Abstract
Patients with systemic sclerosis (SSc) have some potential factors associated with an increased risk of sarcopenia. There has been currently no study to examine such associations in SSc patients. We aimed to determine the prevalence of sarcopenia among SSc patients and examine its association with clinical manifestations and laboratory tests. A cross-sectional study was conducted in 180 adult SSc patients at the Scleroderma Clinic, Khon Kaen University, Thailand, between July 2019 and April 2020. Clinical data, laboratory tests for inflammatory markers, serology, hormone, body composition by dual-energy X-ray absorptiometry, handgrip strength, functional lower extremity strength, and usual gait speed were collected and measured. Sarcopenia was defined according to the criteria of the Asian Working Group for Sarcopenia. One hundred and eighty patients were recruited. Ninety-four cases (52.2%) were the diffuse cutaneous SSc subset. The respective mean age and duration of disease was 58.8 ± 9.4 and 6.2 ± 5.3 years. Sarcopenia was revealed in 41 SSc patients for a prevalence of 22.8% (95% CI 12.1–34.8), while the prevalence was higher in patients with the diffuse cutaneous SSc (dcSSc) compared to the limited cutaneous SSc. BMI at the onset of SSc and C-reactive protein > 5 mg/dL were significantly associated with sarcopenia with a respective OR of 0.60 (95% CI 0.48–0.75) and 3.18 (1.06–9.54). Sarcopenia is common in patients with SSc, but the prevalence is more pronounced in the dcSSc. Inflammatory markers, particularly the CRP level, are strongly associated. BMI at the onset had a negative association with sarcopenia among SSc patients.
Similar content being viewed by others
Introduction
Systemic sclerosis (SSc) is a rare systemic connective tissue disease characterized by skin thickness. Fibrosis is a classical pathological finding that reveals in both skin and internal organ1. The diffuse cutaneous SSc (dcSSc) subset is significantly associated with bad prognosis2,3. The patient with SSc is likely to be at risk for developing sarcopenia. Previous studies demonstrated that several factors for sarcopenia—including malnutrition related to gastrointestinal involvement, chronic inflammation, drugs such as steroid therapy, physical inactivity, and terminal organ failure—also present in patients with SSc4,5.
Sarcopenia is an age-related disease described as a progressive loss of muscle mass and function. A recent study—using definitions from The European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group of Sarcopenia (AWGS)—reported the prevalence of sarcopenia in healthy adults aged ≥ 60 years was 10% and that rates are comparable between men and women6. The prevalence of sarcopenia varied based on ethnicity, measurement methods, and study populations. For instance, Shafiee et al.6 reported that the prevalence of sarcopenia was higher among non-Asian patients than Asian individuals in both sexes and higher when using the Bio-electrical impedance analysis (BIA) to measure muscle mass compared with dual-energy X-ray absorptiometry (DXA) (a respective 19% vs. 10% in men, and 20% vs. 11% in women)6. Another study demonstrated that the prevalence of sarcopenia was highest in nursing-home individuals (51% and 31% in men and women, respectively) compared with community-dwelling individuals (11% and 9% in men and women, respectively), and hospitalized individuals (23% and 24% in men and women, respectively)7,8.
Siegert et al.4 reported that, when using the EWGSOP criteria, the prevalence of sarcopenia in German patients with SSc was 22.5%, and this rate increased with age. The authors found that the potential predictors of sarcopenia—such as disease duration, numbers of medications, numbers of comorbidities—were not different between SSc patients with sarcopenia and non-sarcopenia4. In 62 Italians with SSc, Corallo et al.9 found a prevalence of sarcopenia of 42% by Relative Skeletal Mass Index and 55% by handgrip strength. The clinical predictors of sarcopenia were elderly individuals, long disease duration, malnutrition status, extensive skin thickness, and esophageal involvement9. Other studies7,10, however, suggested that other factors—longer disease duration, abnormal body compositions (low body mass index—BMI) and lean mass (LM), and relative skeletal muscle mass index—SMI), lung and skin involvements—were associated with sarcopenia in patients with SSc.
