Abstract
Accurate staging of gastric cancer is essential for the selection and optimization of therapy. Hydrodistension of the stomach is recommended to improve the accuracy of preoperative staging with contrast-enhanced multidetector computed tomography (MDCT). This study compares the performance of contrast-enhanced gastric water distension versus a nondistension MDCT protocol for T and N staging and serosal invasion in comparison to surgical histopathology. After propensity score matching, 86 patients in each group were included for analysis. The overall accuracy of distension versus nondistension group in T staging was 45% (95% CI 35–56) and 55% (95% CI 44–65), respectively (p = 0.29). There was no difference in the sensitivity and specificity in individual T staging and assessment of serosal invasion (all p > 0.41). Individual stage concordance with pathology was not significantly different (all p > 0.41). The overall accuracy of N staging was the same for distension and nondistension groups (51% [95% CI 40–62]). The majority of N0 staging (78–81%) were correctly staged, whereas N3 staging cases (63–68%) were predominantly understaged. In summary, there was no significant difference in the diagnostic performance of individual TN staging and assessment of serosal invasion using MDCT with or without gastric water distension.
Similar content being viewed by others
Introduction
Gastric cancer is an important cause of cancer-related death in East Asia, South America and Eastern Europe1. While screening in high incidence areas such as Japan and Korea has led to reductions in gastric cancer-associated mortality2,3, surgical or endoscopic resection remains the backbone of curative intent treatment4,5,6. Despite recent treatment strategies such as neoadjuvant and adjuvant chemotherapy, the overall 5-year survival rate remains poor (35–45%)7,8.
Accurate staging of gastric cancer is essential for the selection and optimization of therapy. After initial diagnosis by gastroscopy, diagnostic work-up can consist of endoscopic ultrasonography (EUS) or contrast-enhanced multidetector computed tomography (MDCT)9. A recent meta-analysis consisting of eight studies (n = 1736) compared EUS to MDCT and showed that EUS had higher sensitivity than MDCT (82% versus 41%; p = 0.03) in detecting T1 staging10. However, no significant differences in T2–4 staging were noted between EUS and MDCT. Distension of the stomach with air and/or water to better visualize interruptions to the multilayered pattern of the normal gastric wall has been recommended to improve the accuracy of preoperative staging of gastric cancer through multiplanar reconstructions and virtual gastroscopy11,12,13,14,15,16,17,18. However, such accuracy studies have been limited by small sample sizes19,20,21, patient selection15,19,20, imbalances in the number of patients in each T staging15,19,21, and the lack of head-to-head comparisons with the nondistended stomach on modern MDCT11,12,13. A recent clinical audit of the authors’ hospital cancer registry sought to enumerate the local diagnostic performance of MDCT for gastric cancer staging.
The purpose of this single institution cancer registry study is twofold: first, to analyze the preoperative T and N staging performance of a dedicated water-distended gastric MDCT protocol versus a nondistended MDCT protocol; second, to evaluate the performance of the two protocols in the detection of serosal invasion.
Results
Study cohort
In total 1156 patient records were reviewed, and a final cohort of 516 patients was included after the following exclusions: clinical metastasis at presentation (n = 340), pathological metastasis at surgery (n = 19), no surgical histopathology (n = 55), nonadenocarcinoma histopathology (n = 53), CT performed at referral hospital (n = 125), and CT performed at the emergency department (n = 48).
Propensity score matching
Table 1 tabulates the characteristics of the cohort study at baseline and after propensity score matching. There were class imbalances in the age, sex, origin of CT, pT staging, and pN staging at baseline. The baseline cohort was mostly male (61%, 314/516), with cancers that occurred in the antrum (53.5%, 276/516) and had CT performed as inpatients (74%, 381/516). Early gastric cancer (T1 staging) accounted for 33% (168/516). In the postmatched cohort, both Distension and Nondistension groups consisted of 86 patients each, with 50% (86/172) of cases occurring in the antrum and 32% (55/172) of cases of T1 staging, similar to the baseline cohort.
Diagnostic performance
Interrater reliability for the independent readers is shown in Table 2. In both Distension and Nondistension groups, the ICC was good to excellent for T and N staging, indicating that readers had a high degree of agreement.
