Abstract
Osteopenia is known to be associated with clinical frailty which is linked to inferior outcomes in various clinical scenarios. However, the exact prognostic value of osteopenia in patients undergoing curative intent-surgery for hepatocellular carcinoma (HCC) is not completely understood. This retrospective study was conducted in a cohort of 151 patients who underwent partial hepatectomy for HCC in curative intent at a German university medical center (05/2008–12/2019). Preoperative computed tomography-based segmentation was used to assess osteopenia, and the prognostic impact of pathological changes in bone mineral density (BMD) on perioperative morbidity, mortality, and long-term oncological outcome was analyzed. Five-year overall survival of osteopenic patients was significantly worse compared to those with normal BMD (29% vs. 65%, p = 0.014). In line with this, the probability of disease-free survival at 5 years was significantly worse for patients with osteopenia (21% vs. 64%, p = 0.005). In our multivariable model, osteopenia was confirmed as an independent risk-factor for inferior overall survival (Hazard-ratio 7.743, p = 0.002). Concerning perioperative complications, osteopenic patients performed slightly worse, even though no statistical difference was detected (Clavien-Dindo ≥ 3b; 21% vs. 9%, p = 0.139). The present study confirms osteopenia as an independent risk-factor for inferior survival in patients undergoing partial hepatectomy for HCC in a European cohort. Further studies are warranted to validate these findings.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) has become one of the leading causes of cancer-related death around the globe1. With respect to the central role of the liver in metabolism, most HCC patients are at high-risk of developing pathological alterations of body composition (BC), due to the underlying chronic liver disease2.
Over the past decade, impairment of BC, including depletion of muscle mass (sarcopenia) as well as muscle quality (myosteatosis) have been found to affect perioperative outcomes in various clinical conditions3,4,5. Previous studies have shown a strong association of sarcopenia with poor overall survival (OS) in patients undergoing liver resection for HCC6,7,8 and recent studies conducted by our group detected not only a high prevalence of myosteatosis, but also an association between myosteatosis and poor perioperative outcomes in patients undergoing orthotopic liver transplantation (OLT)9,10. Reduced bone mineral density (BMD), defined as osteopenia, is the most important factor of bone fragility11. Although Dual-energy X-ray absorptiometry (DXA) is the gold standard in examining BMD, CT scan-based attenuation values are increasingly used to characterize BMD, due to its broad availability in oncological patients as part of the pre-operative oncological staging12. Osteopenia is also associated with frailty13, and according to the data of Pereira et al., bone loss may even begin and become clinically detectable before reduction of skeletal muscle mass in patients suffering from chronic diseases14. Recently, studies from Asian cohorts have demonstrated the prognostic value of BMD in the context of mortality in HCC patients undergoing partial hepatectomy or OLT15,16.
Based on the above-mentioned information, the aim of this study was to analyze the prognostic role of BMD in clinical outcomes in a Western-European single-center cohort of HCC patients undergoing partial hepatectomy in curative intent.
Patients and methods
Patients and eligibility
All consecutive patients who underwent partial hepatectomy for HCC at the University Hospital RWTH Aachen (UH-RWTH), Aachen, Germany, between May 2008 and December 2019 were considered for inclusion into this retrospective analysis. Clinical staging was performed prior to elective surgery and patients with systemic or irresectable disease were excluded. Patients where the abdominal staging was performed by MRI were not eligible for the analysis of BMD and therefore have been excluded. The present study was carried out in accordance with the principles of the current version of the Declaration of Helsinki and the good clinical practice (ICH-GCP). The protocol has been approved by the RWTH-Aachen University Institutional Review Board (EK 115/20 and EK 341/21). The IRB ("Ethik-Kommission der RWTH Aachen") waived informed consent due to the retrospective study design and collection of routine clinical data.
Image analysis and segmentation
Bone mineral density (BMD) was determined using imaging data as described by Sharma et al. using a single cross-sectional image at the level of 11th thoracic vertebra15. Up to 12 weeks prior to partial hepatectomy, a computed tomography was performed at the UH-RWTH Aachen for oncological staging. Technical data for CT image acquisition were chosen as the following: 128-section CT scan (SOMATOM Definition Flash, Siemens Healthcare, Erlangen, Germany) with 128 × 0.6 mm section collimation, a gantry rotation time of 0.5 s, a tube potential of 120 kV or a 40-section CT scan (SOMATOM Definition AS, Siemens Healthcare, Erlangen, Germany).
