Abstract
Central line-associated bloodstream infections (CLABSI) cause increased morbidity, mortality, and hospital costs that are partially preventable. The phenomenon of seasonality among CLABSI rates has not been fully elucidated, but has implications for accurate surveillance and infection prevention trials. Longitudinal dynamic cohort of hospitals participating in hospital-wide and intensive care unit bloodstream infection surveillance for at least one full year over 2000 to 2014. Mixed-effects negative binomial regression analysis calculated the peak-to-low ratio between months as an adjusted CLABSI incidence rate ratio (IRR) with 95% confidence intervals (CI). Multivariate regression models examined the associations between CLABSI pathogens and ambient temperature and relative humidity. The study population included 104 hospital sites comprising 11,239 CLABSI. Regression analysis identified a hospital-wide increase in total CLABSI during July–August, with a higher gram-negative peak-to-low incidence rate ratio (IRR 2.52 [95% CI 1.92–3.30], p < 0.001) compared to gram-positive bacteria (IRR 1.29 [95% CI 1.11–1.48], p < 0.001). Subgroup analysis replicated this trend for CLABSI diagnosed in the intensive care unit. Only gram-negative CLABSI rates were associated with increased temperature (IRR + 30.3% per 5 °C increase [95% CI 17.3–43.6], p < 0.001) and humidity (IRR + 22.9% per 10% increase [95% CI 7.7–38.3), p < 0.001). The incidence and proportion of gram-negative CLABSI approximately doubled during the summer periods. Ambient temperature and humidity were associated with increases of hospital-acquired gram-negative infections. CLABSI surveillance, preventive intervention trials and epidemiological studies should consider seasonal variation and climatological factors when preparing study designs or interpreting their results.
Similar content being viewed by others
Introduction
Central venous catheters (CVC) are necessary for medication, fluid, and blood product infusion, and hemodialysis. Unfortunately, these invasive devices can lead to preventable central line-associated bloodstream infections (CLABSI)1. CLABSIs can lead to septic shock, hematogenous bacterial seeding with organ infection, increased length of hospitalization and costs, which impact morbidity and prognosis2. CLABSI rates are primarily dependent on exposure risk: central line catheterization and duration. Considering this exposure, other risk factors include catheter type, insertion site and infection prevention practices. Reported infection rates for non-tunneled short-term CVCs range around 4.4 per 100 catheters, 2.7 per 1000 catheter-days and 1.6 per 10,000 patient-days, but depends on quality of care with adherence to preventive hygienic measures3,4.
Infection seasonality has been well documented for influenza and more recently among bacterial infections. Increased summer incidence has been documented for gram-negative infections5,6, BSI7,8,9,10,11,12,13,14,15, hospital-acquired infections5,6, peritoneal dialysis-related infections16,17, intra-abdominal18 and surgical site infections19,20. One study has identified seasonality for in-hospital CLABSI rates21. CLABSI are largely preventable and can be deduced to human errors with breaks in appropriate aseptic measures during catheter manipulation22,23. This complicates the interpretation of CLABSI seasonality compared to influenza, which has been linked to crowding and climate variables.
The reason for bacterial seasonal fluctuations has not been fully elucidated. Seasonal variation may be a phenomenon manifested by changes and interactions between patient, pathogen, environmental and hospital factors. Proposed explanations include seasonal changes in human behavior such as recreational water exposure, water consumption, or food preparation24, temperature6,15,25, humidity15, moisture within air-conditioned temperature-controlled environments5, and decreased hospital personnel (e.g. nurse-to-patient ratio) which predisposes to non-adherence to infection prevention guidelines25,26,27.
The Belgian Surveillance of Bloodstream Infections in Hospitals program has collected hospital-wide HABSI case-based data since 1992 to improve nationwide surveillance of these severe bacterial infections4. The study objective was to examine the presence of seasonal variation of hospital-wide CLABSI incidence including regression analysis for climatological variables and subgroup analysis per pathogen and CLABSI diagnosed in the intensive care unit (ICU).
Methods
This manuscript is reported according to the STROBE guidelines. All methods were carried out in accordance with relevant guidelines and regulations. The Belgian royal decree of April 2002 mandated bloodstream infection surveillance in acute care hospitals. The committee of Social Security and Health (SCSZG/16/245, deliberation nr. 16/111 of December 20, 2016) approved this surveillance. Informed consent was waived by this same committee.
