Abstract
The effect of anti-vascular endothelial growth factor on neonatal lung development was inconclusive. To evaluate pulmonary function in school-age children who have received intravitreal bevacizumab (IVB) for retinopathy of prematurity (ROP), this study included 118 school-aged children who were grouped into three groups: full-term control children (group 1), preterm children who had not received IVB treatment (group 2) and preterm children with ROP who had received IVB treatment (group 3). Pulmonary function was measured by spirometry and impulse oscillometry. Pulmonary function was significantly better in group 1 than in groups 2 and 3 (all p < 0.05 in forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), forced expiratory flow between 25 and 75% of FVC (FEF25–75), and respiratory resistance at 5 Hz and difference between respiratory resistance at 5 and 20 Hz (R5-R20). There were no statistically significant differences between group 2 and group 3 in all pulmonary function parameters, including FVC, FEV1, ratio of FEV1 to FVC, FEF25-75, R5, R20, R5–R20, and respiratory reactance at 5 Hz. In conclusion, our study revealed that preterm infants receiving IVB for ROP had comparable pulmonary function at school age to their preterm peers who had not received IVB treatment.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is an important infantile retinal disease that causes blindness or severe visual impairment in childhood1,2 and imposes a heavy financial burden in several countries3. Intravitreal anti-vascular endothelial growth factor (anti-VEGF) with bevacizumab monotherapy, compared with conventional laser therapy in infants with stage 3 + retinopathy of prematurity, showed a significant benefit for zone I disease4. Compared with traditional laser monotherapy, intravitreal injection of anti-VEGF (IVI) reduced the recurrence of zone I ROP and the risk of high myopia5,6,7. When combined with laser therapy, anti-VEGF reduced the risk of retinal detachment6. Several studies also proposed that patients who had received anti-VEGF treatment had favorable anatomical outcomes8,9,10.
Regarding the safety issues of using anti-VEGF for ROP, potential adverse events include endophthalmitis, intraocular inflammation and rhegmatogenous retinal detachment11,12. Regarding the systemic safety of this treatment, VEGF plays a crucial role in the development of several organs, including the lungs, brain, kidneys, and liver13. Multiple studies have evaluated the effect of anti-VEGF treatment on the brain, that is, neurodevelopmental outcomes14,15,16,17,18,19,20. While some studies presented negative results19,20, most studies revealed difference in neurodevelopment after anti-VEGF treatment14,15,16,17,18. Among all organs, the lungs contain the highest level of VEGF transcripts12. An animal study also revealed that the blockage of VEGF impaired lung development13. Since serum VEGF levels are reduced for up to 8 weeks after intravitreal anti-VEGF treatment for ROP21,22,23, additional impacts on lung function are a concern in neonates with a history of anti-VEGF treatment. The hypothesis of our study was that the reduction in serum VEGF levels may have an impact on lung development. To date, no clinical study has assessed the long-term pulmonary function of premature patients after they have received anti-VEGF treatment for ROP. Therefore, this study aimed to investigate the pulmonary function of school-age children with or without a history of anti-VEGF treatment for ROP during infancy.
Methods
Study subjects and enrollment and exclusion criteria
This study was approved by the institutional review board of Chang Gung Memorial Hospital in Taoyuan, Taiwan (contract, IRB201900571B0) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each patient’s parent for the enrollment of his or her child in the study.