Table 3 tabulates the T and N staging performance metrics of Distension and Nondistension groups derived from consensus reading. The overall accuracy of Distension versus Nondistension groups in T staging was 45% (95% CI 35–56) and 55% (95% CI 44–65), respectively (p = 0.29), and there was no difference in the overall accuracy for N staging in either group (51% [95% CI 40–62] in both). There were no significant differences in sensitivity and specificity of individual T and N staging between Distension and Nondistension group. Figure 1 shows clinical case examples of T1 to T4b staging cancers in Distention and Nondistention groups.
Clinical images of water-distension versus nondistension MDCT protocols. The water-distension protocol is shown with arterial (a,d,g,j,m) and venous (b,e,h,k,n) phase images. Nondistension protocol is shown with venous phase (c,f,i,l,o). The white arrow points to the primary gastric cancer. In pT4b stage cancers, the invasion of the pancreas (1n) and transverse colon (1o) is noted.
Table 4 compares the diagnostic performance for assessing serosal invasion in Distension and Nondistension groups. The sensitivity was greater than 90%, and the positive predictive value greater than 80% in both groups. However, no significant difference in diagnostic performance was demonstrated between Distension and Nondistension groups.
Discrepancies in clinical and pathological TN staging are shown in Table 5. Concordant staging for T2, T3, T4a and T4b was 60%, 59%, 33% and 50%, respectively, for the Distension group and 67%, 58%, 43%, 80%, respectively, for the Nondistension group. In overstaged pT1 lesions, 24% (5/21) in Distension group and 42% (5/12) in Nondistension group were overstaged by two levels (p = 0.48). There was no significant difference in each individual T or N staging between the two groups (all p > 0.40).
The majority of N0 staging cases were correctly staged, whereas the N3 staging cases were predominantly understaged. This is also reflected in the higher sensitivity of N0 staging in both groups (Distension 78% versus Nondistension 81%, p = 1).
Discussion
This study showed that the overall accuracy of T and N staging, sensitivity and specificity for the identification of individual T and N staging for gastric cancer with MDCT was not significantly different with or without water-distension. The Nondistension group showed a trend toward higher sensitivity than Distension group at each individual T staging (Distension versus Nondistension T1, T2, T3, T4: 34%, 60%, 59%, 55% versus 48%, 67%, 56%, 67%; p > 0.41). This result is in keeping with findings from a recent meta-analysis showing pooled sensitivities of 41%, 48%, 64%, and 61% for T1, T2, T3 and T4 staging respectively10. Sensitivity (Distension versus Nondistension, 90% [95% CI 78–97] versus 94% [84–99]) and specificity (81% [65–92] versus 74% [57–88]) in assessing serosal invasion was not significantly different between groups (p = 0.48 and p = 0.58, respectively). This contrasts with results reported by Hasegawa et al. (using 7 mm axial slices), who showed a lower sensitivity of 55% (43–66), but a higher specificity of 94% (90–96)22.
Clinical T staging concordance with pathology enables proper selection of patients for neoadjuvant chemotherapy. Despite gastric distension with water, stage concordance in the Distension group was all below 60%. There was, however, a trend toward higher stage concordance in the Nondistension group. In a Norwegian study on restaging CT following neoadjuvant chemotherapy, only 24% had stage concordance, 38% overstaged and 38% understaged (kappa = 0.06 [95% CI 0.004–0.12])23. In the Japanese JCOG1302A trial, stage concordance for pT2, pT3, pT4a and pT4b was 40% (94/235), 45% (184/407), 55% (209/380) and 4% (1/25) respectively24. In another series at Shizuoka Cancer Center in Japan, stage concordance for pT1, pT2, pT3 and pT4 was 89% (1951/2197), 25% (133/530), 36% (106/294) and 58% (622/1080) respectively25.
The European MAGIC trial on perioperative chemotherapy found that 8.3% of patients who underwent gastrectomy alone had pT1 disease7. In the present study, 24% (5/21) of the Distension group and 42% (5/12) of the Nondistension group overstaged pT1 as greater than equal to T3 staging (p = 0.48). In a Korean series with modern 64-dectector CT and where 60% (76/127) of lesions were pT1 (due to an extensive country-wide screening program), the mean stage concordance for pT1, pT2, pT3 and pT4a was 97%, 66%, 70%, and 80%, respectively26. As in most series, the higher stage concordance for pT1 may be because the absence of lesions on MDCT in a known gastric cancer patient is often diagnostic for pT1 staging15,27,28.