Study design and setting
A national cohort study of CLABSI epidemiology was performed based on the Belgian Surveillance data of BSI in Hospitals program from January 2000 to December 2014. The full protocol, revised in 2013, is available online in Dutch and French28.
Participants
Participation in the surveillance program entails case-based recording of all CLABSI for a minimum of one trimester per year. Participation was voluntary, but became mandatory in 2014. Eligible hospitals were those with more than 150 beds. To analyze seasonal variation, hospitals included in the cohort performed surveillance for CLABSI (numerator) with hospital-wide patient-day (denominator) data for a full year, i.e. four trimesters within a calendar year, during at least one year.
Case definitions and variables
A laboratory-confirmed BSI required at least two separate samples if the causal micro-organism is a skin commensal and one sample in case of a recognized pathogen (Appendix 1). HABSI were those not present or incubating at the time of admission to the acute care setting (≥ 48 h). An episode in the same patient caused by the same microorganism was considered to be a novel occurrence if there were 14 days between the two episodes. During 2000–2012, CLABSI diagnosis could be classified as confirmed or probable. A definitive CLABSI diagnosis required a concomitant positive CVC tip with identification of the same BSI microorganism (Appendix 2). Probable CLABSI consisted of HABSI with a CVC in place, not secondary to another infection, and considered by the clinician to originate from the CVC. To avoid overdiagnosis, BSI of unknown origin with a central line in place the previous 48 h were not automatically classified as CLABSI unless there was suspicion of catheter infection. Denominator data included number of hospital-wide and ICU patient-days per trimester. CLABSI incidence was reported as a rate per 10 000 patient-days or per 1000 ICU patient-days.
Statistical methods
Mixed-effects negative binomial distribution regression model calculated the adjusted incidence rate ratio (IRR) with 95% confidence intervals (CI) for monthly CLABSI rates. Month was analyzed as a categorical variable, which allows for the identification of a peak-to-low ratio between two months. The comparison of the lowest to the peak incidence rate between months describes the amplitude of the seasonal pattern as an IRR29. This peak-to-low IRR is equal to the peak month’s incidence rate per patient-days divided by the lowest month’s incidence rate. The analysis was performed hospital-wide and stratified for gram-positive, gram-negative and fungal CLABSI. Fixed effects included year, university-affiliated status, hospital bed size, and infection risk exposure expressed as monthly patient-days. Hospitals were applied as random effects to account for varying hospital participation and heterogeneity. The number of patient-days was reported per trimester, but to calculate monthly patient-day risk exposure and incidence rate analysis, patient day trimester data was averaged by dividing by three. Monthly peak-to-low IRR results of the regression analysis predicted monthly CLABSI rate estimates, which were used to graph monthly seasonality by microorganisms. This way both the relative (IRR), absolute changes (mean incidence rate per patient-days), and microorganism proportions (percentage cultured gram-negative bacteria over total CLABSI) could be reported. Sensitivity analysis included separate assessment of CLABSI from 2000 to 2012 that fulfilled the criteria of a definitive diagnosis with concomitant positive catheter tip culture. Subgroup analysis replicated this for CLABSI diagnosed in the ICU.
To assess the climate influence on the infection rate, monthly average ambient temperature (°C), relative humidity (%) and precipitation (mm) from 2000 to 2014 were collected from a meteorological online system based in the center of Belgium30. Two mixed-effects negative binomial regression models were applied to examine the association between CLABSI and climate variables, adjusting for university-status and infection risk exposure. One applied season, temperature, and humidity as fixed effects to assess meteorological influence on CLABSI across all seasons. The second examined weather influences within seasons by applying temperature and humidity-by-season interaction terms15. Seasons were defined as winter (December–February), spring (March–May), summer (June–August), and autumn (September–November). These models were applied to hospital-wide total, gram-positive, gram-negative and fungal CLABSI.
All statistical analyses were done using Stata software, version 14. The mixed-effects negative binomial regression analysis was performed with the menbreg function. Statistical significance was set at p ≤ 0.05.