This retrospective case–control study was conducted between 2016/07/01 and 2020/07/31 at Chang Gung Memorial Hospital, Taoyuan, Taiwan. Three groups of subjects were enrolled in this study: Group 1 consisted of full-term children without ROP, matched to groups 2 and 3 by age at pulmonary function testing, who underwent baseline pulmonary function testing in the Longitudinal Investigation of Global Health in Taiwanese Schoolchildren (LIGHTS) cohort24,25,26,27,28. Group 2 consisted of preterm children with no history of intravitreal bevacizumab (IVB) treatment, and this group was composed of three parts: patients without ROP, patients with untreated ROP, and patients who received laser treatment. Group 3 consisted of premature children with ROP who received anti-VEGF treatment, composed of IVB monotherapy or combined IVB and laser therapy (Fig. 1). The indication for treatment was type 1 ROP as defined by the Early Treatment for ROP Study29,30, that is, zone I ROP of any stage with plus disease (a degree of dilation and tortuosity of the posterior retinal blood vessels meeting or exceeding that of a standard photograph), zone I stage 3 ROP without plus disease, or zone II stage 2 or 3 ROP with plus disease29,30. The technique used for IVB was described previously31,32; that is, 0.625 mg (0.025 mL) bevacizumab was injected intravitreally via the pars plicata under intravenous sedation. For IVB or laser treatment decisions, we suggested that patients with zone I ROP receive IVB treatment according to the BEAT ROP study results4. For type 1 ROP patients with postmenstrual age over 40 weeks, we suggested laser therapy because IVB therapy may increase the risk of tractional retinal detachment33. However, the final decision of IVB or laser treatment was made through shared decision-making after fully explaining the benefits and risks to the patients’ families.
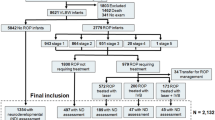
Flowchart showing the inclusion and exclusion of patients during the study period. Abbreviations: IVB, intravitreal injection of bevacizumab; ROP, retinopathy of prematurity. aFull-term children were matched to groups 2 and 3 by age at pulmonary function testing. bOne patient received IVB in the right eye and laser treatment in the left eye. cOne patient received IVB in the right eye and combined IVB and laser treatment in the left eye.
Full-term birth was defined as birth later than 37 weeks of gestational age (GA). Parents of premature children who had been admitted to neonatal intensive care units before and were attending follow-up outpatient clinics were invited to join this study. The status of the off-label use of IVB for ROP treatment was explained to the parents in detail. Pulmonary function testing was arranged for the school-age patients. Preterm patients with prior ROP and IVB treatment were allocated to the target study group (Group 3). Preterm patients without IVB who had been born before 32 weeks of GA (Group 2) were selected for similarity to Group 3 in GA. Full-term children (Group 1) matched to groups 2 and 3 by age at pulmonary testing were enrolled because pulmonary function was highly correlated with age34,35,36. Patients who were unable to finish the pulmonary function test, full-term patients with congenital cardiac or neuromuscular disease and patients whose ROP progressed to stage 4 or 5 after IVB or laser treatment were excluded (Fig. 1).
Pulmonary function measurement
Spirometry is the gold standard and the most commonly used, well-established tool to detect lung disease36. In our study, forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), ratio of FEV1 to FVC (FEV1/FVC), and forced expiratory flow between 25 and 75% (FEF25–75) were measured by using a spirometer (Spirolab II, Medical International Research, Rome, Italy). The measurements of spirometry were described in a prior publication34,37.
An impulse oscillometer (IOS) is a noninvasive tool that is increasingly used in children to measure respiratory resistance (Rrs) and reactance (Xrs)38. We used a commercially equipped IOS (MasterScreen; Jaeger, Wurzburg, Germany). We used oscillometry procedures that have previously been described in detail35. The Rrs was recorded at 5 Hz (R5: representing total airway resistance) and 20 Hz (R20: representing proximal airway resistance). The difference between R5 and R20 was calculated (R5–R20: representing peripheral airway resistance). The Xrs was recorded at 5 Hz (X5).
