Abstract
The present study aimed to investigate indigenous bacteria possibility in recycled paper and cardboard mill (RPCM) wastewater treatment through the isolation and identification of full-scale RPCM indigenous bacteria. The molecular characterization of the isolated bacteria was performed by 16S rRNA gene sequencing. Klebsiella pneumoniae AT-1 (MZ599583), Citrobacter freundii AT-4 (OK178569), and Bacillus subtilis AT-5 (MZ323975) were dominant strains used for RPCM wastewater bioremediation experiments. Under optimal conditions, the maximum values of chemical oxygen demand (COD) and color biodegradation by C. freundii AT-4 were 79.54% and 43.81% after 10 days of incubation, respectively. In the case of B. subtilis strain AT-5 and K. pneumoniae AT-1, the maximum values of COD and color biodegradation were 70.08%, 45.96%, 71.26%, and 32.06%, respectively. The results from optimal conditions regarding efficiency were higher in comparison with the efficiency obtained from the oxidation ditch treatment unit in full-scale RPCM-WWTP. Therefore, the present study introduces the isolated indigenous bacteria strains as a promising candidate for improving the RPCM-WWTP efficiency using bioremediation.
Similar content being viewed by others
Introduction
The pulp and paper industries have been among the major water consumers. Therefore, high water usage produces large volumes of highly colored and toxic wastewater1,2. Based on raw materials, there are two types of the paper industry, including recycled old paper and virgin pulp paper3. The recycled paper mill consumes approximately 20 m3 of water per ton of recycled paper and produces 18 m3 of wastewater. The characteristics of wastewater vary depending on the production method, the raw materials, and applied chemicals in the process4. Compared to wood pulp and paper mills, recycled paper mills and those with a water recycling system have more microbial and organic pollution due to outdoor exposure of recycled papers and water reuse5. Treatment, along with the removal of major pollutants is highly necessary from environmental protection perspectives4,6,7,8.
Various physical, chemical, and biological treatment methods include adsorption2,3,7,8,9, microfiltration8, membrane filtration10, reverse osmosis3,9, coagulation and flocculation2,3,7,8,9,11, electrical coagulation7,10, electrolysis9, chemical precipitation2,7, ion exchange7, oxidation8,9,11, ozonation3,8,9,11, fenton9, photo fenton9, and electro fenton10. However, none of these methods have been developed to be industrially applicable due to high costs, high sludge production, or other drawbacks2,11. The biological treatment methods can also be extremely ineffective when the effluent contains significant amounts of color, resistant organic compounds, and suspended solids1,2.
Biotechnological strategies such as bioremediation have recently received attention. Bioremediation is explained as an approach of using microorganisms to decompose or immobilize contaminants. The detoxification process converts the harmful materials to harmless end products by mineralization, transformation, or alteration mechanisms12,13.
Various studies have been performed to identify microorganisms capable of degrading resistant organic compounds14. Some studies have reported that certain groups of microorganisms, including Bacillus megaterium, Serratia liquefaciens, Bacillus sp., Bacillus subtilis, Pseudomonas stutzeri, Ancylobacter aquaticus, Klebsiella, Methylobacterium spp., Pseudomonas, and Comamonas, are effective in degrading resistant organic compounds, lignin, and chlorinated organic compounds from wastewater1,15,16,17.
Although significant efforts have been made to use prepared isolated microorganisms, less attention has been given to examining the application of recycled paper and cardboard mill (RPCM) native microorganisms. Accordingly, the present study mainly sought to investigate the potential of indigenous bacteria in the RPCM wastewater treatment industry in promoting biological wastewater treatment, which has been performed as batch-scale experiments.
Materials and methods
RPCM treatment plant description and sampling
RPCM industry is located in Yazd (Iran) with the aim of producing various types of packaging paper from recycled materials and has a production capacity of 270 ton/d. The RPCM wastewater treatment plant (RPCM-WWTP) was designed to treat 3000 m3/d of wastewater with an organic loading rate (OLR) of 12,000 kg COD/d. Water consumption and wastewater production equal 4.5–5 m3/ton.d and 3–3.5 m3/ton.d, respectively. The mean removal efficiency of chemical oxygen demand (COD) in the oxidation ditch unit of RPCM-WWTP equals 60.57 ± 16.73%. In addition, the latter biological treatment unit has not been effective in removing color. The arrangement of the RPCM-WWTP and sampling points is in Fig. 1.