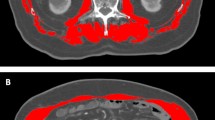
An experienced investigator, who was blinded for the remaining clinical data of the patients, conducted the segmentation in a semi-automated fashion. Briefly, the average pixel density within a single standardized click-and-drag circular region of interest (ROI) defined as the mid-vertebral core sample on the trabecular bone of the 11th thoracic vertebra alone was calculated for all patients using the non-contrast plain phase of the CT scans (Fig. 1)12. To avoid incorrect measurements imaging-related artifacts or regions including the venous plexus have been avoided. Bone mineral density values are displayed in Hounsfield units (HU) where lower attenuation values are associated with poorer bone density.
In this particular study, due to the relatively small cohort and lower event numbers, we decided against the use of newly defined and not validated cutoffs based on the area under the curve analysis and the Youden-index, as it was described by our group in multiple previous reports10,17,18,19,20,21. Therefore, we utilized a previously described and established cut-off value of < 160 HU for male HCC patients based on Sharma et al.15 (Fig. 1). Further, as the distribution of BMD was statistically significant between females and males in our cohort, we defined a cut-off of < 175 HU for females based on their cohort-specific median value to adjust for the gender-specific differences in BMD described before22 (Fig. 1). Further body composition parameters related to the muscle and fat compartments including skeletal muscle index (SMI), visceral fat area (VFA), subcutaneous fat area (SFA) and visceral-to-subcutaneous fat ratio (VSR) were also assessed and reported as described previously5,8,10,20,21.
Clinical data collection and patient follow-up
All clinical data were collected in a prospectively maintained institutional database and analyzed retrospectively. Indication for curative-intent partial hepatectomy was made by a staff hepatobiliary surgeon which was then confirmed by the institutional interdisciplinary tumor board. The partial hepatectomy was performed either laparoscopic or conventionally. Techniques of liver resection including the exact method of parenchymal transection were described by our group in previous studies 23,24,25. The outpatient clinic of the UH-RWTH Aachen as well as the local community based hepatologist network provided the follow-up data used in this study.
Classifications and scores reported in this analysis have been described by our group and by others in previous published studies (including ASA, labMELD, Clavien-Dindo classification-CD and the Comprehensive Complication Index-CCI19,26,27,28, procedural costs29, calculation of transfusion, of the length of hospital stay9,19,30 and long-term follow-up21).
Statistical analysis
The primary endpoint of this study was defined as overall survival (OS) of patients undergoing liver resection for HCC. The incidence of perioperative in-hospital major morbidity (defined by CD ≥ 3b)26, overall perioperative outcome, length of hospital-stay, 90-day mortality, and disease-free survival (DFS) were analyzed and reported as secondary endpoints. Categorial data was reported as absolute and relative frequencies and continuous data were displayed as mean ± standard deviation. Where appropriate, the Chi-square test and Fisher's exact test were used to analyze categorical data. The Student t test, Mann–Whitney U test, and Kruskal–Wallis H test were used to analyze continuous data. Spearman correlation coefficient was used to further analyze the association of BMD and various BC parameter. The associations of survival with BC characteristics were assessed using uni- and multivariable Cox proportional hazards regression models. Survival curves were generated by the Kaplan–Meier method and compared with the log-rank test. Statistical analysis has been performed using SPSS Statistics 24 (IBM Corp., Armonk, NY, USA) and the level of statistical significance was set to p < 0.05.
Results
Study population characteristics
During the defined study period, 151 consecutive patients underwent curative-intend liver surgery for HCC at our institution. Some 51 patients were excluded due to insufficient preoperative imaging which yielded a final study cohort of 100 patients inculding 72 male (72%) and 28 female (28%) patients with a mean age of 67 ± 11 years. Histological cirrhosis has been confirmed in 42 patients and the mean preoperative labMELD was 8 ± 3. Prior to surgery, 22 patients were within the Milan criteria. Some 67 patients were categorized as performance status ASA III or higher and 71 patients suffered from HCC classified as UICC category I or II (n = 36, 35, respectively). A total of 38 patients suffered from more than one intrahepatic tumor. The mean largest tumor diameter was 72 ± 41 mm and the mean number of tumors was 1.9 ± 1.3, retrospectively. Hemihepatecomy (25%) and bisegmentectomy (25%) were the most frequently used operative procedures, followed by atypical resections (24%). In 21% of the cohort, laparoscopic procedure has been performed and R0 resection was achieved in 85% of patients (Table 1).