Statistical analysis
Group differences in continuous variables were identified by using one-way ANOVA. Group differences in categorical variables were identified by using the chi-squared test or Fisher’s exact test when applicable. Univariable linear regression analyses were performed to determine the relationships between individual variables and pulmonary function parameters. Factors that were significant in the univariate analysis were used for a multivariable pretest, and only factors that were still significant were used in the final multivariable analysis. Final multivariable regression analyses were performed to estimate the relationships between variables of interest and pulmonary function and the potential interaction between significant variables in the univariable linear regression. The significance threshold for all tests was set at P < 0.05. All statistical analyses were performed with SPSS software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
Study participants
One hundred eighteen children (65 boys and 53 girls; mean age, 6.67 ± 0.52 years) were included in the final analysis. A flowchart showing how patients were enrolled and excluded from the study is presented in Fig. 1. Among these children’s perinatal history, 50 were full-term infants matched with the preterm infants by test age (Group 1), 33 were preterm infants with no history of IVB treatment (Group 2), and 35 were preterm infants with a history of IVB treatment (Group 3). The ages of the children at testing were similar among 3 groups (p = 0.58). Table 1 shows the demographic data and important confounding factors related to pulmonary function and test status. Both group 2 and group 3 showed less favorable baseline birth demographics and more perinatal complications than group 1. Group 3 showed lower GA (P < 0.001), lower birth weight (BW) (P < 0.001), a lower rate of cesarean section (CS) (P = 0.04), a lower rate of premature rupture of membrane (PROM) (P = 0.03), a lower rate of paternal asthma (P = 0.02), a higher rate of surfactant use (P = 0.005), a higher rate of bronchopulmonary dysplasia (BPD) (P = 0.03), and a longer duration of mechanical ventilator use after birth (P < 0.001) than group 2.
Supplementary table S1 shows the remaining demographics, confounding factors, ROP disease status and treatment status. Group 3 showed lower Apgar scores (P = 0.001 and P = 0.02 at 1 min and 5 min, respectively), a higher incidence of respiratory distress syndrome (RDS) (P = 0.007), a lower incidence of atopic dermatitis (P = 0.003), a higher rate of paternal smoking during pregnancy (P = 0.007), and a longer breastfeeding duration (P = 0.03) than group 2. There were also significant differences between groups 2 and 3 in terms of ROP stage (P < 0.001), zone (P < 0.001), plus disease (P < 0.001) and treatment received (P < 0.001).
Pulmonary function outcomes
Table 2 summarizes the pulmonary function outcomes of the three groups, including FVC, FEV1, FEV1/FVC, FEF25-75, R5, R20, X5 and R5-R20. There were no significant differences among the 3 groups in FEV1/FVC, R20 or X5. However, FVC, FEV1, FEF25–75, R5, and R5–R20 showed significant differences. Compared with group 1, group 2 and group 3 both showed significantly reduced FVC, reduced FEV1, increased R5 and increased R5–R20. Additionally, group 3 showed lower FEF25–75 than group 1. Compared with group 2, group 3 showed trends toward reduced FVC, FEV1, FEV1/FVC, and FEF25–75 and increased R5, R20, and R5-R20. However, the post hoc tests showed that there were no statistically significant differences in any pulmonary outcome parameters between group 2 and group 3.
Supplementary Table S2 shows the univariate linear regression of the relationships between the confounding factors and the pulmonary outcomes. Important factors associated with pulmonary function (IVB history, full term, height) and baseline significant factors (factors that differed between groups 2 and 3 included sex, GA, BW, CS, Apgar scores at 1 min and 5 min, BPD, PROM, RDS, received surfactant, duration of mechanical ventilator use after birth, breastfeeding duration, atopic dermatitis, paternal asthma, present paternal smoking) were analyzed. Then, those factors that had significant predictive power for pulmonary function in the univariate model were used for the pretest multivariate regression model. The factors (sex, test age, BPD, RDS, child height and duration of mechanical ventilator use after birth) that had significant predictive power in the pretest multivariable regression model were selected and used to construct the final multivariable model. FEV1/FVC was not included in the multivariable regression model because there were no significant factors identified in the univariable regression model.
Table 3 shows the final multivariable regression model of the relationship between confounding factors and pulmonary function. Male sex showed a positive relationship with FVC (P < 0.01), FEV1 (P < 0.01) and FEF25–75 (P < 0.01). Child age at pulmonary function test showed a negative association with R5 (P < 0.01) and R5–R20 (P < 0.01). Child height showed a positive association with FVC (P < 0.001), FEV1 (P < 0.001) and FEF25–75 (P < 0.001). Importantly, a history of IVB treatment showed no significant relationship with any of the spirometry or IOS parameters after adjusting for other confounding factors.