As shown, to determine the characterization of communities of heterotrophic bacteria, grab sampling was performed from the main locations, including raw wastewater, internal circulation (IC) reactor, oxidation ditch, and return activated sludge. Then, the samples were immediately transferred to the laboratory into 500-mL sterile glass bottles.
Isolation and bacterial identification
It was theorized that bacteria isolated from their natural habitats can survive in hard conditions by creating several catabolic enzyme systems. Thus, RPCM-WWTP bacteria were isolated and distinguished as an alternative for the improvement of WWT efficiency.
The collected samples were diluted by the serial dilution method (dilution series 10–1 to 10–7)11. Then, 0.1 mL of these solutions was cultured on plates containing a nutrient agar medium16. Next, the plates were incubated at 37 °C for 48 h8. The dominant bacterial colonies (dominant in this study was defined as the highest number of a colony on the plate) were selected and underwent purification. Meanwhile, the isolation process was performed by transferring a supposed colony section on a fresh nutrient agar plate using a sterile loop and culturing as the streak method to achieve pure colonies. Subsequently, the isolated colonies were labeled as RW1-RW4, IC1-IC3, AT1-AT5, and AS1-AS3. Then, the Gram-staining method was used to confirm the purity of the isolated dominant colonies, followed by determining the biochemical characterization of the isolated gram-negative dominant bacterial strains18.
In total, 15 bacteria strains were initially isolated from RPCM-WWTP. The isolated bacterial colonies were diverse in their morphologies. The molecular characterization of the isolated bacteria was performed by 16S rRNA gene sequencing. In addition, DNA was extracted using the boiling method. A NanoDrop spectrophotometer (Thermo scientific LR20-36) was used to evaluate the quantity and quality of the extracted DNA. Then, DNA templates were amplified by the polymerase chain reaction (PCR) using 16S rRNA universal primer set 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′TACGGTTACCTTGTTACGACTT-3′)8. The 20 µL PCR mixture contained 10 µL of Master Mix, 7 µL of deionized water, 1 µL of each primer, and 2 µL of DNA template. The thermal cycling steps were 95 °C for 5 min (denaturation), followed by 30 cycles at 94 °C for 30 s, 55 °C for 35 s (annealing), 72 °C for 1 min (extension), and a final extension step at 72 °C for 5 min (Thermal cycler: Simpliamp, ABI, American). Electrophoresis (FANAVARANE SAHAND AZAR, Iran) and gel dock (compact, Iran) were employed to identify the quality of the DNA strand product. The PCR products were purified by the ExoSAP protocol, and the sequencing was performed using an Applied Biosystems 3500 (ABI) sequencer (Pishgam Biotech Co., Tehran, Iran). To determine the nearest homolog of the isolated bacterial strains, sequence data were analyzed in the BLAST tool (http://www.ncbi.nlm.nih.gov). According to the maximum identity score, the first few sequences were selected and aligned by jPHYDIT software (version 1.0). The retrieved sequences were submitted to Bankit, and the accession numbers were received for the identified isolates19,20. Finally, the phylogenetic tree was plotted using jPHYDIT software (version 1.0).
Bioremediation experiments
Culture preparation
Overall, three bacterial strains out of 15 isolates, including Klebsiella pneumoniae (K. pneumoniae) AT-1 (MZ599583), Citrobacter freundii (C. freundii) AT-4 (OK178569), and Bacillus subtilis (B. subtilis) AT-5 (MZ323975), were used for bioremediation setups (based on their fast growth in the nutrient agar medium and COD and color removal potential).
Experimental setups
A homogenous suspension (as culture) containing 1.5 × 108 CFU/mL (0.5 McFarland) of each bacterium was prepared and separately used for bioremediation. The experiments were performed in conical flasks (250 mL) containing 150 mL of the effluent and culture. Table 1 presents the experimental setup condition of bacterial strains.
