Abstract
The role of liver stiffness measurement (LSM) after sustained virological response (SVR) in HCV patients treated by direct-acting antivirals (DAAs) remains unclear. We aimed to evaluate LSM regression value after SVR and to identify risk factors associated with liver related complications (LRC) or death. This retrospective study analyzed patients with LSM ≥ 10 kPa with LSM by transient elastography pre-DAAs and post-SVR. Patients with previous hepatic decompensation were excluded. Medical records were reviewed to identify primary outcomes. Kaplan–Meier curves and time-to-event Cox proportional-hazard models were performed. 456 patients [65% female, 62 years (IQR 57–68)] were included. During a follow-up of 2.3 years (IQR 1.6–2.7), 28 patients developed 37 outcomes [rate = 29.0 (95% CI 20.0–42.0) per 1000 person-years]. The cumulative incidence of outcomes was significantly lower in patients who regressed LSM ≥ 20% [3.4% (95% CI 1.8–7.0) vs. 9.0% (5.5–14.5), p = 0.028]. In a multivariate Cox-model [HR(95% CI)], male gender [HR = 3.00 (1.30–6.95), p = 0.010], baseline albumin < 3.5 mg/dL [HR = 4.49 (1.95–10.34), p < 0.001] and baseline unfavorable Baveno-VI [HR = 4.72 (1.32–16.83), p = 0.017] were independently associated and LSM regression ≥ 20% after SVR had a trend to reduce the risk of LRC or death [HR = 0.45 (0.21–1.02), p = 0.058]. The use of simple parameters before DAAs and repetition of LSM post-SVR can identify patients with different risks for severe outcome after HCV eradication.
Similar content being viewed by others
Introduction
Direct-acting antivirals (DAA) have changed the natural history of chronic hepatitis C in the last decade1. These regimens are safe and highly effective, leading to rates of sustained virological response (SVR) higher than 90%2,3. Therefore, international guidelines were updated to recommend DAA treatment to all individuals with chronic hepatitis C4,5. A recent large cohort study demonstrated that treatment with DAAs is associated with reduced risk for mortality and hepatocellular carcinoma (HCC), reinforcing the long-term impact of SVR6. However, the burden of liver-related events in patients with hepatitis C virus (HCV) that have successfully cleared the virus persists, particularly in individuals with cirrhosis previous to treatment7.
Historically, liver biopsy remains the reference for liver fibrosis staging and detection of cirrhosis. However, this invasive method has been challenged by several limitations and performing a liver biopsy in SVR patients without abnormal liver tests might not be justified. Liver stiffness measurement (LSM) by transient elastography (TE) is the most validated non-invasive method to assess liver fibrosis in patients with ongoing HCV infection as an alternative to liver biopsy8. LSM by TE can be used to predict severe outcomes in chronic hepatitis C: patients with LSM ≥ 9.5 kPa (advanced fibrosis/cirrhosis) had significantly lower overall survival compared to those in the lower range9. Additionally, combining LSM values with platelet count in HCV-infected patients was extensively validated to identify high-risk esophageal varices10.
Despite the broad validation of the diagnostic and prognostic value of LSM in patients with HCV, the correct interpretation of LSM in patients after SVR remains unclear. Several recent studies have reported a significant regression of LSM after SVR in patients with HCV treated by DAAs11,12. However, it is still controversial whether the decrease of LSM after HCV eradication is related to suppression of viral necro-inflammatory activity rather than regression of liver fibrosis. Moreover, the extent of the long-term clinical impact of LSM reduction after SVR in patients with HCV treated by DAAs remains unclear because most trials had short follow-up periods and such severe outcomes are rare. Establishing an accurate longitudinal non-invasive strategy which could be used to predict severe outcomes after SVR would be clinically and epidemiologically important. The primary aim of this study was to evaluate the prognostic value of LSM regression after SVR by DAAs. The secondary aim was to identify risk factors associated with liver related complications (LRC) or death in patients with HCV after SVR.
Methods
Population and study design
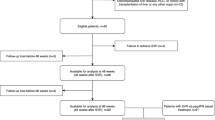
This retrospective observational study was conducted at two tertiary centers for management of patients with viral hepatitis in Rio de Janeiro, Brazil. All adult patients with chronic hepatitis C treated by DAAs between October 2015 and November 2019 were eligible. The exclusion criteria were: (1) HCV treatment failure, loss of follow-up or missing data of SVR; (2) presence of hepatitis B coinfection, liver transplantation or hepatic decompensation (ascites, hepatic encephalopathy, variceal bleeding or HCC) before HCV treatment, (3) non-available LSM before or after DAA and (4) LSM < 10 kPa before HCV treatment. SVR was defined as undetectable HCV RNA at 12 weeks following the conclusion of treatment. LSM was assessed by TE by experimented operators using FIBROSCAN (Echosens, Paris, France). HCC screening and esophageal varices surveillance were performed according to the current international guidelines4,5,13.