Discussion
Our study revealed that, preterm patients with and without a history of IVB treatment showed similar pulmonary function at the age of 6–7 years. We used 2 lung function assessment tools, namely, spirometry and impulse oscillometry, which measured different components of lung function, to determine the outcome of pulmonary function. Pulmonary function did not reveal a significant difference between these 2 groups of patients, although the IVB group had relatively unfavorable baseline demographics, including reduced GA, reduced BW, reduced Apgar scores, increased percentages of RDS and BPD, and extended periods of ventilator use. Male sex, age and height were the 3 most important factors found to be associated with superior pulmonary function at school age. To the best of our knowledge, this is the first study to examine the pulmonary function of ROP patients at school age after anti-VEGF treatment and compare it with that of patients who had not received such treatment. This information is important for clinicians to consider before administering anti-VEGF to preterm children, who face an increased risk of compromised pulmonary function.
VEGF plays an important role in lung disease and development12,13,39,40,41. In the neonatal lungs, VEGF regulates angiogenesis, interacting closely with vascularization and bronchiolar branching41, and enhances type II pneumocyte growth42. Animal studies showed that VEGF receptor blockers or a single dose of anti-VEGF impaired pulmonary vascular growth and postnatal alveolarization and caused pulmonary hypertension in infant rats13,43,44. Additionally, the complex coordinated growth of lung epithelial cells and vessels requires a normal VEGF gradient, and the disruption of temporal and spatial expression of VEGF disrupts lung morphogenesis45. Multiple lung diseases are related to VEGF; for example, asthma, lung cancer, and acute lung injury are related to VEGF overexpression, while emphysema and pulmonary hypertension are related to VEGF receptor blockage39,40. Taken together, VEGF is vital for the healthy development of lung structure and function.
Why did the use of anti-VEGF treatment not further compromise lung function in these preterm children compared to patients with no history of anti-VEGF therapy? Although preterm infants with prior anti-VEGF treatment faced an elevated risk of poor lung function, their lung function was similar to that of preterm infants without anti-VEGF treatment by the time they reached school age. First, it is possible that the coordinated timely release of VEGF, rather than a high level of VEGF, was vital to lung vascularity development, and a VEGF blockage alone had no impact on neonatal oxygen requirements46. Also, although serum VEGF levels were suppressed for 2 months after the use of IVB21,22,47, these suppressive effects are relatively short compared to the full period of lung development. Lung development starts from a postmenstrual age of 4 weeks and continues into early adolescence48. More than 85% of alveoli are formed after birth, and the lungs have the potential to continue growing even in adulthood48,49. Vollsæter et al. showed a similar slope of lung function development from mid-childhood to adulthood between preterm and term-born groups50. Longitudinal studies up to adolescence and 21 years of age also showed gradual improvement in lung function among preterm patients51,52. Other studies showed that lung alveolar growth had sufficient plasticity to catch up to normal after impairment by drugs, toxins or malnutrition53,54,55.
Furthermore, in addition to VEGF, there are multiple other factors that regulate lung development, including Wnt signaling, retinoic acid signaling, fibroblast growth factor signaling, and histone acetylation56. The impact of altered VEGF levels can be mitigated by complex molecular signaling in lung development. Additionally, VEGF signaling can be regulated by multiple factors, such as platelet-derived growth factor, transforming growth factor, insulin growth factor-I, fibroblast growth factor, keratinocyte growth factor, estrogens and IL-1β39. There is also a VEGF homolog known as placental growth factor, whose function is similar to that of VEGF39. Future studies need to examine the impact of VEGF suppression on lung homeostasis of various signaling factors.