Body composition assessment
The median time between the CT imaging used for segmentation and surgery was 19 [6–47] days. In our cohort, the mean BMD was 153 ± 53 HU with a mean BMI of 26 ± 4. The mean SMI, a parameter to characterize muscle mass and sarcopenia, was 45 ± 9 cm2/m2 for all included patients.
Concerning demographics and clinical characteristics, osteopenic patients were significantly older than non-osteopenic patients (70 ± 9 vs. 61 ± 14 years; p < 0.001, Table 1) and presented with a higher number of tumor nodules (2 ± 1.4 vs. 1.6 ± 1.3; p = 0.030, Table1). While BMI and SMI did not differ between groups (p = 0.359; p = 0.479, Table 1), muscle quality (L3Muscle-RA) was significantly inferior in osteopenic patients (31 ± 10 vs. 36 ± 9 HU; p = 0.049, Table 1) and the amount of visceral fat (VFA) was substantially higher in osteopenic patients, even though the difference was not significant (191 ± 126 vs. 139 ± 90; p = 0.070, Table1). In line with these findings, patients age and VFA were significantly associated with BMD using the Spearman ‘s correlation coefficient and corresponding correlations plots (r = − 0.445, p = 0.000; r = 0.246, p = 0.014, Fig. 2). Detailed patient characteristics are displayed in Table 1.
Perioperative outcome and osteopenia
In terms of perioperative outcomes, no difference was detected between the osteopenic and non-osteopenic subcohorts. Despite the lack of statistical significance, there was a tendency towards an increased perioperative morbidity in osteopenic patients. Major postoperative complications (CD ≥ 3b) occurred in 21% of the osteopenic patients and in 9% of non-osteopenics (p = 0.139, Table 2). The distribution of major morbidity is demonstrated in Table 3. Similar, CCI was higher but not significantly different in patients with osteopenia (24 ± 31 vs. 17 ± 25, p = 0.381, Table 2). In line with the findings above, mean hospital stay was 5 days longer in osteopenic patients but did not differ significantly (16 ± 15 vs. 11 ± 7, p = 0.103, Table 2), likewise the estimated procedural costs (14.2 ± 7.8 vs. 12.0 ± 6.8 TEuro, p = 0.147). Need of intraoperative FFP and RBC transfusion was similar between the groups (2.1 ± 2.5 vs. 2.1 ± 2.9 units p = 0.826; 1 ± 1.7 vs. 1.1 ± 1.9 units p = 0.906, Table 2). Five patients (15%) with normal BMD and 9 (13%) osteopenic patients died within the first 90-days following surgery (p = 0.909, Table 2, respectively).
Impact of osteopenia on long-term overall and disease-free survival
The median OS of all included patients in this study was 42 months with a median DSF of 37 months and a median follow-up period of 52 months. 5-year OS of osteopenic patients was significantly inferior when compared to those with normal BMD (29% vs. 65%, p = 0.014; Fig. 3, respectively). In line with the findings above, the probability of patient DFS at 5 years was significantly worse for patients with osteopenia compared with patients above the defined cut-offs of BMD (21% vs. 64%, p = 0.005; Fig. 3, respectively).
Further, due to the sex-related differences in BMD values we performed a subgroup analysis based on gender. In male patients suffering from osteopenia, 5-year OS was significantly impaired when compared with non-osteopenic males (0% vs. 64%, p = 0.008; Fig. 4). Disease-free survival in male patients was likewise significant impaired (24% vs. 66%, p = 0.007; Fig. 4). Interestingly, the findings above could not be confirmed in the female sub-cohort. While 5-year OS was largely comparable in female patients (58% vs. 66%, p = 0.374; Fig. 4), osteopenic females showed inferior DFS, even though the difference did not reach the levels of statistical significance (29% vs. 60%, p = 0.363; Fig. 4).