Based on data in Table 1, mineral salts (Na2HPO4, 2.4 g/L; K2HPO4, 2.0 g/L; NH4NO3, 0.1 g/L; MgSO4, 0.01 g/L; CaCl2, 0.01 g/L) and glucose (0.5%, w/v) were used as co-substrates. The solution pH was adjusted (pH = 7.2) and sterilized for 20 min at 121 °C14. Glucose was added after sterilization using a sterile syringe filter (20 µm). The inoculated flasks were incubated in a shaker incubator at 140 rpm and 30 °C for 10 days.
The influent of the oxidation ditch reactor (corresponding to COD = 1056 mg/L and color = 610 ADMI; The American Dye Manufacturer’s Institute) was investigated to perform bioremediation experiments. Colony forming unit (CFU/mL) counts method were performed to measure bacterial growth by the serial dilution method14. The samples were withdrawn on days 2, 4, 6, and 10 for the analysis of bacterial growth, periodic monitoring of operating parameters, and reduction in COD and color.
Chemicals and physicochemical analyses
Culture media and chemicals were of analytical grade and purchased from Merck Company. All parameters were analyzed according to Standard Methods for the Examination of Water and Wastewater Instruction18. The pH parameter was measured by a pH meter (L2012, Labtron Co, Iran). The turbidity meter was used to determine the turbidity (A-TUR-1.16, AndisheSazan Electricity Industry, Iran), and the ADMI method was utilized to measure the color (DR6000, HACH, USA). Further, COD was measured by the closed reflux method (Method: 5220-D). Furthermore, dissolved oxygen (DO), electrical conductivity (EC), and temperature were determined by a multi-meter (HQ40d, HACH, USA). Bioremediation experiments were conducted in triplicate, and the data are presented as the mean ± standard deviation.
Results and discussion
RPCM industrial wastewater characteristics
Six samples in three months were analyzed to investigate the qualitative characteristics of RPCM raw wastewater. Table 2 provides the main characteristics of raw wastewater. RPCM influent wastewater had high turbidity, total suspended solids (TSS), color, and COD, along with a dark brown color and pH ≈ 6.4 (Table 2). Previous studies have reported that pulp and paper wastewater had high levels of COD, lignin, and cellulose, while pH was in the neutral range9,17,21,22. The discharge of effluents containing these compounds has adverse effects on receiving aquatic environments9,23.
Identification of dominant bacterial strains
Isolation and phenotype identification
Table 3 presents the characteristics of bacterial enumeration in the operation unites of RPCM-WWTP. According to Table 3, raw wastewater had the most abundant bacterial community with a mean value of 4 × 108 CFU/mL.
The same bacterial morphotypes were observed in all samples using the Gram-staining method (rod-shaped). The isolated colonies were composed of gram-negative and positive heterotrophic bacteria. Table 4 provides the percentage distribution of cultured G (−) and G ( +) dominant strains in the operation units of RPCM-WWTP based on the Gram-staining method. The most percentage distribution of Gram-negative and Gram-positive bacteria was related to oxidation ditch and IC reactor unites, respectively (Table 4).
The biochemical characterization of the isolated Gram-negative dominant bacterial community is presented in Table 5. Based on the results, the biochemical characterization of isolated Gram-negative strains included negative tests of oxidase, gelatin, and indole, along with the positive test of citrate utilization. In addition, the motility test of strains AT-1, IC-2, and AT-3 was negative. Table 5 also provides the results of other biochemical tests, including triple sugar iron (TSI), lysine iron agar (LIA), phenylalanine deaminase (PAD), urea, H2S, and Methyl red/Voges Proskauer (MR/VP).
Molecular identification
In this study, a total of 15 dominant bacteria colonies were identified from raw wastewater, oxidation ditch, IC reactor, and return activated sludge, including 4, 3, 5, and 3 colonies, respectively. Figure 2 illustrates the BLAST analysis of the partial 16S rRNA sequence and phylogenetic tree of 15 isolated bacteria strains of the RPCM treatment plant. The BLAST analysis demonstrated 99–100% identity with the bacterial species of Bacillus, Pseudomonas, Enterobacter, Alcaligenes, Citrobacter, Acinetobacter, Klebsiella, and Brucella.