However, we still cannot fully exclude the possibility that anti-VEGF has a minor effect on the distal airway because VEGF plays an important role in lung alveolarization and type II pneumocyte development41,42. FEF25–75 is related to dysfunction of the distal airway57, and R5–R20 represents distal airway resistance38. In our study, we noted that, compared to preterm infants with no history of IVB treatment, preterm infants with a history of IVB anti-VEGF treatment showed a minor trend toward reduced FEF25–75 (IVB: 1.12; non-IVB: 1.35; p = 0.11) and increased R5–R20 (IVB: 4.12; non-IVB: 4.08; p = 1.00), but the difference was not statistically significant. Also, longer mechanical ventilation need was observed in Group 3. However, whether anti-VEGF have temporary detrimental effect to infant in perinatal period was uncertain due to complicated confounding factors such as BPD. Future studies are needed to assess the possibilities of a minor effect of IVB on late lung alveolarization.
This study was limited by being a single-center study with a small sample size and a retrospective study design. Also, only infants who survived to school age were included in our study, which meant that the anti-VEGF effect to those most vulnerable patients with mortality was not accounted in our study. Additionally, patients receiving IVB anti-VEGF treatment alone and in conjunction with laser therapy were combined into the same group due to the small sample size of the subgroup. Ultimately, the R2% value of Table 3 was medium, which meant it was not a perfect model to predict pulmonary function but still provided us with information on the relationship between the variables and pulmonary function. However, our study had the strength of detailed documentation of baseline data and various risk factors. In addition, instead of only one pulmonary function test, two tests including spirometry and impulse oscillometry, were conducted in these children when they reached school age.
In conclusion, preterm infants with a history of IVB treatment showed similar pulmonary outcomes at school age compared to preterm infants without IVB treatment. This is the first study to examine the impact of pulmonary function following anti-VEGF treatment for ROP in preterm children when they reach school age. Our findings do not imply that it is “absolutely safe” to use anti-VEGF in these patients. Judicious use of anti-VEGF for ROP is still recommended until its systemic impact is fully understood. Future prospective randomized studies are needed to confirm the impact of VEGF suppression on the pulmonary function of these vulnerable patients.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Sommer, A. et al. Challenges of ophthalmic care in the developing world. JAMA Ophthalmol. 132, 640–644. https://doi.org/10.1001/jamaophthalmol.2014.84 (2014).
Blencowe, H., Lawn, J. E., Vazquez, T., Fielder, A. & Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res. 74(Suppl 1), 35–49. https://doi.org/10.1038/pr.2013.205 (2013).
Rothschild, M. I. et al. The economic model of retinopathy of prematurity (EcROP) screening and treatment: Mexico and the United States. Am. J. Ophthalmol. 168, 110–121. https://doi.org/10.1016/j.ajo.2016.04.014 (2016).
Mintz-Hittner, H. A., Kennedy, K. A. & Chuang, A. Z. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 364, 603–615. https://doi.org/10.1056/NEJMoa1007374 (2011).
Geloneck, M. M. et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: A randomized clinical trial. JAMA Ophthalmol. 132, 1327–1333. https://doi.org/10.1001/jamaophthalmol.2014.2772 (2014).
Sankar, M. J., Sankar, J. & Chandra, P. Anti-vascular endothelial growth factor VEGF drugs for treatment of retinopathy of prematurity. Cochrane Database Syst. Rev. 2018, 009734. https://doi.org/10.1002/14651858.CD009734.pub3 (2018).
Mintz-Hittner, H. A. & Geloneck, M. M. Review of effects of anti-VEGF treatment on refractive error. Eye Brain 8, 135–140. https://doi.org/10.2147/eb.S99306 (2016).
Moran, S. et al. Bevacizumab versus diode laser in stage 3 posterior retinopathy of prematurity. Acta Ophthalmol. 92, e496-497. https://doi.org/10.1111/aos.12339 (2014).
Lepore, D. et al. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: Fluorescein angiographic findings. Ophthalmology 125, 218–226. https://doi.org/10.1016/j.ophtha.2017.08.005 (2018).