Data collection
Demographic, clinical and biological data were collected by trained investigators using an electronic case-report form at Research Electronic Data Capture (REDCap, https://projectredcap.org/). Clinical features included comorbidities, data of HCV history/treatment and liver stiffness by TE before and after DAA therapy. Laboratory parameters (including liver enzymes, albumin, creatinine, fasting glucose and platelet count) performed at least three months before or after each LSM were collected. All medical records from included patients were reviewed by trained investigators to identify the prospective incidence of the following primary outcomes after SVR: LRC (ascites, hepatic encephalopathy, variceal bleeding, or HCC), liver transplantation or death.
Liver stiffness measurement
TE examinations were performed before and after HCV treatment in patients with a 3-h fasting by experienced operators. Liver fibrosis staging by TE was considered as reliable for analysis if the following criteria had been met: (1) at least 10 valid measurements; (2) an interquartile range (IQR) lower than 30% of the median of LSM and (3) a success rate of more than 60%. TE examination obtained closest to the date of start and the end of DAA treatment was considered for LSM before treatment and after SVR, respectively. Patients with LSM ≥ 10 kPa before HCV treatment were classified as compensated advanced chronic liver disease (c-ACLD). Additionally, those patients with LSM ≥ 20 kPa or platelet count < 150 × 109/mm3 before HCV treatment were considered as having an unfavorable Baveno VI status13.
Statistical analysis
Continuous variables were reported as median (interquartile range, IQR) and discrete variables were reported as absolute (n) and relative frequency (%). The duration of follow-up was calculated from the end of treatment to the date of clinical outcome or last visit at one of the two centers until November 30, 2019. If several LRC occurred during the follow-up, the first one was considered for the analysis. The incidence rates of the primary outcomes (per 1,000 person-years) were calculated. The relative risk of incidence of outcomes was evaluated according to surrogate markers of liver dysfunction/fibrosis before HCV treatment and LSM regression post-SVR. A receiver operator characteristic (ROC) curve analysis was performed and the optimal threshold of LSM regression (%) to predict LRC or death was identified using the point nearest to the upper left corner of the ROC curve14. Kaplan–Meier curves were plotted and the log-rank test was calculated for univariate analysis. We used the time to event Cox proportional-hazard model to identify factors associated with LRC or death (hazard-ratio, HR) after checking that the main variables verified the proportional-hazard assumption using the Schoenfeld residuals. Variables found to be associated (p value ≤ 0.10) with LRC or death in the univariate analysis were entered into the multivariate Cox models adjusted for age and gender15. The variable Baveno VI status (favorable vs. unfavorable) replaced the variables LSM and platelet count at baseline in the models due to collinearity. The analysis was performed using STATA package, version 15, 2017 (StataCorp LP, College Station, TX, USA). Significance level was determined when p ≤ 0.05 assuming two-tailed tests.
Ethics approval and consent to participate
The study was conducted according to the good clinical practice guidelines and the Declaration of Helsinki and was approved with waiver of informed consent by the Ethical Committees from the Federal Hospital of Bonsucesso (IRB number 51736815.3.3001.5253) and the National Institute of Infectious Diseases Evandro Chagas (IRB number 51736815.3.0000.5262).
Results
A total of 1,131 patients with chronic hepatitis C were treated by DAA in two tertiary centers of Rio de Janeiro (Brazil) from October 2015 to November 2019. One hundred forty-four patients were excluded due to treatment failure (n = 26), death before SVR evaluation (n = 5), loss of follow-up after SVR (n = 87) or missing SVR data (n = 26). Furthermore, 120 patients were excluded because of cirrhosis decompensation previously to DAA therapy and 110 patients due to the absence of paired LSM evaluations, before and after HCV treatment. A total of 301 patients were excluded due to LSM < 10 kPa (absence of c-ACLD) before HCV treatment. Figure 1 is a flow chart that summarizes the study enrollment. A total of 456 patients [65% female, median age of 62 years (IQR 57–68), median ALT levels of 80 UI/L (IQR 54–132), 5.5% with HIV coinfection, 88.6% infected by genotype (GT) 1 and 67.5% treated by sofosbuvir/daclatasvir (SOF/DCV)] were included in the study. Additionally, a total of 250 patients [55% (95% CI 51–60)] had an unfavorable Baveno VI status before HCV treatment. Table 1 summarizes demographic and clinical characteristics of patients included in the study. An 17.0% decrease in LSM from before HCV treatment to after SVR was the optimal threshold of LSM regression to predict the incidence of LRC or death defined as the point nearest to the upper left corner of the ROC curve. This threshold wielded (95%CI)) an AUROC of 0.60 (0.51–0.69) as well as sensitivity, specificity, positive and negative predictive values of 32% (16–52), 88% (85–91), 15% (7–27) and 95% (93–97), respectively.