Phylogenetic tree of the dominant bacterial strains of RPCM-WWTP based on the partial 16s rRNA gene sequence representing the relationship between other neighboring members (the Genbank accession numbers were exhibited in parenthesis): (a) raw wastewater, (b) IC reactor, (c) oxidation ditch, and (d) return activated sludge. Note. RPCM: Recycled paper and cardboard mill; WWTP: Wastewater treatment plant; IC: Internal circulation.
Based on the molecular characterization of isolated bacteria by 16S rRNA gene sequencing, most of the identified species belonged to the genus of Bacillus and Pseudomonas. In addition, the bacterial species of Enterobacter, Alcaligenes, Citrobacter, Acinetobacter, Klebsiella, and Brucella were identified (Fig. 2), which can be effective in the biodegradation of organic compounds such as COD, lignin, and color24,25,26. Moreover, they can be more effective in removing COD and color from RPCM wastewater by bioremediation. The use of foreign microorganisms for bioremediation has been successful, but their efficiency depends on different factors, including the ability to compete with indigenous microorganisms, predators, and various environmental factors12,27. Therefore, bioremediation by a dominant indigenous microbial community isolated from industrial wastewater that has been adapted to this environment can be a promising and effective method for the decomposition of wastewater pollutants. Additionally, previous studies highlighted the ability of some of these microorganisms to decompose various pollutants of municipal and industrial wastewater. For example, Bacillus species have shown promised applications for the degradation of industrial wastewater pollutants by bioremediation. Some other studies reported that certain microbial groups such as Serratia liquefaciens, Bacillus megaterium, and Bacillus sp. can be effective in removing resistant organic compounds from industrial wastewater1. In this study, indigenous bacterial strains including K. pneumoniae AT-1, C. freundii AT-4, and B. subtilis AT-5, were chosen for the bioremediation experiments of the RPCM effluent.
Bioremediation experiments
Monitoring of RPCM effluent degradation by bacterial strains
The bioremediation experiments were conducted for 10 days on the RPCM effluent at pH = 7.2 in three setups, including effluent + culture, effluent + culture + mineral salt, and effluent + culture + mineral salt + glucose and the RPCM effluent treated with K. pneumoniae AT-1, C. freundii AT-4, and B. subtilis AT-5.
In general, the bacterial growth during RPCM effluent treatment in setups revealed good growth at an incubation time of 2–4 days, and growth was significant up to six days of incubation. Figure 3 displays the bacterial growth in optimal conditions (glucose-containing setup). As shown, growth was considerable up to six days of incubation, and afterward, it demonstrated a low trend. Other researchers reported that the usage of glucose and sucrose (0.5–1.0%) as co-substrates is effective in the bacterial growth and treatment efficiency of the pulp and paper mill effluent7,14.
In optimal conditions with glucose, pH reduced up to 5.5, and then it slowly increased up to ~ 7.7 after six days of incubation and reached 8.5 at the end of the treatment (Fig. 4). In addition, pH variations in other setups had an increase of up to ~ 9 during four days of incubation and consequently reduced up to 8.7. The effluent pH changed during RPCM effluent degradation due to bacterial metabolic activities. In glucose-free cultures, a pH increase of up to ~ 9 occurred within four days of incubation. The effluent was treated at neutral pH; some bacteria can increase the pH of the environment due to the consumption of the available organic matter in the effluent as a carbon source, leading to the generation of alkali enzymes28. However, a decrease in the pH value was observed in the glucose-containing setup. The acidic pH might be due to glucose consumption in the effluent, resulting in the generation of acidic products29. Moreover, the partial fermentation process led to the generation of the acidic compound11,29. As the simple form of carbon sources (i.e., glucose) decreased, the substrate shifted from glucose to the organic carbon source and metabolic products, resulting in a gradual increase in pH8.
In the first two days, COD and color decreased due to glucose consumption, but subsequently, heterotrophic bacteria were forced to consume organic compounds, leading to a significant reduction in COD and color. This finding is in accordance with the obtained results from the glucose-free setup.
DO was one of the other measured factors during bioremediation (Fig. 5). The minimum DO was observed in the K. pneumoniae AT-1 culture after six days of incubation (1 mg/L). During RPCM effluent treatment, DO was reduced at the initial phase of bacterial growth due to the consumption of DO present in the effluent. The minimum dose of DO was 1 mg/L in the culture of K. pneumoniae AT-1 at six days of incubation.