Yoon, J. M. et al. outcomes after laser versus combined laser and bevacizumab treatment for type 1 retinopathy of prematurity in zone I. Retina 37, 88–96. https://doi.org/10.1097/iae.0000000000001125 (2017).
Falavarjani, K. G. & Nguyen, Q. D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye Lond. 27, 787–794. https://doi.org/10.1038/eye.2013.107 (2013).
Monacci, W. T., Merrill, M. J. & Oldfield, E. H. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am. J. Physiol. 264, C995-1002. https://doi.org/10.1152/ajpcell.1993.264.4.C995 (1993).
Khalili, S., Shifrin, Y., Pan, J., Belik, J. & Mireskandari, K. The effect of a single anti-vascular endothelial growth factor injection on neonatal growth and organ development: In vivo study. Exp. Eye Res. 169, 54–59. https://doi.org/10.1016/j.exer.2018.01.020 (2018).
Ahmed, K., Ali, A. S., Delwadia, N. & Greven, M. A. Neurodevelopmental outcomes following intravitreal bevacizumab with laser versus laser photocoagulation alone for retinopathy of prematurity. Ophthalmic Surg. Lasers Imaging Retin. 51, 220–224. https://doi.org/10.3928/23258160-20200326-03 (2020).
Araz-Ersan, B. et al. Preliminary anatomical and neurodevelopmental outcomes of intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. Curr. Eye Res. 40, 585–591. https://doi.org/10.3109/02713683.2014.941070 (2015).
Fan, Y. Y. et al. Neurodevelopmental outcomes after intravitreal bevacizumab therapy for retinopathy of prematurity: A prospective case-control study. Ophthalmology 126, 1567–1577. https://doi.org/10.1016/j.ophtha.2019.03.048 (2019).
Kennedy, K. A. & Mintz-Hittner, H. A. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J. aapos. 22, 61-65.e61. https://doi.org/10.1016/j.jaapos.2017.10.006 (2018).
Raghuram, K. et al. Neurodevelopmental outcomes in infants treated with intravitreal bevacizumab versus laser. J. Perinatol. 39, 1300–1308. https://doi.org/10.1038/s41372-019-0420-z (2019).
Morin, J. et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 137, e20153218. https://doi.org/10.1542/peds.2015-3218 (2016).
Natarajan, G. et al. neurodevelopmental outcomes of preterm infants with retinopathy of prematurity by treatment. Pediatrics 144, e20183537. https://doi.org/10.1542/peds.2018-3537 (2019).
Wu, W. C. et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol. 133, 391–397. https://doi.org/10.1001/jamaophthalmol.2014.5373 (2015).
Wu, W. C. et al. Serum vascular endothelial growth factor after bevacizumab or ranibizumab treatment for retinopathy of prematurity. Retina 37, 694–701. https://doi.org/10.1097/iae.0000000000001209 (2017).
Huang, C. Y. et al. Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefes. Arch. Clin. Exp. Ophthalmol. 256, 479–487. https://doi.org/10.1007/s00417-017-3878-4 (2018).
Lu, H. Y. et al. Association between maternal age at delivery and allergic rhinitis in schoolchildren: A population-based study. World Allergy Organ. J. 13, 100127. https://doi.org/10.1016/j.waojou.2020.100127 (2020).
Yao, T. C. et al. Association of prenatal exposure to fine particulate matter pollution with childhood eczema. Allergy 76, 2241–2245. https://doi.org/10.1111/all.14738 (2021).
Chang-Chien, J. et al. Metabolomic differences of exhaled breath condensate among children with and without asthma. Pediatr. Allergy Immunol. 32, 264–272. https://doi.org/10.1111/pai.13368 (2021).
Ho, C. H. et al. Early-life weight gain is associated with non-atopic asthma in childhood. World Allergy Organ. J. 15, 100672. https://doi.org/10.1016/j.waojou.2022.100672 (2022).