In comparing pT3-4 staging accuracy in the JCOG1302A trial, stage concordance was similar for MDCT with 1-mm or 5-mm slice thickness (44% [37–50] versus 42% [39–45]), but worse for MDCT with gastric air distension (39% [34–44] versus 45% [42–48])29. The sensitivity was higher for 1-mm versus 5-mm (90% [84–94] versus 84% [82–87]) and higher for CT with air distension (87% [82–91] versus 84% [81–87]). Overall, there were no remarkable differences in the comparisons. Of note, in this trial, more than 2 times the number of CTs performed was without air distension than with air distension. Our findings are in keeping with the JCOG1302A trial; gastric distension for pT3–4 lesions did not improve staging concordance (all p > 0.44). Indeed, the classic three-layered pattern of the gastric wall in contrast-enhanced CT images11,26,30, which is critical for the evaluation of T1–2 lesions and accentuated with gastric distension, is not applicable in T3–4 lesions.
The diagnostic performance of N staging was not affected with or without gastric distension with water. Indeed except for N0 staging (> 78%), N staging sensitivity was uniformly poor (< 60%). Size (> 8 or > 10 mm), morphology, clustering and enhancement are often used to determine whether nodal metastasis is present, but invariably, small lymph nodes may be sites of metastasis. The literature reported a sensitivity ranging from 4 to 17% for T1 tumors31,32 to 63% for T2–4 tumors33 and 67–87% when T1–4 tumors are considered34,35,36. The use of PET/CT does not improve upon sensitivity of N staging (38–40%)37,38. In one series, the N staging concordance was 73% (2244/3066), 29% (105/368), 23% (79/348) and 72% (97/134) for N0, N1, N2, and N3 respectively25. In the JCOG1302A trial, the N0 staging concordance was 66% (278/423)24. In the current study, pN0 staging concordance was 78% (25/32) and 81% (26/32) for Distension and Nondistension groups, respectively. The majority of pN1–3 staging in both groups was understaged (48–68%).
There are a few limitations in this study. First, the water-distension protocol involved supine-only imaging irrespective of the location of the tumor. In this study, antrum and body tumors accounted for more than three quarters of all tumors. Second, virtual gastroscopy or tailored multiplanar reconstruction relative to the primary lesion was not performed as these techniques required additional reading and time at the workstation; this is not part of the standard workflow in our institution. Third, pT1 accounted for one-third of cases, which reflects the real-world prevalence in our population. While EUS staging may be preferable and more sensitive for T1 lesions, MDCT still forms an important part of preoperative staging. Finally, the independent readers were aware of the location of the tumor identified by endoscopy, this may have affected the diagnostic performance. However, in routine clinical practice, final clinical staging is performed by members of the gastric cancer multidisciplinary tumor board with access to endoscopic images for review.
In conclusion, the overall accuracy, individual T and N staging sensitivity and specificity of MDCT with or without gastric distension with water were not significantly different. The sensitivity and specificity in the detection of serosal invasion on MDCT were not affected with or without gastric distension with water.
Material and methods
Study design and study cohort
This was a single institution retrospective review of the gastric cancer registry from 2010 to 2015, which encompasses the 7th edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system. Patient demographics, origin of CT image (inpatient, outpatient, outside referral, and emergency department), site of tumor (antrum, body, fundus or diffuse [greater than two contiguous sites]), clinical (based on the initial CT stage from multidisciplinary tumor board) and pathological TNM stage, histopathological subtype based on WHO and Lauren’s classification (intestinal, diffuse, and mixed), and the CT protocol were evaluated.
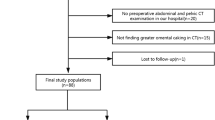
The registry includes all patients who were diagnosed or referred to our hospital for the treatment of gastric cancer. The following exclusion criteria were applied: clinical metastasis at presentation, pathological metastasis at surgery, no surgical histopathology, nonadenocarcinoma histopathology, CT performed at the referral hospital, and CT performed at the emergency department. CT images from referral hospitals and from the emergency department were excluded because of inconsistent image quality and/or reconstruction parameters. The study design flowchart is shown in Fig. 2.
We sought to compare the diagnostic performance of two CT protocols (described in detail below) in the evaluation of T and N staging to that of surgically-resected specimens. This study was approved by the Chang Gung Medical Foundation Institutional Review Board and a waiver of informed consent was approved as the research involves no more than minimal risk to subjects. All study methods were carried out in accordance with relevant guidelines and regulations. The STARD guidelines were used to ensure the reporting of this diagnostic accuracy study39.