Effect of additional co-substrate on COD and color biodegradation in the RPCM effluent
To confirm whether the effluent alone can be a contributor to bacterial growth and removal of COD and color, the experiments were conducted in the effluent and bacterial culture of K. pneumoniae AT-1, C. freundii AT-4, and B. subtilis AT-5. The bacterial growth was slow in the effluent alone, but bacteria could utilize the organic compounds of the RPCM effluent for growth.
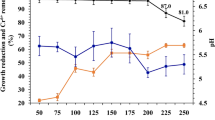
COD and color biodegradation rates, as the main factors of RPCM wastewater industries, are displayed in Fig. 6. Based on the results, the biodegradation of COD and color by the B. subtilis strain AT-5 after a 10-day incubation period was 35.8 ± 6.16% and 27.53 ± 3.96%, respectively. The biodegradation of COD and color by K. pneumoniae AT-1 and C. freundii AT-4 were 31.21 ± 4.3% and 24.8 ± 2.87%, as well as 30 ± 5.39% and 24.47 ± 3.1%, respectively. According to the results of the first setup without adding the co-substrate, the removal of COD and color by the B. subtilis strain AT-5 was higher compared to the other strains. These results indicate that K. pneumoniae AT-1 and C. freundii AT-4 strains can also degrade some of COD and color without using the co-substrate. However, previous research reported that the bacterial culture cannot grow and remove pollutants without adding carbon and nitrogen sources8.
Figure 7 depicts the effect of mineral salt on the performance of bacterial strains. As shown, the biodegradation of COD and color by C. freundii AT-4 after the incubation period of 10 days reached 61.10 ± 11.3% and 32.84 ± 4.4%, respectively. COD and color biodegradation by K. pneumoniae AT-1 and B. subtilis strain AT-5 were 44.47 ± 5.5%, 31.75 ± 3.66%, 48.68 ± 10.9%, and 30.43 ± 3.12%, respectively. In the second setup, the biodegradation of COD and color increased by all three bacteria (Fig. 7). In the case of C. freundii AT-4, the addition of the mineral salt had a significant effect on the growth and COD removal compared to the setup without the mineral salt. C. freundii AT-4 demonstrated the maximum COD biodegradation of 61.1%, while the maximum COD biodegradation of C. freundii AT-4 in culture without the mineral salt was 30.5%. C. freundii AT-4 had more COD and color biodegradation compared to the other two bacteria.
Figure 8 illustrates the results of the simultaneous effect of the mineral salt and glucose as the co-substrate compound. According to the findings, by adding mineral salt and glucose, the biodegradation of COD and color by C. freundii AT-4 after the incubation time of 10 days reached 79.54 ± 7.88% and 43.81 ± 6.46%, respectively. COD and color biodegradation by K. pneumoniae AT-1 and B. subtilis strain AT-5 were 71.26 ± 6.97%, 32.01 ± 3.21%, 70.08 ± 8.37%, and 45.98 ± 4.31%, respectively.
Based on these results, the presence of glucose as an additional carbon source had a significant effect on bacterial growth and COD removal by all three bacteria. C. freundii AT-4 had more COD biodegradation in comparison to K. pneumoniae AT-1 and B. subtilis strain AT-5. In the case of color biodegradation, B. subtilis strain AT-5 and C. freundii AT-4 were more effective in removing color compared to K. pneumoniae AT-1. The results of this study indicated that in the presence of the mineral salt and glucose, the indigenous bacteria of RPCM-WWTP have more ability to degrade the COD and color of the effluent. Similar findings were reported in the other research studies7.
These results represented that the bacteria used glucose and glucose along with color, respectively, leading to a decrease in the color concentration of the effluent. One study reported similar results during the bacterial treatment of the effluent29. In another study, adsorption on the cell surface has been explained as a contributor to color removal9.