Follow-up and incidence of clinical outcomes
Baseline LSM was performed in a median of 13.7 months (IQR 7.6–24.7) before the start of HCV treatment by DAA and LSM post-SVR in a median of 4.8 months (IQR 3.4–9.8) after the conclusion of treatment. During a median follow-up of 2.3 years (IQR 1.6–2.7) after SVR, 28 patients developed a total of 37 primary outcomes (LRC or death): HCC [n = 10 (27%), hepatic encephalopathy [n = 9 (24%)], ascites [n = 9 (24%)], variceal bleeding [n = 5 (14%)] or death [n = 4 (11%)]. No patient underwent liver transplantation. The overall rate of incidence of clinical outcomes was 29.0 (95% CI 20.0–42.0) per 1,000 person-years. Surrogate markers of liver dysfunction (albumin levels and platelet count) and/or liver fibrosis (LSM) before HCV treatment and LSM regression post-SVR were evaluated to stratify the risk of primary outcomes (LRC or death). Considering the baseline assessment before HCV treatment, the relative risk [RR (95%CI)] of incidence of outcomes was significantly higher in patients with serum albumin levels < 3.5 mg/dL [vs. albumin ≥ 3.5 mg/dL; RR = 4.51 (2.06–9.89), p < 0.001] and those individuals with unfavorable Baveno VI status [vs. favorable; RR = 6.58 (1.99–21.78), p < 0.001]. On the other hand, the risk of incidence of outcomes was significantly lower in those patients who had at least 20% regression of LSM post-SVR [19.3 per 1,000 person-years (95%CI 10.7–34.9)] compared with those without [43.0 per 1,000 person-years (95% CI 26.7–69.1)] [RR = 0.45 (0.21–0.96), p = 0.034] (Table 2). Patients with serum albumin level lower than 3.5 mg/dL before treatment had a higher cumulative incidence of clinical outcomes (95%CI) at 2 years compared with those with albumin ≥ 3.5 mg/dL [17.8% (10.5–29.3) vs 3.6% (2.0–6.7), log-rank p < 0.001] (Fig. 2A). Additionally, patients with unfavorable Baveno VI status at baseline had significantly higher incidence of LRC or death compared with those with favorable Baveno VI status [9.6% (6.3–14.4) vs 1.1% (0.3–4.2), log-rank p < 0.001] (Fig. 2B). In contrast, those patients who obtained a regression of at least 20% of LSM post-SVR had significantly lower cumulative incidence of clinical outcomes at 2-year follow-up compared with those without LSM regression [3.4% (1.8–7.0) vs. 9.0% (5.5–14.5), p = 0.028] (Fig. 3).