To date, there is a paucity of evidence on the association between sarcopenia and relevant parameters in patients with SSc, including clinical features of SSc, serology, inflammatory markers, and concomitant medications. The current study aimed to determine the prevalence of sarcopenia and examine the associations between its clinical factors and sarcopenia in adult Thai patients with SSc.
Methods
Setting and subjects
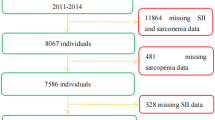
The study was designed as a cross-sectional investigation among adult patients with SSc who, between July 2019 and April 2020, attended the Scleroderma Clinic, Khon Kaen University, Khon Kaen, Thailand. Excluded from the study were patients with (1) overlap syndrome; (2) neurological diseases (including neurodegenerative, motor neuron diseases, cerebrovascular disease, multiple sclerosis, muscular dystrophy, spinal cord disease, anterior horn cell disease, or peripheral neuropathy); (3) immobilization syndrome or unable to walk due to severe joint contracture or painful ulcer affecting the measurement of muscle strength; (4) active myositis (muscle weakness with rising of muscle enzyme, inflammatory myositis from muscle biopsy, and abnormal electromyography); (5) severe infection or sepsis; (6) any stage of cancer; or, (7) pregnant or lactating.
Measurements
The participants were invited to meet a research nurse who administered the questionnaire and informed consent form. Demographic data were recorded, including age, sex, underlying diseases, smoking and alcohol intake patterns, and current medications. Clinical assessments were performed in all eligible patients, including symptoms on the visit such as Raynaud’s phenomenon, digital ulcers, skin tightness, synovitis, hand deformity, tendon friction rub, stomach and esophageal involvement.
Blood samples were collected, and measurements included serology (anti-centromere antibody (ACA), anti-topoisomerase I antibody (ATA)), complete blood count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), vitamin D [25,(OH)D] level, testosterone (in men), estradiol (in women), cortisol, and thyroid function test (free T4, free T3, and thyroid-stimulating hormone—TSH). Upon enrollment, the following were measured body composition using dual-energy X-ray absorptiometry (DXA), handgrip strength, functional lower extremity strength, and usual gait speed.
Body composition
Body composition (muscle mass and fat mass) for each patient was measured by DXA (Lunar Prodigy bone densitometer, GE Healthcare, Madison, WI, USA; CV = 1% for whole body values). According to the DXA results, we calculated the appendicular skeletal muscle mass (ASM) for each patient as the sum of the upper and lower limb muscle mass without bone and fat tissue. Appendicular skeletal mass index (ASMI) was calculated as the ASM divided by height squared (ASM/height2, kg/m2)11,12. According to the Asian Working Group for Sarcopenia (AWGS), the cut-off point for ASMI for low muscle mass is < 7.0 kg/m2 for men and < 5.4 kg/m2 for women11,13. The fat free mass index (FFMI) was calculated to identify muscle loss using the fat free mass divided by height squared. The cut-off value for low FFMI is < 15.5 kg/m2 for men and < 12.6 kg/m2 for women14.
Muscle strength assessment
Handgrip strength test
Handgrip strength (in kg) was measured using the Takei T.K.K.5001 handgrip dynamometer (Takei Scientific Instruments Co., Ltd, Tokyo, Japan). Participants stood in a neutral position, arms by their side with full elbow extension, then the participants were asked to press the dynamometer for 5 s for each measurement. All measurements were carried out alternatively between the left and right hands three times with no rest. Maximum strength values for both hands were recorded. According to the AWGS 2019, the cut-off point for low handgrip strength is < 28 kg for men and < 18 kg for women15.
Physical performance assessment
In the study, a 6-m gait speed was used for the assessment of physical performance. Each participant walked normally with and without instruments, depending on the safety and comfort of the participant16. The test was repeated thrice, and each recorded time calculated and used for interpretation. The cut-off point for low gait speed was 1.0 m/s for both men and women15.
Both muscle strength and physical performance assessments were conducted by experienced physical therapists, nurses, and research assistants. Each test was performed only by the participant when he/she felt comfortable and had normal vital signs. A participant was removed from the study if any adverse events resulted from the tests (i.e., severe muscle injury, dyspnea uncorrected after rest or requiring oxygen therapy), or they could not complete all the measurements.