According to the obtained data, the indigenous bacteria of RPCM-WWTP were capable of significantly removing COD and color. Tiku et al. found that the usage of glucose and sucrose (0.5–1.0%) as co-substrates was effective in the bacterial growth and removal efficiency of the pulp mill effluent. However, additional sources of carbon have not been employed by the researchers due to excessive growth of biomass, leading to high turbidity in the samples7. Likewise, Tyagi et al. applied a bacterial-fungal consortium (Micrococcus luteus, Bacillus subtilis, and Phanerochaete chrysosporium) to remove biochemical oxygen demand (BOD), COD, and lignin from pulp and paper wastewater and reported biodegradation rates of 87.2%, 94.7%, and 97%, respectively16. The efficiency of Aneurinibacillus aneurinilyticus, Paenibacillus sp., and Bacillus sp. was investigated in treating the paper mill effluent. Based on the results, COD removal by these bacterial strains was obtained 52–78% after six days of incubation25. In the treatment of the pulp paper mill effluent, Bacillus cereus and Serratia marcescens demonstrated biodegradation of color (45–52%), lignin (30–42%), BOD (40–70%), COD (50–60%), and phenol (32–40%), in a 7-day incubation period26.
Effect of the incubation period on the COD and color biodegradation of the RPCM effluent
Given that the retention time is an important factor for the effective wastewater treatment of RPCM, this study investigated the effect of the incubation period (2, 4, 6, and 10 days) on the removal of COD and the color of the effluent in three setups (Figs. 6, 7 and 8). In general, during effluent treatment, increasing the incubation period increased the biodegradation of COD and color. An increase in the incubation period led to an increase in the COD removal rate up to 79.55%, corresponding to C. freundii AT-4. This fluctuation was 70% and 55.3% for Bacillus subtilis strain AT-5 and K. pneumoniae AT-1, respectively. However, during 6–10 days of incubation, there were insignificant rates in setups 1 and 2 and an increase of about 15% in the third setup.
In color biodegradation, the experiment results were similar to the previous stage. Increasing the incubation period to six days caused a significant increase in biodegradation efficiency, but there was no significant increase after six days of incubation. The maximum biodegradation of color after six days of incubation in optimal conditions was obtained by C. freundii AT-4 (43.8%), Bacillus subtilis strain AT-5 (45.7%), and K. pneumoniae AT-1 (32.02%), respectively. Previous studies have reported the incubation periods of 68,14, 726,30, 911, and 1031days for the removal of color from the pulp and paper effluent by different bacterial and fungal strains. In other studies, the removal rate of COD was 94.7%, 85%, and 78% by a bacterial-fungal consortium after an incubation period of nine days16, Serratia liquefaciens strain LD-5 with an incubation period of six days8, and Paenibacillus sp. strain LD-1 isolated from the soil sample after an incubation period of six days, respectively14. Table 6 summarizes the comparison results between the maximum biodegradation of COD and color by indigenous bacterial strains used in this study and other bacterial strains reported in similar studies.
Table 6 provides the maximum biodegradation of COD and color by C. freundii AT-4, Bacillus subtilis strain AT-5, and K. pneumoniae AT-1 used in the present study compared to other bacteria reported in the literature. Based on the findings, the maximum biodegradation of COD and color by indigenous bacterial strains was promising. Therefore, these bacterial strains can be applied for the removal of COD and color from RPCM wastewater. Given that these strains are part of the microbial consortium of RPCM wastewater and are adapted to the environmental conditions of WWTP, they can be suitable alternatives for the efficiency improvement of WWTP under optimal conditions.
Conclusion
Overall, the promising biodegradation of RPCM wastewater treatment was investigated via the isolation and cultivation of its indigenous bacteria after the identification of K. pneumoniae AT-1, C. freundii AT-4, and B. subtilis strain AT-5 used for bioremediation experiments. Native strains could degrade contamination based on the COD and color of RPCM industry wastewater. Under optimal conditions, the maximum biodegradation of COD and color by C. freundii AT-4 was 79.54% and 43.81% after 10 days of incubation, respectively. Regarding B. subtilis strain AT-5 and K. pneumoniae AT-1, the maximum COD and color biodegradation was obtained at 70.08% and 45.96%, as well as 71.26% and 32.06%, respectively. The isolated strains of K. pneumoniae AT-1, C. freundii AT-4, and B. subtilis strain AT-5 were capable of COD and color degradation from the RPCM effluent under optimal conditions. The obtained results under optimal conditions were highly considerable in comparison with the efficiency results obtained from the oxidation ditch treatment unit in full-scale RPCM-WWTP. Therefore, these strains can be as promoting candidates for the bioremediation of RPCM-WWTP.