CT protocols
Owing to the reimbursement policy of the national health care system, patients whose CT was performed at the time of presentation had restricted access to secondary dedicated staging CT after the diagnosis of gastric cancer. In this group of patients, gastric distension was not performed prior to CT acquisition. For the purpose of this study, we defined two types of protocols: a water-distension dual-phase protocol for staging of newly diagnosed gastric cancer (hereafter referred to as “Distension”), and a nonwater distended (“Nondistension”) protocol.
Per the Distension protocol, the patient ingest up to 1000 mL of purified water prior to CT examination to distend the stomach. Scan acquisition was supine-only covering the whole stomach during arterial phase and the abdomen to the pelvis during the venous phase. Axial, coronal and sagittal arterial phase images were routinely reconstructed into 3 mm slice thickness and interval; axial venous phase images were reconstructed into 5 mm slice thickness and interval.
Protocols designated as Nondistension included contrast-enhanced single and dual-phase CT of the abdomen and pelvis without prior distension of the stomach. For this protocol, axial and coronal images from venous phase images were reconstructed into 5 mm thickness and interval. All CT examinations were performed with 16- or 64-detector CT scanners.
Propensity score matching
The study cohort consisted of imbalanced groups (Distension versus Nondistension 402:114). Propensity-score matching was performed logistic regression model with the following covariates: age, sex, pT staging, pN staging, Lauren’s classification, tumor site, CT origin, year of diagnosis. The standardized mean difference was used to evaluate matching between groups, with a value of 0.1 or higher indicating an imbalance. Propensity score matching was performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) with the MatchIt package (version 3.0.2)40 using the nearest neighbor matching method and a caliper distance of 0.2 without replacement.
Secondary image and tumor stage evaluation by independent readers
Over the 5-year study period, different and/or additional radiologists joined the gastric cancer multidisciplinary tumor board and rendered the clinical TNM stage. To mitigate bias and evaluate the reliability and reproducibility of image readings by different radiologists, additional image review by 4 radiologists who were members of the gastric cancer multidisciplinary tumor board (C.M.C, W.H.C, Y.S.L, Y.C.L with 11, 7, 7, and 3 years of experience, respectively) was performed. The clinical TN stage established during the tumor board conference was included as a 5th independent reader. All readers performed the image interpretation separately and were independently blinded to the pathological TN staging. Images from the same CT protocol were batched and then randomized for reading on two separate occasions. Readers noted the clinical T (1, 2, 3, 4a and 4b), and clinical N (0, 1, 2, 3) staging based on the AJCC/UICC 7th edition.
Statistical analysis
Continuous variables are presented as the means ± standard deviation or, when the distributions are skewed, as the medians and interquartile ranges. Categorical variables were compared using the Chi-Square test or Fisher’s Exact test. In the independent reader study, interrater reliability with intraclass correlation coefficient (ICC) estimates and their 95% confident intervals were calculated using R with the irr package (version 0.84.1) based on a mean-rating (k = 5), absolute-agreement, 2-way random effects model. Intraclass correlation coefficient values less than 0.5 were indicative of poor reliability, values between 0.5 and 0.75 indicated moderate reliability, values between 0.75 and 0.9 indicated good reliability, and values greater than 0.9 indicate excellent reliability41. Consensus TN staging was calculated from the mode (majority consensus) of all 5 reader observations. Diagnostic performance (sensitivity, specificity, positive predictive value, negative predictive value) with 95% confidence intervals was calculated from the consensus TN staging. Clinical versus pathological TN staging concordance (under stage, correct stage, over stage) was calculated. The statistical significance was set at p < 0.05.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Kim, H. et al. Effectiveness of gastric cancer screening on gastric cancer incidence and mortality in a community-based prospective cohort. Cancer Res. Treat. 50, 582–589. https://doi.org/10.4143/crt.2017.048 (2018).
Hamashima, C. et al. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One 8, e79088. https://doi.org/10.1371/journal.pone.0079088 (2013).
Hatta, W., Gotoda, T., Koike, T. & Masamune, A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig. Endosc. 32, 180–190. https://doi.org/10.1111/den.13531 (2020).