Operational definitions
SSc was diagnosed according to the 2013 ACR/EULAR Classification for Scleroderma17 and classified as per LeRoy et al.18 as dcSSc or limited cutaneous SSc (lcSSc). Sarcopenia was defined according to the criteria of the Asian Working Group for Sarcopenia (AWGS) 201915. Participants who met the criteria for both low handgrip strength or low gait speed and low muscle mass were considered to have “sarcopenia”, and participants with low handgrip strength, low gait speed, and low muscle mass as having “severe sarcopenia”.
Pulmonary involvement was fulfilled when interstitial fibrosis is detected by high resolution computed tomography (HRCT). In this study, pulmonary arterial hypertension (PAH) was diagnosed by: (a) the mean pulmonary arterial pressure (mPAP) is > 20 mmHg at rest; and, (b) a pulmonary artery wedge pressure of ≤ 15 mmHg and a pulmonary vascular resistance of ≥ 3 Wood units, as confirmed by right heart catheterization19. According to the WHO definition, low body mass index (BMI) was < 18.5 kg/m2 in the present study20. Anemia was defined as < 12 g/dL in non-pregnant women and < 13 g/dL in men21. High CRP and ESR were considered when the levels were > 5 mg/dL and > 25 mm/h, respectively22,23. Serum 25(OH)D level < 30 ng/mL was defined as vitamin D insufficiency24,25. The definition of a low cortisol level was fulfilled when it was < 5 µg/dL. The definition of high thyroid-stimulating hormone (TSH) was fulfilled when it was > 4.2 mIU/L. The definition of hypothyroidism was fulfilled when TSH was > 4.2 mIU/L, and Free T4 was < 0.93 ng/dL.
Sample size calculation
The sample size was initially determined based on the prevalence of sarcopenia and the study’s primary objective. Based on an estimated 22%, the prevalence of sarcopenia in SSc4 (with a precision of 10% and a confidence level of 95%) required at least 66 cases be included. The secondary objective of the study sought to determine the factors associated with sarcopenia in SSc. It referred to a ratio of 1 suspected associated factor using an equation. According to the literature review, ten sarcopenic patients would be needed to study at least four factors associated with sarcopenia10,26. Thus, at least 180 SSc patients were required for the current study.
Statistical analysis
Continuous variables were presented as means and standard deviations. The prevalence of sarcopenia was expressed as a percentage and 95% confidence interval (95% CI). We compared SSc patients with sarcopenia to those without sarcopenia. A Chi-square test or Fischer’s exact test was performed to evaluate the association between dichotomous variables. A Student’s t-test or Wilcoxon-Mann–Whitney test was used to examine differences for continuous data between two groups as appropriate. The association between sarcopenia (dependent variable) and the independent variables (SSc characteristics and laboratory tests) was expressed as the odds ratio (OR) and its corresponding 95% CI. A multivariate logistic regression model was run, and the following covariables were included based on their clinical relevance. Any additional significant variables (p-value < 0.1) from the univariate analysis were also included in the model. Statistical significance was defined as a P-value < 0.05. Statistical analyses were performed using STATA version 16.0 (StataCorp., College Station, TX, USA).
Ethics approval and consent to participate
The Human Research Ethics Committee of Khon Kaen University reviewed and approved the study as per the Helsinki Declaration and the Good Clinical Practice Guidelines (HE621103). All eligible patients signed informed consent before enrollment.
Results
One hundred and eighty patients were recruited in the study, for which the female to male ratio was 1.9:1. The majority of patients (94 cases; 52.2%) had the diffuse cutaneous SSc subset. The respective mean age and mean duration of disease was 58.8 ± 9.4 and 6.2 ± 5.3 years. Sarcopenia—as defined by the AWGS definition—was revealed in 41 SSc patients for a prevalence of 22.8% (95% CI 12.1–34.8), and 73.2% (30/41) were classified as having severe sarcopenia. Around half of the patients who had sarcopenia were ≥ 60 years of age. The demographic data are presented in Table 1.