The results of this study can be helpful for microbial management strategies and increase the efficiency and stability of the treatment process. To obtain more detailed results, it is required to conduct further studies using other variables affecting the inoculation process to treat this type of wastewater.
Data availability
The datasets generated and analyzed during the current study are available in the (http://www.ncbi.nlm.nih.gov) repository [accession numbers MZ613189, MZ603671, MZ603715, MZ603673, MZ613313, MZ613316, MZ613319, MZ599595, MZ603616, MZ323975, MZ599583, OK178569, MZ598482, MZ597866 & OK178561].
Abbreviations
- AS:
-
Activated sludge
- AT:
-
Aeration tank
- BOD:
-
Biochemical oxygen demand
- COD:
-
Chemical oxygen demand
- DO:
-
Dissolved oxygen
- EC:
-
Electrical conductivity
- IC reactor:
-
Internal circulation reactor
- IC:
-
Internal circulation reactor
- LIA:
-
Lysine iron agar
- MR/VP:
-
Methyl red/Voges Proskauer
- OLR:
-
Organic loading rate
- PAD:
-
Phenylalanine deaminase
- RPCM:
-
Recycled paper and cardboard mill
- RPCM-WWTP:
-
Recycled paper and cardboard mill wastewater treatment plant
- RW:
-
Raw wastewater
- TSI:
-
Triple sugar iron
- TSS:
-
Total suspended solids
References
Cai, F., Lei, L. & Li, Y. Different bioreactors for treating secondary effluent from recycled paper mill. Sci. Total Environ. 667, 49–56 (2019).
Malaviya, P. & Rathore, V. Bioremediation of pulp and paper mill effluent by a novel fungal consortium isolated from polluted soil. Bioresour. Technol. 98, 3647–3651 (2007).
Bakraoui, M. et al. Biogas production from recycled paper mill wastewater by UASB digester: Optimal and mesophilic conditions. Biotechnol. Rep. 25, e00402 (2020).
Zwain, H. M., Aziz, H. A., Ng, W. J. & Dahlan, I. Performance and microbial community analysis in a modified anaerobic inclining-baffled reactor treating recycled paper mill effluent. Environ. Sci. Pollut. Res. 24, 13012–13024 (2017).
Granhall, U. et al. Bacterial community diversity in paper mills processing recycled paper. J. Ind. Microbiol. Biotechnol. 37, 1061–1069 (2010).
Hosseini, Z. et al. Bioremediation of cardboard recycling industrial wastewater using isolated native fungal strains. Desalin. Water Treat 154, 201–208 (2019).
Tiku, D. K. et al. Holistic bioremediation of pulp mill effluents using autochthonous bacteria. Int. Biodeterior. Biodegrad. 64, 173–183 (2010).
Haq, I., Kumar, S., Kumari, V., Singh, S. K. & Raj, A. Evaluation of bioremediation potentiality of ligninolytic Serratia liquefaciens for detoxification of pulp and paper mill effluent. J. Hazard. Mater. 305, 190–199 (2016).
Hossain, K. & Ismail, N. Bioremediation and detoxification of pulp and paper mill effluent: a review. Res. J. Environ. Toxicol. 9, 113 (2015).
Liang, J. et al. Performance and microbial communities of a novel integrated industrial-scale pulp and paper wastewater treatment plant. J. Clean Prod. 278, 123896 (2021).
Chandra, R. & Singh, R. Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem. Eng. J. 61, 49–58 (2012).
Adams, G. O., Fufeyin, P. T., Okoro, S. E. & Ehinomen, I. Bioremediation, biostimulation and bioaugmention: a review. Int. J. Environ. Bioremediat. Biodegrad. 3, 28–39 (2015).
Wasi, S., Tabrez, S. & Ahmad, M. Use of Pseudomonas spp. for the bioremediation of environmental pollutants: A review. Environ. Monit. Assess. 185, 8147–8155 (2013).
Raj, A., Kumar, S., Haq, I. & Singh, S. K. Bioremediation and toxicity reduction in pulp and paper mill effluent by newly isolated ligninolytic Paenibacillus sp.. Ecol. Eng. 71, 355–362 (2014).