Results
Surveillance from 2000 to 2014 led to the identification of 11,239 CLABSI with 12 401 cultured microorganisms. The cohort of hospitals performing surveillance for an entire year consisted of 104 hospital sites (Table 1). This cohort included 7816 CLABSI in 7563 patients with a total of 8618 cultured microorganisms. Selection of hospitals performing surveillance consecutively for an entire calendar year led to exclusion of 3420 (30.4%) CLABSI. Missing patient-day data only led to a further loss of 3 CLABSI (0.1%) hospital-wide. For the subgroup analysis, missing ICU patient-day data (314 of 3299) was interpolated based on the median number of monthly ICU patient-days. The median CLABSI rate was 1.0 (IQR 0.5–1.6) per 10 000 patient-days, with an ICU CLABSI rate of 1.04 (IQR 0.51–1.62) per 1000 patient-days.
Gram-positive pathogens constituted the majority of cases, followed by gram-negative and fungal microorganisms (Table 2). The most common were coagulase-negative staphylococci, Staphylococcus aureus, and Candida spp. Less common microorganisms included Enterobacterales, Acinetobacter spp., and viridans group streptococci. Enterococcus faecium consisted of 19.1% of all enterococcal infections. 12.1% of CLABSI were polymicrobial. During 2000–2012 CLABSI were classified as definitive or probable. This period accounted for 7533 CLABSI, among which 3492 (46.4%) were classified as definitive with positive catheter tip culture.
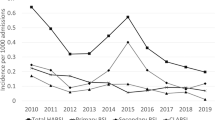
Mixed-effects regression analysis identified a sinusoidal pattern with an approximately 50% increase in total CLABSI from February up to August (Table 3). Seasonal variation was most pronounced among gram-negative microorganisms; when comparing August to March there was a two-and-a-half-fold increase in incidence rate during August (IRR 2.52 [95% CI 1.92–3.30], p < 0.001, Fig. 1). Although the gram-positive IRR increased significantly, this relatively small incidence rate ratio (IRR 1.29 [95% CI 1.11–1.48], p < 0.001) translated into an absolute rate increase of approximately 0.25 HABSI per 10,000 patient-days during the months of July–September. The change in relative IRR was statistically significant for fungal CLABSI, yet the absolute rate change was negligible. Even with this combined increase of total CLABSI during the summer months, a proportionate increase in the percentage of CLABSI in favor of gram-negative pathogens remained. Gram-negative bacteria represented 18.8% of total CLABSI in February, and this percentage increased to a maximum of 32.6% in August. Subgroup analysis of CLABSI diagnosed in the ICU also recognized gram-negative CLABSI seasonality with increasing incidence over the summer months which peaked during October (Appendix 3).
Seasonal variation of hospital-wide central line-associated bloodstream infection incidence, per microorganism. Composite monthly incidence rates of central line-associated bloodstream infection (CLABSI) based on the hospital-wide mixed-effects regression analysis results (Table 3). Total CLABSI seasonality during July–August was associated with both gram-positive and gram-negative increases. The rate of total CLABSI classifies polymicrobial BSI as a single CLABSI.
Across 2000–2014, the spread (5th to 95th percentile) of average temperature across all months was 2.4 °C to 19.3 °C (median 11 °C) and 67% to 90% for relative humidity (median 79%). When assessing the impact of meteorological factors on CLABSI incidence rates over all seasons, a significant association was found between temperature and the total CLABSI rate. Notably, subgroup analysis revealed that this relation was due to the gram-negative pathogens. Gram-negative CLABSI demonstrated IRR increases of 30.3% (17.3–43.6, p < 0.001) per rise of 5 °C ambient temperature across all seasons. Except for during winter, this association was present within all seasons, which represents an incidence rate difference between a warm versus a cool summer (Table 4).
Likewise, increases in 10% relative humidity was associated with increased gram-negative (IRR 22.9%, 7.7–38.3, p < 0.001), but not gram-positive nor fungal CLABSI rates. As with temperature, humidity’s role in gram-negative incidence rates was further demonstrated within all separate seasons including the winter. Precipitation was not associated with CLABSI incidence. Analysis of CLABSI with a definite diagnosis affirmed the seasonal variation among total, gram-positive and gram-negative CLABSI (Appendix 4).