Finally, univariable Cox regression analyses revealed that pre-operative labMELD, intraoperative FFP and RBC transfusion and osteopenia were significantly associated with 5-year overall survival (Table 4). In the multivariable model, gender (HR 3.128 95% CI 1.159–8.444, p = 0.024, Table 4), pre-operative labMELD (HR 2.200 95% CI 1.030–4.699, p = 0.042, Table 4) and osteopenia (HR 7.743 95% CI 2.186–27.431., p = 0.002, Table 4) have been discovered to be independent predictors of inferior overall survival and demonstrated statistically significant results with meaningful hazard ratios (Table 4). Concerning DFS being outside the Milan criteria, AST, ALT, intraoperative FFP, negative R-0 status and osteopenia were found to be significantly associated with 5-year DFS in the univariable Cox regression analyses (Table 5). However, in the multivariable analysis, osteopenia lost its significant association with disease free survival while being outside the Milan criteria (HR 4.357 95% CI 1.493–12.714, p = 0.015, Table 5), intraoperative FFP transfusion (HR 3.693 95% CI 1.515–9.003, p = 0.004, Table 5) and not R0 resection (HR 3.356 95% CI 1.223–9.206, p = 0.019, Table 5) were still associated with disease free survival.
Discussion
The present study shows the value of BMD and associated osteopenia as a clinical risk-factor in predicting oncological outcomes following partial hepatectomy for HCC in a Western-European cohort. While there was no significant difference in terms of perioperative morbidity between osteopenic patients and those with normal BMD, the prognostic value of BMD seems to be accentuated in the long run.
HCC is an important oncological entity with a worldwide increasing incidence25. Tumor recurrence and impaired long-term survival following liver resection are remaining key problems in the treatment of HCC patients23,24,31. Identification of novel risk-factors associated with inferior outcomes is of utmost clinical importance to optimize preoperative selection of surgical candidates and better balance the operative risk with the expected survival benefit.
Bone mineral density is known to be the most frequently used parameter to characterize the loss of bone mass and an important morphological component of patient frailty32. Although, DXA is the gold standard method in the diagnostics of osteopenia and osteoporosis, a growing body of evidence supports the use of radiation attenuation values of the trabecular bone based on routine staging CT-scans in oncological patients22,33,34.
While Sharma et al. were the first to explore the association between impaired BMD and HCC prognosis in a liver transplant setting, the Japanese group of Miyachi et al. has recently found an association between osteopenia and poor long-term outcome after partial hepatectomy for HCC15,16. Both groups used a general BMD cut-off of 160 HU to define osteopenia. However, due to a well-documented gender-specific difference in BMD values, the use of a non-gender specific cutoff was an important limitation of these previous two studies. Therefore, in our study we decided to implement sex-specific cut-offs for osteopenia which was similar to the strategy used recently by Sharshar et al. in a Japanese cohort of patients with pancreatic cancer22,35. While we used the well-established and frequently described cut-off of 160 HU for men, we chose a median-based cut-off of 175 HU for female patients16.
Using these cut-off values, we could show that osteopenic patients had significantly worse OS and DFS and osteopenia was identified as an independent risk-factor for inferior OS in our multivariable model. This is in line with the above-mentioned previous studies from Asian cohorts15,16. However, osteopenia was significantly associated with inferior DFS in the univariable Cox regression analysis, it could not be confirmed as independent risk-factor for inferior DFS in the multivariable model.
Next, we carried out a gender-specific subgroup analysis for overall and recurrence-free survival. While osteopenic male patients presented with a significantly inferior OS, this difference was not present in the female sub-cohort. Although, this gender-specific difference cannot be explained completely using our data, these findings are in line with those of Miyachi et al.16. In this Japanese study, these discrepancies were explained by the higher age of female patients with a more prominent age-related bone loss. However, in our present cohort, female patients were actually younger than males (median 63 vs. 69 years). Another possible explanation why BMD failed to stratify our female sub-cohort into high and low-risk groups may lie in the relatively low sample size of the female sub-cohort.
Various patient-related factors are known to influence BMD. These include for example race and menopausal status. As the study was carried out in a Western European hospital, the examined cohort was relatively homogenous concerning race. In line, most female patients were post-menopausal with only 3 females younger than 58 years.
