0000158953.57966.c0 (2005).
Gerdesmeyer, L. et al. Chronic radiculopathy. Use of minimally invasive percutaneous epidural neurolysis according to Racz. Schmerz 19, 285–295. https://doi.org/10.1007/s00482-004-0371-x (2005).
Thome, C. et al. Outcome after less-invasive decompression of lumbar spinal stenosis: A randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J. Neurosurg. Spine 3, 129–141. https://doi.org/10.3171/spi.2005.3.2.0129 (2005).
Zucherman, J. F. et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur. Spine J. 13, 22–31. https://doi.org/10.1007/s00586-003-0581-4 (2004).
Lee, J., Hida, K., Seki, T., Iwasaki, Y. & Minoru, A. An interspinous process distractor (X STOP) for lumbar spinal stenosis in elderly patients: Preliminary experiences in 10 consecutive cases. J. Spinal Disord. Tech. 17, 72–77. https://doi.org/10.1097/00024720-200402000-00013 (2004).
Manchikanti, L. et al. One day lumbar epidural adhesiolysis and hypertonic saline neurolysis in treatment of chronic low back pain: A randomized, double-blind trial. Pain Physician 7, 177–186 (2004).
Mariconda, M., Fava, R., Gatto, A., Longo, C. & Milano, C. Unilateral laminectomy for bilateral decompression of lumbar spinal stenosis: A prospective comparative study with conservatively treated patients. J. Spinal Disord. Tech. 15, 39–46. https://doi.org/10.1097/00024720-200202000-00006 (2002).
Simotas, A. C., Dorey, F. J., Hansraj, K. K. & Cammisa, F. Jr. Nonoperative treatment for lumbar spinal stenosis. Clinical and outcome results and a 3-year survivorship analysis. Spine 25, 197–203. https://doi.org/10.1097/00007632-200001150-00009 (2000).
Heavner, J. E., Racz, G. B. & Raj, P. Percutaneous epidural neuroplasty: Prospective evaluation of 0.9% NaCl versus 10% NaCl with or without hyaluronidase. Region Anesth. Pain Med. 24, 202–207. https://doi.org/10.1016/S1098-7339(99)90128-1 (1999).
Amundsen, T. et al. Lumbar spinal stenosis clinical and radiologic features. Spine 20, 1178–1186. https://doi.org/10.1097/00007632-199505150-00013 (1995).
Porter, R. W. & Miller, C. G. Neurogenic claudication and root claudication treated with calcitonin. A double-blind trial. Spine 13, 1061–1064. https://doi.org/10.1097/00007632-198809000-00015 (1988).
Porter, R. W. & Hibbert, C. Calcitonin treatment for neurogenic claudication. Spine 8, 585–592. https://doi.org/10.1097/00007632-198309000-00004 (1983).
Beurskens, A. J., de Vet, H. C. & Koke, A. J. Responsiveness of functional status in low back pain: A comparison of different instruments. Pain 65, 71–76. https://doi.org/10.1016/0304-3959(95)00149-2 (1996).
Breivik, E. K., Bjornsson, G. A. & Skovlund, E. A comparison of pain rating scales by sampling from clinical trial data. Clin. J. Pain 16, 22–28. https://doi.org/10.1097/00002508-200003000-00005 (2000).
Dyck, P. & Doyle, J. B. Jr. “Bicycle test” of van Gelderen in diagnosis of intermittent cauda equina compression syndrome case report. J. Neurosurg. 46, 667–670. https://doi.org/10.3171/jns.1977.46.5.0667 (1977).
Jensen, M. P., Turner, L. R., Turner, J. A. & Romano, J. M. The use of multiple-item scales for pain intensity measurement in chronic pain patients. Pain 67, 35–40. https://doi.org/10.1016/0304-3959(96)03078-3 (1996).
Childs, J. D., Piva, S. R. & Fritz, J. M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 30, 1331–1334. https://doi.org/10.1097/01.brs.0000164099.92112.29 (2005).
Manchikanti, L. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management, part I: Introduction and general considerations. Pain Physician 11, 161–186 (2008).
Manchikanti, L., Benyamin, R. M., Helm, S. & Hirsch, J. A. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 3: Systematic reviews and meta-analyses of randomized trials. Pain Physician 12, 35–72 (2009).