Discussion
This cohort study analyzed CLABSI seasonality over 15 years. Hospital-wide total CLABSI exhibited sinusoidal seasonal variation with rising rates from March to August. Gram-positive CLABSI demonstrated slightly higher rates during the summer to autumn period; yet the most marked rise was among gram-negative pathogens, which displayed both an increased absolute rate change and proportion out of total CLABSI. Gram-negative bacteria were responsible for less than one fifth of CLABSI and this percentage increased to nearly one third during the August summer peak. Sensitivity analyses confirmed these findings when examining CLABSI with a definitive diagnosis by catheter tip culture or paired peripheral and CVC blood cultures. Climate variables of ambient temperature and humidity were shown to be associated with the total in-hospital CLABSI incidence rate after adjustment for hospitals and seasons. Gram-negative bacteria were the only family of microorganisms to consistently exhibit associations with temperature and humidity. Subgroup analysis of CLABSI diagnosed in the ICU also demonstrated gram-negative increases during the summer with a delayed peak during October.
Strengths of this study include long-term surveillance over multiple different hospitals, detailed microorganism identification, sensitivity and subgroup analyses, and mixed-effects regression analysis for adjusted peak-to-low monthly IRRs and climatological variables15,29.
Main limitations included lack of patient comorbidity, number of catheter-days, data on nurse-to-patient ratio and in-hospital climate data. Furthermore, the assumption was made that reported quarterly data on patient-days was equally distributed across the calendar months. Another known limitation is the diagnostic difficulty of CLABSI, with bloodstream infections falsely attributed to the catheter instead of occult gram-negative intra-abdominal abscesses or mucosal barrier injury. Nonetheless, our findings were reproduced among subgroup analysis for CLABSI diagnosed with catheter tip or paired blood cultures.
Because of insufficient data on number of catheter-days, number of patient-days had to be used to adjust for different risk exposure. Fluctuations in device-usage or nurse-staffing between seasons may influence the CLABSI incidence rate. Nonetheless, higher ambient temperature within the summer season remained significantly positively associated with CLABSI rates. Furthermore, although relative humidity was lower during the summer it was still positively associated with higher gram-negative CLABSI rates.
Although much of the literature has focused on CLABSI prevention, little has been reported on seasonality of these hospital infections27,31. Multiple previous studies have identified seasonal variation with summer increases in gram-negative BSI, but not fully examined HABSI nor BSI per infectious focus14,15. Peritoneal dialysis catheter-related peritonitis have demonstrated increased gram-negative IRRs during warmer months and gram-positive bacteria during the spring16,17. Other studies have shown summer increases in gram-negative outpatient healthcare-associated BSI, among which a large proportion were associated with intravascular catheters13,14,32,33. Higher risk for gram-negative BSI development has been linked to climate, such as geographic proximity to the equator, even after adjusting for healthcare expenditure differences26. Higher gram-negative BSI have been found during warmer months5,6,11 along with temperature associations6,15,26.
However, these studies did not focus on CVCs, hospital-acquired or inpatient catheter infections, or CLABSI due to different microorganisms. One recent study within a European tertiary-care hospital demonstrated a CLABSI incidence increase during the summer period over two surveillance years21. Unlike this study, theirs described an association with precipitation and not temperature nor humidity. However, limitations included a short-term duration and that no stratification per microorganism species could be performed.
This is the first large study to describe an increased gram-negative inpatient CLABSI incidence during the summer and association with climatological factors. Previous studies have found higher gram-negative BSI during warmer months5,6,11 with temperature associations6,15,26, but did not perform CLABSI subgroup analysis.
How increases in ambient temperature and humidity may translate to higher CLABSI rates is not fully elucidated. Disruption of the sterile catheter dressing has previously been defined as an important risk factor in the pathogenesis of CLABSI through colonization of the external lumen34. During the summer the warmer, damper skin environment may predispose to catheter dressing disruption. This could then lead to bacterial catheter colonization and subsequently CLABSI35. Combined with a decreased nurse-to-patient ratio, higher device-utilization rates, and frequent or inadequate catheter dressing changes, the summer climate changes may lead to an increased CLABSI rate27,31,35. However, even though the majority of CLABSI are skin commensals, the regression analysis did not identify an association between climate and gram-positive CLABSI.
Multidrug-resistant gram-negative bacteria have been described as waterborne pathogens causing healthcare-associated infections, commonly linked to contaminated sinks as a reservoir36. A narrative review found that 9.7% to 68.1% of random ICU water samples were positive for P. aeruginosa, and between 14.2 and 50% of patient infections were due to genotypes found in ICU water37,38. The summer months may improve the growth and density of gram-negative flora, leading to higher colonisation pressure among humans or the environment, increasing the risk of catheter colonization and CLABSI development.