Lee, H. J. et al. Cord blood IgE predicts allergic sensitization, elevation of exhaled nitric oxide and asthma in schoolchildren. Pediatr. Allergy Immunol. 33, e13838. https://doi.org/10.1111/pai.13838 (2022).
Tasman, W. S. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 121, 1684–1694. https://doi.org/10.1001/archopht.121.12.1684 (2003).
Good, W. V. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. Discuss. 102, 233–248 (2004).
Wu, W. C. et al. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in taiwan. Am. J. Ophthalmol. 155, 150-158.e151. https://doi.org/10.1016/j.ajo.2012.06.010 (2013).
Wu, W. C. et al. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in taiwan. Ophthalmology 118, 176–183. https://doi.org/10.1016/j.ophtha.2010.04.018 (2011).
Yonekawa, Y. et al. Progressive retinal detachment in infants with retinopathy of prematurity treated with intravitreal bevacizumab or ranibizumab. Retina 38, 1079–1083. https://doi.org/10.1097/iae.0000000000001685 (2018).
Chang, S. M. et al. Reference equations for spirometry in healthy Asian children aged 5 to 18 years in Taiwan. World Allergy Organ. J. 12, 100074. https://doi.org/10.1016/j.waojou.2019.100074 (2019).
Lai, S. H. et al. Reference value of impulse oscillometry in Taiwanese preschool children. Pediatr. Neonatol. 56, 165–170. https://doi.org/10.1016/j.pedneo.2014.09.002 (2015).
Beydon, N. et al. An official American thoracic society/european respiratory society statement: Pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 175, 1304–1345. https://doi.org/10.1164/rccm.200605-642ST (2007).
Yao, T. C. et al. Obesity disproportionately impacts lung volumes, airflow and exhaled nitric oxide in children. PLoS ONE 12, e0174691. https://doi.org/10.1371/journal.pone.0174691 (2017).
Bickel, S., Popler, J., Lesnick, B. & Eid, N. Impulse oscillometry: Interpretation and practical applications. Chest 146, 841–847. https://doi.org/10.1378/chest.13-1875 (2014).
Voelkel, N. F., Vandivier, R. W. & Tuder, R. M. Vascular endothelial growth factor in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L209-221. https://doi.org/10.1152/ajplung.00185.2005 (2006).
Tuder, R. M. & Yun, J. H. Vascular endothelial growth factor of the lung: Friend or foe. Curr. Opin. Pharmacol. 8, 255–260. https://doi.org/10.1016/j.coph.2008.03.003 (2008).
Woik, N. & Kroll, J. Regulation of lung development and regeneration by the vascular system. Cell Mol. Life Sci. 72, 2709–2718. https://doi.org/10.1007/s00018-015-1907-1 (2015).
Luttun, A. & Carmeliet, P. Angiogenesis and lymphangiogenesis: highlights of the past year. Curr. Opin. Hematol. 11, 262–271. https://doi.org/10.1097/01.moh.0000126936.58889.95 (2004).
Le Cras, T. D., Markham, N. E., Tuder, R. M., Voelkel, N. F. & Abman, S. H. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L555-562. https://doi.org/10.1152/ajplung.00408.2001 (2002).
Jakkula, M. et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L600-607. https://doi.org/10.1152/ajplung.2000.279.3.L600 (2000).
Akeson, A. L. et al. Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev. Biol. 264, 443–455. https://doi.org/10.1016/j.ydbio.2003.09.004 (2003).
Ovali, F., Yetik, H., Tuten, A., Topcuoglu, S. & Gunay, M. Effects of intravitreal anti-VEGF therapy on the clinical course of bronchopulmonary dysplasia. Iran. J. Pediatr. 26, e4637. https://doi.org/10.5812/ijp.4637 (2016).
Hong, Y. R. et al. plasma concentrations of vascular endothelial growth factor in retinopathy of prematurity after intravitreal bevacizumab injection. Retina 35, 1772–1777. https://doi.org/10.1097/iae.0000000000000535 (2015).