Japanese Gastric Cancer, A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastr. Cancer 20(1–19), 2017. https://doi.org/10.1007/s10120-016-0622-4 (2014).
Network, N. C. C. Clinical Practice Guidelines in Oncology. Gastric Cancer (Version 3.2020), https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (2020).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355, 11–20. https://doi.org/10.1056/NEJMoa055531 (2006).
Hartgrink, H. H. et al. Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch gastric cancer group trial. J. Clin. Oncol. 22, 2069–2077. https://doi.org/10.1200/JCO.2004.08.026 (2004).
Smyth, E. C. et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27, v38–v49. https://doi.org/10.1093/annonc/mdw350 (2016).
Nie, R. C. et al. Endoscopic ultrasonography compared with multidetector computed tomography for the preoperative staging of gastric cancer: A meta-analysis. World J. Surg. Oncol. 15, 113. https://doi.org/10.1186/s12957-017-1176-6 (2017).
Chen, C. Y. et al. Gastric cancer: Preoperative local staging with 3D multi-detector row CT—Correlation with surgical and histopathologic results. Radiology 242, 472–482. https://doi.org/10.1148/radiol.2422051557 (2007).
Shimizu, K., Ito, K., Matsunaga, N., Shimizu, A. & Kawakami, Y. Diagnosis of gastric cancer with MDCT using the water-filling method and multiplanar reconstruction: CT-histologic correlation. AJR Am. J. Roentgenol. 185, 1152–1158. https://doi.org/10.2214/AJR.04.0651 (2005).
Kim, H. J. et al. Gastric cancer staging at multi-detector row CT gastrography: Comparison of transverse and volumetric CT scanning. Radiology 236, 879–885. https://doi.org/10.1148/radiol.2363041101 (2005).
Chen, C. Y., Wu, D. C., Kang, W. Y. & Hsu, J. S. Staging of gastric cancer with 16-channel MDCT. Abdom. Imaging 31, 514–520. https://doi.org/10.1007/s00261-005-0218-7 (2006).
Lee, I. J. et al. Diagnostic performance of 64-channel multidetector CT in the evaluation of gastric cancer: Differentiation of mucosal cancer (T1a) from submucosal involvement (T1b and T2). Radiology 255, 805–814. https://doi.org/10.1148/radiol.10091313 (2010).
Kumano, S. et al. T-staging of gastric cancer of air-filling multidetector-row CT: Comparison with hydro-multidetector-row CT. Eur. J. Radiol. 81, 2953–2960. https://doi.org/10.1016/j.ejrad.2011.12.039 (2012).
Shen, Y. et al. Evaluation of early gastric cancer at multidetector CT with multiplanar reformation and virtual endoscopy. Radiographics 31, 189–199. https://doi.org/10.1148/rg.311105502 (2011).
Kim, A. Y., Kim, H. J. & Ha, H. K. Gastric cancer by multidetector row CT: Preoperative staging. Abdom. Imaging 30, 465–472. https://doi.org/10.1007/s00261-004-0273-5 (2005).
Tsuda, K. et al. Intramural invasion of gastric cancer: Evaluation by CT with water-filling method. J. Comput. Assist. Tomogr. 19, 941–947. https://doi.org/10.1097/00004728-199511000-00019 (1995).
Rossi, M. et al. Local invasion of gastric cancer: CT findings and pathologic correlation using 5-mm incremental scanning, hypotonia, and water filling. AJR Am. J. Roentgenol. 172, 383–388. https://doi.org/10.2214/ajr.172.2.9930788 (1999).
Bhandari, S. et al. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: A comparison with conventional endoscopy, EUS, and histopathology. Gastrointest. Endosc. 59, 619–626. https://doi.org/10.1016/s0016-5107(04)00169-5 (2004).
Hasegawa, S. et al. A prospective validation study to diagnose serosal invasion and nodal metastases of gastric cancer by multidetector-row CT. Ann. Surg. Oncol. 20, 2016–2022. https://doi.org/10.1245/s10434-012-2817-1 (2013).
Sando, A. D., Fougner, R., Gronbech, J. E. & Bringeland, E. A. The value of restaging CT following neoadjuvant chemotherapy for resectable gastric cancer. A population-based study. World J. Surg. Oncol. 19, 212. https://doi.org/10.1186/s12957-021-02313-3 (2021).