Significantly more SSc patients with the dcSSc subset that were male and positive for the ATA test had sarcopenia. The clinical manifestations at onset that were more prevalent among patients with sarcopenia than without included digital ulcer, hand deformity, low BMI (< 18.5 kg/m2), high mRSS, low serum albumin, and anemia. The only clinical manifestation at enrollment prevalent among sarcopenic SSc patients was high TSH. The serum albumin, lean mass, fat free mass, and fat free mass indices were significantly lower in patients with sarcopenia than non-sarcopenic patients (Table 2).
After doing the univariate analysis, the covariates significantly associated with sarcopenia in SSc patients were included in the multivariate model (Table 3). After adjusting for the significant variables from the univariate logistic regression analysis and relevant clinical confounders, high CRP at enrollment (OR 3.18, 95% CI 1.06–9.54) and low BMI at onset (OR 0.60, 95% CI 0.48–0.75) remained significantly associated with sarcopenia in SSc patients.
Discussion
According to the AWGS definition, we found that the prevalence of sarcopenia was 22.8% (95% CI 1.21–3.48) among Thai SSc patients. There was no significant difference with respect to age and disease duration between patients with and without sarcopenia. The prevalence of sarcopenia in the study was comparable to a previous study conducted in Germany (22.5%)4. Our study likewise did not find any differences in age and disease duration between patients with and without sarcopenia. The previous study, however, included 17 cases of mixed connective tissue disease or undifferentiated connective tissue disease, and the majority had lcSSc (54.3%) while the majority in our study had dcSSc; thus, the study populations were different. The SSc subset does not influence the comparison of the prevalence of sarcopenia between the Thai and German SSc patients. The comparable prevalence of sarcopenia is supported by our logistic regression analysis that indicated that neither the dcSSc nor lcSSc subset was associated with sarcopenia.
In general, then, sarcopenia is an age-related disease. The prevalence of sarcopenia in healthy adults age ≥ 60 years—using the definitions proposed by the EWGSOP and AWGS—are around 10% (95% CI 8–12%) in men and 10% (95% CI 8–13%) in women4. According to our findings, the prevalence of sarcopenia in SSc appeared to be higher than in the healthy population for people over the age of 60. Furthermore, nearly half of our SSc patients in the younger age group had sarcopenia. There was no age-matched control group in the study. As a result, it is impossible to conclude whether the prevalence of sarcopenia was higher in SSc than in control. However, we suggest that attending physicians should be aware of the risk of sarcopenia in SSc patients, not just the elderly, but also the middle-aged.
Unexpectedly, we found that the severity of disease in SSc patients was not associated with sarcopenia despite their being a mortality risk (viz., extensive skin tightness evaluated by mRSS, internal organ fibrosis (pulmonary fibrosis and gastrointestinal involvement), and vasculopathy (renal crisis and pulmonary arterial hypertension)26. The factor that might be a complication or related to SSc disease itself—i.e., low BMI at onset—was a risk for sarcopenia development in SSc. Notwithstanding, the relationship between sarcopenia and SSc is complex and remains uncertain. The pathophysiological process of sarcopenia in SSc is thought to be skeletal muscle involvement, especially muscle weakness, which was revealed in up to 90% of SSc patients27,28. Other possible mechanisms include microangiopathy, inflammatory infiltration, and muscular interstitial fibrosis28. Malnutrition status from gastrointestinal involvement, chronic inflammation, physical inactivity, medical treatment, and organ failure might also contribute to the development of sarcopenia in SSc5. Our study revealed that low BMI at onset and high CRP at enrollment were associated with sarcopenia. This finding supports the role of malnutrition and inflammation in the development of sarcopenia in SSc patients. According to our study design (a cross-sectional study), we can only speculate upon the progression and/or outcome of sarcopenia in SSc patients in cases of improved nutritional status. A longitudinal study on the outcome of sarcopenia among SSc patients would provide important information.