Schittny, J. C. Development of the lung. Cell Tissue Res. 367, 427–444. https://doi.org/10.1007/s00441-016-2545-0 (2017).
Burri, P. H. Fetal and postnatal development of the lung. Annu. Rev. Physiol. 46, 617–628. https://doi.org/10.1146/annurev.ph.46.030184.003153 (1984).
Vollsæter, M., Røksund, O. D., Eide, G. E., Markestad, T. & Halvorsen, T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 68, 767–776. https://doi.org/10.1136/thoraxjnl-2012-202980 (2013).
Narang, I., Rosenthal, M., Cremonesini, D., Silverman, M. & Bush, A. Longitudinal evaluation of airway function 21 years after preterm birth. Am. J. Respir. Crit. Care Med. 178, 74–80. https://doi.org/10.1164/rccm.200705-701OC (2008).
Koumbourlis, A. C. et al. Longitudinal follow-up of lung function from childhood to adolescence in prematurely born patients with neonatal chronic lung disease. Pediatr. Pulmonol. 21, 28–34. https://doi.org/10.1002/(sici)1099-0496(199601)21:1%3c28::Aid-ppul5%3e3.0.Co;2-m (1996).
Tschanz, S. A., Makanya, A. N., Haenni, B. & Burri, P. H. Effects of neonatal high-dose short-term glucocorticoid treatment on the lung: A morphologic and morphometric study in the rat. Pediatr. Res. 53, 72–80. https://doi.org/10.1203/00006450-200301000-00014 (2003).
Avdalovic, M. V. et al. Ozone exposure during the early postnatal period alters the timing and pattern of alveolar growth and development in nonhuman primates. Anat. Rec. Hoboken 295, 1707–1716. https://doi.org/10.1002/ar.22545 (2012).
Kalenga, M., Tschanz, S. A. & Burri, P. H. Protein deficiency and the growing rat lung. I. Nutritional findings and related lung volumes. Pediatr. Res. 37, 783–788. https://doi.org/10.1203/00006450-199506000-00018 (1995).
Mullassery, D. & Smith, N. P. Lung development. Semin. Pediatr. Surg. 24, 152–155. https://doi.org/10.1053/j.sempedsurg.2015.01.011 (2015).
Ciprandi, G. & Cirillo, I. The pragmatic role of FEF(25–75) in asymptomatic subjects, allergic rhinitis, asthma and in military setting. Expert. Rev. Respir. Med. 13, 1147–1151. https://doi.org/10.1080/17476348.2019.1674649 (2019).
Acknowledgements
This research was supported in part by the Chang Gung Memorial Hospital, Taoyuan, Taiwan (grant no.: CMRPG3K0421, CMRPG3I0071~3 and CMRPG3J0121~3); and the Ministry of Science and Technology, Taiwan (grant no.: MOST 109-2314-B-182A-019-MY3 and MOST 109-2314-B-182-042-MY3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would like to thank Ms. Hsiao-Jung Tseng for statistical assistance; Ms. Tseng is affiliated with the Clinical Trial Center, Chang Gung Memorial Hospital (funded by the Ministry of Health and Welfare of Taiwan; grant MOHW109-TDU-B-212-114005).
Author information
Authors and Affiliations
Contributions
C.Y.H., W.C.W. and T.C.Y. made the study design. C.Y.H., W.C.W. and T.C.Y. collected the data. C.Y.H. and H.J.T. performed statistical analysis. C.Y.H., W.C.W., T.C.Y. and S.H.L. drafted the original manuscript text. W.C.W, T.C.Y. and S.H.L. reviewed and edited the manuscript. W.C.W. and T.C.Y. supervised the study. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, CY., Lai, SH., Tseng, HJ. et al. Pulmonary function in school-age children following intravitreal injection of bevacizumab for retinopathy of prematurity. Sci Rep 12, 18788 (2022). https://doi.org/10.1038/s41598-022-22338-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22338-2
This article is cited by
-
Spätfolgen der Frühgeborenenretinopathie im Kindesalter
Die Ophthalmologie (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.