Karn, S. K., Chakrabarty, S. & Reddy, M. Pentachlorophenol degradation by Pseudomonas stutzeri CL7 in the secondary sludge of pulp and paper mill. J. Environ. Sci. 22, 1608–1612 (2010).
Tyagi, S. et al. Bioremediation of pulp and paper mill effluent by dominant aboriginal microbes and their consortium. Int. J. Environ. Res. 8, 561–568 (2014).
Toczyłowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review. Renew. Sustain. Energy Rev. 78, 764–772 (2017).
APHAA, WEF. Standard Methods for the Examination of Water and Wastewater Vol. 21 (American Public Health Association, 2005).
Sharma, S. & Malaviya, P. Bioremediation of tannery wastewater by chromium resistant novel fungal consortium. Ecol. Eng. 91, 419–425 (2016).
Vijayalakshmidevi, S. & Muthukumar, K. Improved biodegradation of textile dye effluent by coculture. Ecotoxicol Environ. Saf. 114, 23–30 (2015).
Moussavi, G. & Aghanejad, M. The performance of electrochemical peroxidation process for COD reduction and biodegradability improvement of the wastewater from a paper recycling plant. Sep. Purif. Technol. 132, 182–186 (2014).
Birjandi, N., Younesi, H. & Bahramifar, N. Treatment of wastewater effluents from paper-recycling plants by coagulation process and optimization of treatment conditions with response surface methodology. Appl. Water Sci. 6, 339–348 (2016).
Haq, I., Kumar, S., Raj, A., Lohani, M. & Satyanarayana, G. Genotoxicity assessment of pulp and paper mill effluent before and after bacterial degradation using Allium cepa test. Chemosphere 169, 642–650 (2017).
Kim, E., Yulisa, A., Kim, S. & Hwang, S. Monitoring microbial community structure and variations in a full-scale petroleum refinery wastewater treatment plant. Bioresour. Technol. 306, 123178 (2020).
Raj, A., Reddy, M. K. & Chandra, R. Decolourisation and treatment of pulp and paper mill effluent by lignin-degrading Bacillus Sp.. J. Chem. Technol. Biotechnol. 82, 399–406 (2007).
Chandra, R., Raj, A., Yadav, S. & Patel, D. K. Reduction of pollutants in pulp paper mill effluent treated by PCP-degrading bacterial strains. Environ. Monit. Assess. 155, 1–11 (2009).
Tyagi, M., da Fonseca, M. M. R. & de Carvalho, C. C. J. B. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22, 231–241 (2011).
Bitton, G. Wastewater Microbiology (Wiley, 2005).
Abhishek, A., Dwivedi, A., Tandan, N. & Kumar, U. Comparative bacterial degradation and detoxification of model and kraft lignin from pulp paper wastewater and its metabolites. Appl. Water Sci. 7, 757–767 (2017).
Shi, Y. et al. Directed bioconversion of Kraft lignin to polyhydroxyalkanoate by Cupriavidus basilensis B-8 without any pretreatment. Process Biochem. 52, 238–242 (2017).
Barapatre, A. & Jha, H. Decolourization and biological treatment of pulp and paper mill effluent by lignin-degrading fungus Aspergillus flavus strain F10. Int. J. Curr. Microbiol. Appl. Sci. 5, 19–32 (2016).
Acknowledgements
This research was supported by Shahid Sadoughi University of Medical Sciences, Yazd, Iran, (Grant No: 6782) as part of a PhD dissertation (IR.SSU.SPH.REC.1398.096).
Author information
Authors and Affiliations
Contributions
M.GH. performed the experiments and drafted the manuscript. M.T.GH. and A.H.M. contributed to design experiments and material preparation. Further, M.T.GH., A.H.M., F.T., A.J.N., and M.H.E. interpreted the data. In addition, S.J. analyzed the experimental results. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gholami, M., Ghaneian, M.T., Teimouri, F. et al. Indigenous bacteria as an alternative for promoting recycled paper and cardboard mill wastewater treatment. Sci Rep 12, 16767 (2022). https://doi.org/10.1038/s41598-022-21362-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21362-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.