The literature includes some reports of atherosclerotic-related diseases in sarcopenia, particularly among Japanese and Korean patients29,30. Hypertension, diabetes mellitus, cardiovascular disease, and chronic kidney disease were reported to be associated with sarcopenia29,30,31. Another study revealed that sarcopenia was associated with multiple comorbidities, cigarette smoking, and low serum levels of testosterone in men11. According to the present study, we did not find any significant associations between sarcopenia and comorbidity, cigarette smoking, testosterone level, or medical treatment in SSc patients (particularly steroids and/or immunosuppressants).
The present findings should be interpreted in light of some potential limitations. First, there was no definition for sarcopenia in SSc patients, thus we adapted the definition from the AWGS. The resulting prevalence might not be repeated using other definitions and/or methodologies. Second, there lacked a control group. Therefore, it is impossible to conclude whether the prevalence of sarcopenia was higher in SSc than in control. Third, we did not include patients with active myositis which would be an overlap syndrome (SSc with polymyositis); hence, we cannot examine the correlation of recent or current myositis and sarcopenia. Forth, we did not confirm adrenal insufficiency by adrenocorticotropic hormone (ACTH) stimulation test, so we cannot state the degree of adrenal insufficiency in our patients or whether there is any difference in adrenal insufficiency between patients with and without sarcopenia. Fifth, due to facility limitations, we do not have data on malabsorption for all SSc patients. Finally, we did not examine the correlation between disease activity and sarcopenia, and further study is needed.
The strengths of the study follow. The study enrolled the correct number of patients according to the sample size calculation for answering both the study’s primary and secondary objectives, thus validating the co-prevalence of having SSc and sarcopenia. We also included parameters of interest into the analysis for possible factors associated with sarcopenia in SSc, namely: SSc subset, clinical characteristics of SSc, serology, inflammatory markers, sex hormone, thyroid hormone, adrenal hormone, coexisting diseases and concomitant medications. Our findings have value for evaluating sarcopenia development in SSc patients and could provide insights into giving patients better care and planning future studies.
Conclusion
Sarcopenia is common in SSc patients and has impact on muscle strength, as well as muscle and physical function. Abnormal body composition is also frequently found in patients with sarcopenia. SSc patients with a low BMI and a high CRP are at risk of sarcopenia and should be monitored closely. Early detection of sarcopenia could lead to preventive measures with the aim of avoiding disability, impaired functional status, and hospitalization.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Apipattarakul, R. et al. Clinical characteristics and mortality rate of Thai elderly-onset systemic sclerosis. Clin. Exp. Rheumatol. 36(Suppl 113), 76–81 (2018).
Tyndall, A. J. et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann. Rheum. Dis. 69, 1809–1815 (2010).
Foocharoen, C., Mahakkanukrauh, A., Suwannaroj, S. & Nanagara, R. Spontaneous skin regression and predictors of skin regression in Thai scleroderma patients. Clin. Rheumatol. 30, 1235–1240 (2011).
Siegert, E. et al. Prevalence of sarcopenia in systemic sclerosis: Assessing body composition and functional disability in patients with systemic sclerosis. Nutr. Burbank Los Angel. Cty. Calif. 55–56, 51–55 (2018).
Wang, C. & Bai, L. Sarcopenia in the elderly: Basic and clinical issues. Geriatr. Gerontol. Int. 12, 388–396 (2012).
Shafiee, G. et al. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J. Diabetes Metab. Disord. 16, 21 (2017).
Marighela, T. F., de S. Genaro, P., Pinheiro, M. M., Szejnfeld, V. L. & Kayser, C. Risk factors for body composition abnormalities in systemic sclerosis. Clin. Rheumatol. 32, 1037–1044 (2013).
Papadopoulou, S. K., Tsintavis, P., Potsaki, P. & Papandreou, D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J. Nutr. Health Aging 24, 83–90 (2020).
Corallo, C. et al. Sarcopenia in systemic sclerosis: The impact of nutritional, clinical, and laboratory features. Rheumatol. Int. 39, 1767–1775 (2019).
Caimmi, C. et al. Malnutrition and sarcopenia in a large cohort of patients with systemic sclerosis. Clin. Rheumatol. 37, 987–997 (2018).
Chen, L.-K. et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 15, 95–101 (2014).