Although, the mechanisms behind the association of bone loss and low BMD in the surgical and oncological setting remains to be fully elucidated16,36,37. A possible explanation for bone loss might be a paraneoplastic effect. Due to the impact of the tumor itself and its treatment on bone metabolism, cancer is known to be linked to bone loss. In this context, independently of sex or cancer type, the risk of osteoporosis is noticeably higher in patients suffering from cancer than in the general population38. It is assumed to be of immunological nature based on a relatively poorly understood cross-talk between bone, the immune system and the tumor itself16,36. This is supported by the observation that certain anti-resorptive drugs used in the treatment of osteoporosis also have significant anti-tumor effects via various immunological pathways. Inflammatory cytokines produced by tumors promote osteoclastogenesis39,40,41. Thus, cancer-related pro-inflammatory microenvironment accelerates bone loss. In this particular context, the association between cyclooxygenases, prostaglandin E2 as well as further mediators of cancer-related inflammation and accelerated bone density loss has been described before42.
Several studies have reported a correlation between reduced muscle mass (sarcopenia) and BMD. The group of Szulc et al. found that sarcopenia was associated with thinner bone cortices and a higher risk of falls in elderly male patients43. Although, in our study an association with SMI could not be confirmed, we found a highly significant negative correlation with patient age and VFA. No correlation was detected between the other analyzed BC parameters and BMD. Thus, the findings are partially in line with a recent publication by Sharshar et al. who reported a strong correlation between BMD and patient age, VFA and myosteatosis (IMAC and psoas muscle index) in an Asian cohort undergoing surgery for pancreatic cancer22.
Correlation of BC parameters and perioperative outcome has been described in various clinical conditions including chronic liver disease and OLT3,9,44. In our cohort, patients suffering from osteopenia developed more major complications (CD > 3b) although this difference was not significant. Nonetheless, CCI was higher and as a result, osteopenic patients stayed longer in hospital and the estimated procedural costs were slightly higher. Even though no statistically difference was found in terms of postoperative morbidity, presumably due to our relatively small sample-size, it can be assumed that osteopenic patients may present with an increased risk of developing complications when undergoing liver surgery for HCC than those with normal BMD. This should be assessed further in future studies.
Certain limitations of this study should be acknowledged here. First, it is important to reflect whether osteopenia cutoffs utilized in our analysis were adequate to properly identify patients at risk for poor outcomes. It is known that not only age, nutrition status, sex but also race and other specific factors related to this particular cohort and tumor-entity might strongly affect BMD values and their distribution42. These confounding factors could not be addressed properly in this retrospective dataset and should be explored further in prospective clinical trials with controlled data collection. Second, preoperative CT images used for BMD measurement were taken at various time points as part of the clinical routine and were analyzed in a retrospective and uncontrolled fashion. We could not explore longitudinal changes of BMD either, due to the limited availability of follow-up CT scans.
Notwithstanding these limitations, to the best of our knowledge, this study is the first report to evaluate the value and limitations of osteopenia as a risk factor of clinical outcomes following curative-intent liver surgery for HCC in a western-European single-center cohort. The use of BMD as a prognostic marker lies in its simplicity. Although, it may never replace the subjective assessment of “fitness for surgery” by an experienced hepatobiliary surgeon or hepathologist, in combination with other body composition and frailty parameters it may serve as a useful clinical tool to improve pre-operative patient selection in HCC. Further prospective clinical trials are warranted to validate these findings and assess functional components of frailty and BC at the same time. Most pre-habilitation and enhanced-recovery programs are currently focusing on the muscle compartment, physical function measured predominantly by parameters of muscle function and fitness5. In the context of the present findings, an interesting direction of future research would be to develop therapeutic and pre-habilitation approaches directed specifically towards frail osteopenic patients.
Data availability
All relevant data were reported within the manuscript. Further supporting data will be provided upon written request addressed to the corresponding author.
Abbreviations
- ALT:
-
Alanine aminotransferase
- ASA:
-
American society of anesthesiologists
- AST:
-
Aspartate aminotransferase
- BC:
-
Body composition
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CCI:
-
Comprehensive complication index
- CD:
-
Clavien-Dindo classification
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- CUSA:
-
Cavitron ultrasonic surgical aspirator
- FFP units:
-
Fresh frozen plasma units
- GCP:
-
Good clinical practice
- GGT:
-
Gamma glutamyltransferase
- HCC:
-
Hepatocellular carcinoma
- HU:
-
Hounsfield unit
- ICU:
-
Intensive care unit
- L3:
-
Third lumbar level
- MELD:
-
Model of end-stage liver disease
- OR:
-
Odds-ratio
- PVE:
-
Portal vein embolization
- POD:
-
Postoperative day
- TACE:
-
Transarterial chemoembolization
- TARE:
-
Transarterial radioembolization
- RA:
-
Radiation attenuation
- RBC units:
-
Red blood cell units
- SE:
-
Standard error
- SFA:
-
Subcutanous fat area
- SMI:
-
Skeletal muscle index
- TEur:
-
Thousand Euros
- UH-RWTH:
-
University hospital of the RWTH university
- UICC:
-
Union for international cancer control
- VFA:
-
Viseral fat area
- VSR:
-
Visceral-to-subcutaneous fat ratio
References
White, D. L. et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 152(4), 812–820 (2017).