This is the first multicenter cohort to identify seasonal variation of gram-negative CLABSI. These findings have important implications on multiple facets concerning infection prevention and surveillance. New trials investigating CLABSI prevention interventions should account for seasonal variation as a confounding factor, since implementation during a peak summer month could lead to an infection rate decrease falsely attributed to the intervention instead of regression to the mean1. CLABSI surveillance monitoring solely during low incidence rate months should be avoided; surveillance sampling during random trimesters has been validated as an accurate method for incidence measurement39. Although quality improvement strategies should be continuously performed, new initiatives could be started during peak incidence months where there may be more room for improvement40. CLABSI documentation has become an important quality of care indicator due to their preventable nature23. Further studies should assess this variation could be due to changes in nurse-staffing or device-usage. Comparison between international surveillance databases is required to quantify similar associations between climate and gram-negative bacteria, especially considering the rising prevalence of multiple drug resistance and difficult-to-treat infections.
Data availability
The data that support the findings of this study are available from the department of Healthcare-Associated Infections and Antimicrobial Resistance within Sciensano. However, restrictions apply to the availability of the data, which were used under license for this current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the responsible department.
References
Blot, K. et al. Prevention of central line-associated bloodstream infections through quality improvement interventions: A systematic review and meta-analysis. Clin. Infect. Dis. 59, 96–105 (2014).
Blot, S. I. et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin. Infect. Dis. 41, 1591–1598 (2005).
Maki, D. G., Kluger, D. M. & Crnich, C. J. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin. Proc. 81, 1159–1171 (2006).
Blot, K. et al. Increasing burden of Escherichia coli, Klebsiella pneumoniae, and Enterococcus faecium in hospital-acquired bloodstream infections (2000–2014): A national dynamic cohort study. Infect. Control Hosp. Epidemiol. 40, 705–709 (2019).
McDonald, C., Banerjee, S., & Jarvis, W. et al. Seasonal Variation of Acinetobacter Infections: 1987–1996, 1–5. http://cid.oxfordjournals.org/content/29/5/1133.full.pdf+html. (2015).
Perencevich, E. N. et al. Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect. Control Hosp. Epidemiol. 29, 1124–1131 (2008).
Gradel, K. O. et al. Seasonal variation of Escherichia coli, Staphylococcus aureus, and Streptococcus pneumoniae bacteremia according to acquisition and patient characteristics: A population-based study. Infect. Control Hosp. Epidemiol. 37, 946–953 (2016).
Al-Hasan, M. N. et al. Seasonal variation in Escherichia coli bloodstream infection: A population-based study. Clin. Microbiol. Infect. 15, 947–950 (2009).
Al-Hasan, M. N. et al. Epidemiology and outcome of Klebsiella species bloodstream infection: A population-based study. Mayo Clin. Proc. 85, 139–144 (2010).
Al-Hasan, M. N. et al. Temporal trends in Enterobacter species bloodstream infection: A population-based study from 1998–2007. Clin. Microbiol. Infect. 17, 539–545 (2011).
Anderson, D. J. et al. Seasonal variation in Klebsiella pneumoniae bloodstream infection on 4 continents. J. Infect. Dis. 197, 752–756 (2008).
Chazan, B. et al. Seasonal variation in Escherichia coli bloodstream infections in northern Israel. Clin. Microbiol. Infect. 17, 851–854 (2011).
Kato, K. et al. Seasonal trend and clinical presentation of Bacillus cereus bloodstream infection: Association with summer and indwelling catheter. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1371–1379 (2014).
Alcorn, K. et al. Seasonal variation in health care-associated bloodstream infection: Increase in the incidence of gram-negative bacteremia in nonhospitalized patients during summer. Am. J. Infect. Control. 41, 1205–1208 (2013).
Eber, M. R. et al. Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PLoS ONE 6, e25298 (2011).
Buttigieg, J. et al. Seasonal variation in the peritoneal dialysis-related infections: A Single center experience in the mediterranean. Ther. Apher. Dial. 20, 501–506 (2016).
Cho, Y. et al. Seasonal variation in peritoneal dialysis-associated peritonitis: A multi-centre registry study. Nephrol. Dial. Transplant. 27, 2028–2036 (2012).
Zangbar, B. et al. Seasonal variation in emergency general surgery. Ann. Surg. 263, 1–76 (2015).