Fukagawa, T. et al. A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastr. Cancer 21, 68–73. https://doi.org/10.1007/s10120-017-0701-1 (2018).
Bando, E. et al. Validation of the prognostic impact of the new tumor-node-metastasis clinical staging in patients with gastric cancer. Gastr. Cancer 22, 123–129. https://doi.org/10.1007/s10120-018-0799-9 (2019).
Kim, J. W. et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur. Radiol. 22, 654–662. https://doi.org/10.1007/s00330-011-2283-3 (2012).
Fukuya, T. et al. Efficacy of helical CT in T-staging of gastric cancer. J. Comput. Assist. Tomogr. 21, 73–81. https://doi.org/10.1097/00004728-199701000-00014 (1997).
Yu, J. S. et al. Value of nonvisualized primary lesions of gastric cancer on preoperative MDCT. AJR Am. J. Roentgenol. 189, W315-319. https://doi.org/10.2214/AJR.07.2672 (2007).
Sato, Y. et al. Diagnosis of invasion depth in resectable advanced gastric cancer for neoadjuvant chemotherapy: An exploratory analysis of Japan clinical oncology group study: JCOG1302A. Eur. J. Surg. Oncol. 46, 1074–1079. https://doi.org/10.1016/j.ejso.2020.02.038 (2020).
Minami, M., Kawauchi, N., Itai, Y., Niki, T. & Sasaki, Y. Gastric tumors: radiologic-pathologic correlation and accuracy of T staging with dynamic CT. Radiology 185, 173–178. https://doi.org/10.1148/radiology.185.1.1523303 (1992).
Fujikawa, H. et al. Diagnostic value of computed tomography for staging of clinical T1 gastric cancer. Ann. Surg. Oncol. 21, 3002–3007. https://doi.org/10.1245/s10434-014-3667-9 (2014).
Feng, X. Y. et al. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer—Results of a single institution study of 610 Chinese patients. PLoS One 8, e78846. https://doi.org/10.1371/journal.pone.0078846 (2013).
Terashima, M. et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastr. Cancer 22, 1044–1052. https://doi.org/10.1007/s10120-019-00941-z (2019).
Wang, M. et al. Pre-operative lymph node status of gastric cancer evaluated by multidetector computed tomography. Int. J. Clin. Exp. Med. 8, 18213–18224 (2015).
Seevaratnam, R. et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastr. Cancer 15(Suppl 1), S3-18. https://doi.org/10.1007/s10120-011-0069-6 (2012).
Mocellin, S. & Pasquali, S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD009944.pub2 (2015).
Park, K., Jang, G., Baek, S. & Song, H. Usefulness of combined PET/CT to assess regional lymph node involvement in gastric cancer. Tumori 100, 201–206. https://doi.org/10.1700/1491.16415 (2014).
Kim, E. Y. et al. The value of PET/CT for preoperative staging of advanced gastric cancer: Comparison with contrast-enhanced CT. Eur. J. Radiol. 79, 183–188. https://doi.org/10.1016/j.ejrad.2010.02.005 (2011).
Bossuyt, P. M. et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Radiology 277, 826–832. https://doi.org/10.1148/radiol.2015151516 (2015).
Ho, D. E., Imai, K., King, G. & Stuart, E. A. MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 42, 1–28. https://doi.org/10.18637/jss.v042.i08 (2011).
Portney, L. G. & Watkins, M. P. Foundations of Clinical Research: Applications to Practice, 3rd edn, 892 (Pearson/Prentice Hall, 2015).
Acknowledgements
The authors thank all members of the Cancer Center, Chang Gung Memorial Hospital, for their invaluable help. The authors also acknowledge the assistance of the dedicated radiology staff at Chang Gung Memorial Hospital at Linkou.
Author information
Authors and Affiliations
Contributions
Study conceptualization: C.-M.C. Image analysis: Y.-H.L., W.-H.C., Y.-C.L., C.-M.C. Data collection: A.-H.C. Statistical analysis: Y.-H.L., C.-M.C. Manuscript draft: Y.-H.L., A.-H.C., C.-M.C. Manuscript revisions: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, YH., Chan, WH., Lai, YC. et al. Gastric hydrodistension CT versus CT without gastric distension in preoperative TN staging of gastric carcinoma: analysis of single-center cancer registry. Sci Rep 12, 11321 (2022). https://doi.org/10.1038/s41598-022-15619-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15619-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.