Bunchorntavakul, C. & Reddy, K. R. Review article: Malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment. Pharmacol. Ther. 51(1), 64–77 (2020).
Kaibori, M. et al. Effect of intramuscular adipose tissue content on prognosis in patients undergoing hepatocellular carcinoma resection. J. Gastrointest. Surg. 19(7), 1315–1323 (2015).
Kudou, K. et al. Prognostic significance of sarcopenia in patients with esophagogastric junction cancer or upper gastric cancer. Ann. Surg. Oncol. 24(7), 1804–1810 (2017).
Reichelt, S. et al. Body composition and the skeletal muscle compartment in liver transplantation: Turning challenges into opportunities. Am. J. Transplant. 28, 1943–1957 (2022).
Harimoto, N. et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J. Surg. 100(11), 1523–1530 (2013).
Peng, P. D. et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 13(7), 439–446 (2011).
Meister, F. A. et al. The role of sarcopenia and myosteatosis in short- and long-term outcomes following curative-intent surgery for hepatocellular carcinoma in a European cohort. Cancers (Basel) 14(3), 720 (2022).
Czigany, Z. et al. Myosteatosis to predict inferior perioperative outcome in patients undergoing orthotopic liver transplantation. Am. J. Transplant. 20(2), 493–503 (2020).
Meister, F. A. et al. Various myosteatosis selection criteria and their value in the assessment of short- and long-term outcomes following liver transplantation. Sci. Rep. 11(1), 13368 (2021).
Kanis, J. A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359(9321), 1929–1936 (2002).
Pickhardt, P. J. et al. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 158(8), 588–595 (2013).
Verschueren, S. et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos. Int. 24(1), 87–98 (2013).
Pereira, F. B., Leite, A. F. & de Paula, A. P. Relationship between pre-sarcopenia, sarcopenia and bone mineral density in elderly men. Arch. Endocrinol. Metab. 59(1), 59–65 (2015).
Sharma, P. et al. Bone mineral density predicts posttransplant survival among hepatocellular carcinoma liver transplant recipients. Liver Transplant. 22(8), 1092–1098 (2016).
Miyachi, Y. et al. Bone mineral density as a risk factor for patients undergoing surgery for hepatocellular carcinoma. World J. Surg. 43(3), 920–928 (2019).
Amygdalos, I. et al. Clinical value and limitations of the preoperative C-reactive-protein-to-albumin ratio in predicting post-operative morbidity and mortality after deceased-donor liver transplantation: A retrospective single-centre study. Transpl. Int. 34(8), 1468–1480 (2021).
Amygdalos, I. et al. Low postoperative platelet counts are associated with major morbidity and inferior survival in adult recipients of orthotopic liver transplantation. J. Gastrointest. Surg. 24, 1996 (2019).
Boecker, J. et al. Potential value and limitations of different clinical scoring systems in the assessment of short- and long-term outcome following orthotopic liver transplantation. PLoS ONE 14(3), e0214221 (2019).
Czigany, Z. et al. Myosteatosis to predict inferior perioperative outcome in patients undergoing orthotopic liver transplantation. Am. J. Transplant. 20(2), 493–503 (2019).
Czigany, Z. et al. The role of recipient myosteatosis in graft and patient survival after deceased donor liver transplantation. J. Cachexia Sarcopenia Muscle 12, 358–367 (2021).
Sharshar, M. et al. Impact of the preoperative bone mineral density on the outcomes after resection of pancreatic cancer. Surg. Today 50(7), 757–766 (2020).
Bednarsch, J. et al. Prognostic evaluation of HCC patients undergoing surgical resection: An analysis of 8 different staging systems. Langenbecks Arch. Surg. 406(1), 75–86 (2021).