Manchikanti, L., Datta, S., Smith, H. S. & Hirsch, J. A. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 6. Systematic reviews and meta-analyses of observational studies. Pain Physician 12, 819–850 (2009).
Manchikanti, L. et al. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 5 Diagnostic accuracy studies. Pain Physician 12, 517–540 (2009).
Manchikanti, L., Hirsch, J. A. & Smith, H. S. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 2: Randomized controlled trials. Pain Physician 11, 717–773 (2008).
Adamova, B., Vohanka, S. & Dusek, L. Differential diagnostics in patients with mild lumbar spinal stenosis: The contributions and limits of various tests. Eur. Spine J. 12, 190–196. https://doi.org/10.1007/s00586-002-0503-x (2003).
Chow, J. H. & Chan, C. C. Validation of the Chinese version of the oswestry disability index. Work 25, 307–314 (2005).
Deen, H. G. et al. Use of the exercise treadmill to measure baseline functional status and surgical outcome in patients with severe lumbar spinal stenosis. Spine 23, 244–248. https://doi.org/10.1097/00007632-199801150-00019 (1998).
Deen, H. G. Jr. et al. Test-retest reproducibility of the exercise treadmill examination in lumbar spinal stenosis. Mayo Clin. Proc. 75, 1002–1007. https://doi.org/10.4065/75.10.1002 (2000).
Fairbank, J. C., Couper, J., Davies, J. B. & O’Brien, J. P. The Oswestry low back pain disability questionnaire. Physiotherapy 66, 271–273 (1980).
Fritz, J. M. & Irrgang, J. J. A comparison of a modified Oswestry low back pain disability questionnaire and the Quebec back pain disability scale. Phys. Ther. 81, 776–788. https://doi.org/10.1093/ptj/81.2.776 (2001).
Holm, I., Friis, A., Storheim, K. & Brox, J. I. Measuring self-reported functional status and pain in patients with chronic low back pain by postal questionnaires: A reliability study. Spine 28, 828–833 (2003).
Roland, M. & Morris, R. A study of the natural history of low-back pain. Part II: Development of guidelines for trials of treatment in primary care. Spine 8, 145–150. https://doi.org/10.1097/00007632-198303000-00005 (1983).
Tomkins, C. C., Battie, M. C., Rogers, T., Jiang, H. & Petersen, S. A criterion measure of walking capacity in lumbar spinal stenosis and its comparison with a treadmill protocol. Spine 34, 2444–2449. https://doi.org/10.1097/BRS.0b013e3181b03fc8 (2009).
Whitehurst, M., Brown, L. E., Eidelson, S. G. & D’Angelo, A. Functional mobility performance in an elderly population with lumbar spinal stenosis. Arch. Phys. Med. Rehabil. 82, 464–467. https://doi.org/10.1053/apmr.2001.20828 (2001).
Steffen, T. M., Hacker, T. A. & Mollinger, L. Age- and gender-related test performance in community-dwelling elderly people: Six-minute walk test, berg balance scale, timed up & go test, and gait speeds. Phys. Ther. 82, 128–137. https://doi.org/10.1093/ptj/82.2.128 (2002).
Stromqvist, B., Fritzell, P., Hagg, O., Jonsson, B., Swedish Society of Spinal S. The Swedish spine register: Development, design and utility. Eur. Spine J. 18(Suppl 3), 294–304. https://doi.org/10.1007/s00586-009-1043-4 (2009).
Lassale, B., Bitan, F. & Bex, M. Résultats fonctionnels et facteur de prognostic du traitement chirurgical des sténoses lombaires dégéneratives. Rev. Chir. Orthop. 74, 85–88 (1988).
Deyo, R. A. Measuring the functional status of patients with low back pain. Arch. Phys. Med. Rehabil. 69, 1044–1053 (1988).
Exner, V. & Keel, P. Measuring disability of patients with low-back pain–validation of a German version of the Roland & Morris disability questionnaire. Schmerz 14, 392–400. https://doi.org/10.1007/s004820000010 (2000).