Koljonen, V. et al. “Dog days” surgical site infections in a Finnish trauma hospital during 2002–2005. J. Hosp. Infect. 71, 290–291 (2009).
Durkin, M. J. et al. Seasonal variation of common surgical site infections: Does season matter?. Infect. Control Hosp. Epidemiol. 36, 1011–1016 (2015).
Schreiber, P. W. et al. Seasonal differences in central line-associated bloodstream infection incidence rates in a Central European setting: Results from prospective surveillance. Am. J. Infect. Control. 47, 1011–1013 (2019).
Pronovost, P. et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N. Engl. J. Med. 355, 2725–2732 (2006).
Umscheid, C. A. et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect. Control Hosp. Epidemiol. 32, 101–114 (2011).
Freeman, J. T., Anderson, D. J. & Sexton, D. J. Seasonal peaks in Escherichia coli infections: Possible explanations and implications. Clin. Microbiol. Infect. 15, 951–953 (2014).
Richet, H. Seasonality in Gram-negative and healthcare-associated infections. Clin. Microbiol. Infect. 18, 934–940 (2012).
Fisman, D. et al. geographical variability in the likelihood of bloodstream infections due to gram-negative bacteria: Correlation with proximity to the equator and health care expenditure. PLoS ONE 9, e114548–e114618 (2014).
Safdar, N., Kluger, D. M. & Maki, D. G. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters: Implications for preventive strategies. Medicine 81, 466–479 (2002).
WIV-ISP. Surveillance Nationale des Septicémies dans les Hôpitaux Belges. (2017)
Christiansen, C. F. et al. Methods to assess seasonal effects in epidemiological studies of infectious diseases-exemplified by application to the occurrence of meningococcal disease. Clin. Microbiol. Infect. 18, 963–969 (2012).
MeteoBelgië. Monthly Climatological Parameter in Ukkel. https://www.meteobelgie.be/klimatologie/grafische-gegevens/ukkel-vanaf-1833.html. Accessed 25 Feb 2018.
O’Grady, N. P. et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 52, e162–e193 (2011).
Toscano, C. M. et al. Gram-negative bloodstream infections in hematopoietic stem cell transplant patients: the roles of needleless device use, bathing practices, and catheter care. Am J Infect Control 37, 327–334 (2009).
Smith, T. L. et al. Bloodstream infections in pediatric oncology outpatients: A new healthcare systems challenge. Infect. Control Hosp. Epidemiol. 23, 239–243 (2002).
Mermel, L. A. Prevention of intravascular catheter-related infections. PLoS ONE 132, 391–402 (2000).
Timsit, J.-F. et al. Dressing disruption is a major risk factor for catheter-related infections. Crit. Care Med. 40, 1707–1714 (2012).
Kanamori, H., Weber, D. J. & Rutala, W. A. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin. Infect. Dis. 62, 1423–1435 (2016).
Trautmann, M., Lepper, P. M. & Haller, M. Ecology of Pseudomonas aeruginosa in the intensive care unit and the evolving role of water outlets as a reservoir of the organism. Am. J. Infect. Control 33, S41–S49 (2005).
Cervia, J. S., Canonica, F. & Ortolano, G. Water as a source of health care-associated infections. Arch. Intern. Med. 167, 92 (2007).
Fontela, P. S. et al. Surveillance length and validity of benchmarks for central line-associated bloodstream infection incidence rates in intensive care units. PLoS ONE 7, e36582–e36586 (2012).
Valencia, C. et al. Poor adherence to guidelines for preventing central line-associated bloodstream infections (CLABSI): Results of a worldwide survey. Antimicrob. Resist. Infect. Control 5, 49 (2016).
Funding
SB holds a research mandate of the Specific Research Fund at Ghent University.
Author information
Authors and Affiliations
Contributions
K.B. designed the study; performed data analysis and interpretation; wrote and revised the report. N.H. directed the surveillance program; designed the study; performed data interpretation; and revised the report. S.B. designed the study; performed data interpretation; and revised the report. D.V. performed data interpretation and revised the report. M.L. directed the surveillance program; designed the study; performed data interpretation; and revised the report. All authors critically reviewed and approved the final report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blot, K., Hammami, N., Blot, S. et al. Gram-negative central line-associated bloodstream infection incidence peak during the summer: a national seasonality cohort study. Sci Rep 12, 5202 (2022). https://doi.org/10.1038/s41598-022-08973-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08973-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.