Lurje, G. et al. Prognostic factors of disease-free and overall survival in patients with hepatocellular carcinoma undergoing partial hepatectomy in curative intent. Langenbecks Arch. Surg. 403(7), 851–861 (2018).
Lurje, I. et al. Treatment strategies for hepatocellular carcinoma (-) a multidisciplinary approach. Int. J. Mol. Sci. 20(6), 1465 (2019).
Clavien, P. A. et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 250(2), 187–196 (2009).
Slankamenac, K. et al. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann. Surg. 258(1), 1–7 (2013).
Bednarsch, J. et al. The role of ALPPS in intrahepatic cholangiocarcinoma. Langenbecks Arch. Surg. 404(7), 885–894 (2019).
Staiger, R. D. et al. The comprehensive complication index (CCI(R)) is a novel cost assessment tool for surgical procedures. Ann. Surg. 268(5), 784–791 (2018).
Amygdalos, I. et al. Low postoperative platelet counts are associated with major morbidity and inferior survival in adult recipients of orthotopic liver transplantation. J. Gastrointest. Surg. 24(9), 1996–2007 (2020).
Miller, H. et al. Impact of angiogenesis- and hypoxia-associated polymorphisms on tumor recurrence in patients with hepatocellular carcinoma undergoing surgical resection. Cancers (Basel) 12(12), 3826 (2020).
Kenny, A. M. et al. Association between level of frailty and bone mineral density in community-dwelling men. J. Clin. Densitom 9(3), 309–314 (2006).
Jang, S. et al. Opportunistic osteoporosis screening at routine abdominal and thoracic CT: Normative L1 trabecular attenuation values in more than 20 000 adults. Radiology 291(2), 360–367 (2019).
Ziemlewicz, T. J., Binkley, N. & Pickhardt, P. J. Opportunistic osteoporosis screening: Addition of quantitative CT bone mineral density evaluation to CT colonography. J. Am. Coll. Radiol. 12(10), 1036–1041 (2015).
Chen, C. C. et al. Gender interactions between vertebral bone mineral density and fat content in the elderly: Assessment using fat-water MRI. J. Magn. Reson. Imaging 51(5), 1382–1389 (2020).
Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7(4), 292–304 (2007).
Cheng, M. L. & Fong, L. Effects of RANKL-targeted therapy in immunity and cancer. Front. Oncol. 3, 329 (2014).
Reuss-Borst, M. et al. Prevalence of osteoporosis among cancer patients in Germany: Prospective data from an oncological rehabilitation clinic. Osteoporos. Int. 23(4), 1437–1444 (2012).
Takahashi, K. et al. Prognostic significance of preoperative osteopenia in patients undergoing esophagectomy for esophageal cancer. World J. Surg. 45(10), 3119–3128 (2021).
Jones, D. H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440(7084), 692–696 (2006).
Wang, Y. et al. Eosinophils attenuate hepatic ischemia-reperfusion injury in mice through ST2-dependent IL-13 production. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abb6576 (2021).
Yao, S. et al. Bone mineral density correlates with survival after resection of extrahepatic biliary malignancies. Clin. Nutr. 38(6), 2770–2777 (2019).
Szulc, P. et al. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men—The MINOS study. J. Bone Min. Res. 20(5), 721–729 (2005).
Montano-Loza, A. J. et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia Sarcopenia Muscle 7(2), 126–135 (2016).
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare funding from the START-program (#108/21 to FAM, #23/19 to ZC), the Clinician Scientist program (to ZC) and the FF-Med program (to FAM) of the Faculty of Medicine RWTH Aachen University without involvement of the funders in study design, data collection, data analysis, manuscript preparation or decision to publish.
Author information
Authors and Affiliations
Contributions
The study was designed by the initiating study team (F.A.M., S.V., Z.C.). Data collection and analysis were performed by F.A.M., J.B., S.V., Z.C. Manuscript was drafted by F.A.M., S.V., Z.C. Further authors (A.M., P.B., S.A.L., T.F.U., W.J.L., D.J., J.B., U.P.N.) have substantially contributed to the final version of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meister, F.A., Verhoeven, S., Mantas, A. et al. Osteopenia is associated with inferior survival in patients undergoing partial hepatectomy for hepatocellular carcinoma. Sci Rep 12, 18316 (2022). https://doi.org/10.1038/s41598-022-21652-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21652-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.