Kucukdeveci, A. A., Tennant, A., Elhan, A. H. & Niyazoglu, H. Validation of the Turkish version of the Roland-Morris disability questionnaire for use in low back pain. Spine 26, 2738–2743 (2001).
Patrick, D. L. et al. Assessing health-related quality of life in patients with sciatica. Spine 20, 1899–1908. https://doi.org/10.1097/00007632-199509000-00011 (1995).
Roland, M. & Fairbank, J. The Roland-Morris disability questionnaire and the oswestry disability questionnaire. Spine 25, 3115–3124. https://doi.org/10.1097/00007632-200012150-00006 (2000).
Tait, R. C., Chibnall, J. T. & Krause, S. The pain disability index: Psychometric properties. Pain 40, 171–182. https://doi.org/10.1016/0304-3959(90)90068-o (1990).
Tait, R. C., Pollard, C. A., Margolis, R. B., Duckro, P. N. & Krause, S. J. The pain disability index: Psychometric and validity data. Arch. Phys. Med. Rehabil. 68, 438–441 (1987).
Aaronson, N. K. et al. Translation, validation, and norming of the dutch language version of the SF-36 health survey in community and chronic disease populations. J. Clin. Epidemiol. 51, 1055–1068. https://doi.org/10.1016/s0895-4356(98)00097-3 (1998).
Fukuhara, S., Bito, S., Green, J., Hsiao, A. & Kurokawa, K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 51, 1037–1044. https://doi.org/10.1016/s0895-4356(98)00095-x (1998).
Fukuhara, S., Ware, J. E. Jr., Kosinski, M., Wada, S. & Gandek, B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J. Clin. Epidemiol. 51, 1045–1053. https://doi.org/10.1016/s0895-4356(98)00096-1 (1998).
McHorney, C. A., Kosinski, M. & Ware, J. E. Jr. Comparisons of the costs and quality of norms for the SF-36 health survey collected by mail versus telephone interview: Results from a national survey. Med. Care 32, 551–567. https://doi.org/10.1097/00005650-199406000-00002 (1994).
Stewart, A. L. et al. Functional status and well-being of patients with chronic conditions. Results from the medical outcomes study. JAMA 262, 907–913 (1989).
Ware, J. E. Jr. SF-36 health survey update. Spine 25, 3130–3139. https://doi.org/10.1097/00007632-200012150-00008 (2000).
Ware, J. E. Jr. et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the medical outcomes study. Med. Care 33, AS264-279 (1995).
Ware, J. E. Jr. & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30, 473–483 (1992).
Greenough, C. G. & Fraser, R. D. Assessment of outcome in patients with low-back pain. Spine 17, 36–41. https://doi.org/10.1097/00007632-199201000-00006 (1992).
Holt, A. E., Shaw, N. J., Shetty, A. & Greenough, C. G. The reliability of the low back outcome score for back pain. Spine 27, 206–210. https://doi.org/10.1097/00007632-200201150-00017 (2002).
Daltroy, L. H., Cats-Baril, W. L., Katz, J. N., Fossel, A. H. & Liang, M. H. The North American spine society lumbar spine outcome assessment Instrument: Reliability and validity tests. Spine 21, 741–749. https://doi.org/10.1097/00007632-199603150-00017 (1996).
Pose, B., Sangha, O., Peters, A. & Wildner, M. Validation of the North American spine society instrument for assessment of health status in patients with chronic backache. Z. Orthop. Ihre. Grenzgeb. 137, 437–441. https://doi.org/10.1055/s-2008-1037387 (1999).
Author information
Authors and Affiliations
Contributions
Drs. M.M.W., D.R., and F.B. designed the study, conducted the title and abstract search and extracted the data. All authors interpreted the study results. Drs. M.M.W., D.R., and F.B. drafted the first version of the manuscript. Drs. J.M.B., U.H., N.H.U., M.F., and J.S. commented on the manuscript. All authors approved the final manuscript and this submission and declared to have no competing financial interests.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wertli, M.M., Rossi, D., Burgstaller, J.M. et al. Validity of outcome measures used in randomized clinical trials and observational studies in degenerative lumbar spinal stenosis. Sci Rep 13, 1068 (2023). https://doi.org/10.1038/s41598-022-27218-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27218-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.