Barbat-Artigas, S., Plouffe, S., Pion, C. H. & Aubertin-Leheudre, M. Toward a sex-specific relationship between muscle strength and appendicular lean body mass index?. J. Cachexia Sarcopenia Muscle 4, 137–144 (2013).
Limpawattana, P., Kotruchin, P. & Pongchaiyakul, C. Sarcopenia in Asia. Osteoporos. Sarcopenia 1, 92–97 (2015).
Campbell, T. M. & Vallis, L. A. Predicting fat-free mass index and sarcopenia in assisted-living older adults. Age Dordr. Neth. 36, 9674 (2014).
Chen, L.-K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300-307.e2 (2020).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31 (2019).
van den Hoogen, F. et al. Classification criteria for systemic sclerosis: An ACR-EULAR collaborative initiative. Arthritis Rheum. 65, 2737–2747 (2013).
LeRoy, E. C. et al. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 15, 202–205 (1988).
Simonneau, G. et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 53, 1801913 (2019).
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363, 157–163 (2004).
Cappellini, M. D. & Motta, I. Anemia in clinical practice-definition and classification: Does hemoglobin change with aging?. Semin. Hematol. 52, 261–269 (2015).
Lapić, I., Padoan, A., Bozzato, D. & Plebani, M. Erythrocyte sedimentation rate and C-reactive protein in acute inflammation. Am. J. Clin. Pathol. 153, 14–29 (2020).
Bitik, B. et al. Differential diagnosis of elevated erythrocyte sedimentation rate and C-reactive protein levels: A rheumatology perspective. Eur. J. Rheumatol. 2, 131–134 (2015).
Gallagher, J. C. & Sai, A. J. Vitamin D insufficiency, deficiency, and bone health. J. Clin. Endocrinol. Metab. 95, 2630–2633 (2010).
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 (2007).
Marighela, T. F., de S. Genaro, P., Pinheiro, M. M., Szejnfeld, V. L. & Kayser, C. Risk factors for body composition abnormalities in systemic sclerosis. Clin. Rheumatol. 32, 1037–1044 (2013).
Paik, J. J., Mammen, A. L., Wigley, F. M. & Gelber, A. C. Myopathy in scleroderma, its identification, prevalence, and treatment: Lessons learned from cohort studies. Curr. Opin. Rheumatol. 26, 124–130 (2014).
Ranque, B., Authier, F.-J., Berezne, A., Guillevin, L. & Mouthon, L. Systemic sclerosis-associated myopathy. Ann. N. Y. Acad. Sci. 1108, 268–282 (2007).
Sanada, K. et al. A cross-sectional study of sarcopenia in Japanese men and women: Reference values and association with cardiovascular risk factors. Eur. J. Appl. Physiol. 110, 57–65 (2010).
Chin, S. O. et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS ONE 8, e60119 (2013).
Beaudart, C., Rizzoli, R., Bruyère, O., Reginster, J.-Y. & Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health Arch. Belg Sante Publique 72, 45 (2014).
Acknowledgements
The authors thank (a) the Research and Graduate Studies, Khon Kaen University, the Scleroderma Research Group, Khon Kaen University, and the Thai Rheumatism Association for the support, and (b) Mr. Bryan Roderick Hamman for assistance with the English-language presentation under the aegis of the Khon Kaen University Publication Clinic, Thailand.
Funding
The study received funding support from and Division of Rheumatology, Faculty of Medicine, Khon Kaen University, Thailand.
Author information
Authors and Affiliations
Contributions
A.S. did the data collection and drafted the manuscript. C.F. designed the study and drafted and edited the manuscript. D.T. and K.S. did the data collection. C.P. did data analysis, edited, and proof the final manuscript. C.F., A.M., S.S., P.S., and C.P. proofread the manuscript. The authors consent to publication and grant the Publisher exclusive license of the full copyright.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sangaroon, A., Foocharoen, C., Theerakulpisut, D. et al. Prevalence and clinical association of sarcopenia among Thai patients with systemic sclerosis. Sci Rep 12, 18198 (2022). https://doi.org/10.1038/s41598-022-21914-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21914-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.