Factors associated with primary outcomes
The presence of male gender, higher baseline AST levels [per 10 UI/L], higher LSM values, baseline serum albumin < 3.5 mg/dL, baseline platelet count < 150 × 109/mm3, presence of baseline unfavorable Baveno VI status and regression of LSM post-SVR were associated (p < 0.10) with the incidence of outcomes in the univariate Cox proportional-hazard analysis (Table 3). Those variables were inserted in the multivariate Cox proportional-hazard models adjusted for age and gender. The variables LSM and platelet count before HCV treatment were replaced by the variable “Baveno VI status” in the multivariate models due to strong collinearity. The following factors were independently associated with the incidence of LRC or death after SVR in HCV treated with DAAs [HR (95%CI)]: male gender [vs female, HR = 3.00 (1.30–6.95), p = 0.010], baseline serum albumin < 3.5 mg/dL [vs ≥ 3.5 mg/dL, HR = 4.49 (1.95–10.34), p < 0.001] and baseline unfavorable Baveno VI [vs favorable, HR = 4.72 (1.32–16.83), p = 0.017] (Table 3). In this multivariate model LSM regression of at least 20% after SVR had a trend to reduce the risk of LRC or death [HR = 0.45 (95%CI 0.21–1.02), p = 0.058]. Additionally, LSM regression post-SVR of at least 20% significantly reduced the risk of LRC or death in multivariate Cox models adjusted for age-and-gender [HR = 0.43 (0.20–0.92), p = 0.029] and those adjusted for age, gender and baseline albumin level [HR = 0.44 (0.20–0.99), p = 0.049]. We observed similar results in a sensitivity analysis considering only patients with unfavorable Baveno VI status before HCV treatment (n = 250 and incidence of primary outcomes in 25 patients): low albumin level was independently associated with an increased risk [HR = 5.56 (95% CI 2.22–13.97)] and LSM regression after SVR with a decreased risk of LRC or death [HR = 0.31 (95% CI 0.12–0.80)] (Supplementary Table 1). Moreover, we repeated the analysis considering the LSM more distantly performed from the end of the HCV treatment [median time = 12.5 months (IQR 10.3–14.9)] to evaluate the impact of LSM regression on incidence of LRC or death. LSM post-SVR remained stable, since the median value (IQR) for the first and the last LSM post-SVR were 12.4 kPa (8.5–19.9) and 12.0 kPa (7.9–19.2), respectively. Male gender [vs female, HR = 2.96 (95% CI 1.29–6.82), p = 0.011], baseline serum albumin < 3.5 mg/dL [vs ≥ 3.5 mg/dL, HR = 4.33 (1.89–9.94), p = 0.001], baseline unfavorable Baveno VI [vs favorable, HR = 4.72 (1.32–16.8), p = 0.017] and at least 20% of LSM regression post-SVR [vs < 20% LSM regression, HR = 0.38 (0.17–0.86), p = 0.021] were independently associated with the incidence of clinical outcomes after SVR in HCV treated with DAAs in this multivariate analysis (Supplementary Table 2). We observed similar results when repeating those analyses and sub-analyses in the HCV mono-infected cohort [n = 431 patients and incidence of primary outcomes in 27 patients] (Supplementary Tables 3 and 4). Regarding the evolution of laboratory parameters, persistent elevation of AST levels after SVR increased the risk of outcomes [HR = 10.8 (95% CI 1.30–89.76), p = 0.028] and patients with low albumin levels or low platelet count pre-treatment remained at high risk for development of clinical outcomes even if they recovered these parameters after SVR [albumin: HR = 3.64 (95% CI 1.43–9.28) and platelet count: HR = 3.67 (95% CI 0.92–14.68)].
Discussion
The current study highlighted that albumin levels lower than 3.5 mg/dL and/or presence of unfavorable Baveno VI status (LSM ≥ 20 kPa or platelet count < 150 × 109/mm3) before HCV treatment were associated with an increased risk of LRC or death after SVR adjusted for confounding factors in patients with c-ACLD. Additionally, the regression of at least 20% of LSM after SVR was associated with a significant decrease in incidence of LRC or death in patients with c-ACLD treated by DAAs. Our study findings have implications for optimizing management of patients with HCV after SVR. Patients with abnormal liver function tests, low platelet count and/or LSM ≥ 20 kPa before HCV treatment remain at high risk for development of severe outcomes even after SVR. On the other hand, the regression of LSM ≥ 20% during follow-up after HCV eradication seems to be a significant protective factor. The threshold of 20% for LSM regression after SVR compared to before treatment was chosen as the rounded point nearest to the upper left corner of the ROC curve for LSM decrease to predict LRC or death.
Large sample size studies, such as the ERCHIVES (n = 12,467) and ANRS CO22 Hepather (n = 9,895) cohorts, confirmed that interferon-free HCV treatment significantly decreases all-cause mortality6,16. However, the evaluation of the impact of LSM regression after SVR compared with LSM before HCV treatment was not feasible in both studies. Early regression in LSM after SVR might be related to changes in hepatic inflammation rather than fibrosis. However, a large Canadian cohort of HIV-HCV coinfected study that prospectively examined long-term changes in LSM before and after SVR due to DAAs suggested that LSM following SVR likely reflects true reversal of fibrosis17.
We acknowledge that the prognostic value of post-SVR LSM remains controversial. Pons et al.reported that albumin level at baseline and regression of LSM after SVR during follow-up were independently associated with the risk of HCC in 572 patients with LSM ≥ 10 kPa before treatment during a median follow-up of 2.9 years18. In addition, the regression of LSM at SVR compared to 30-days before the start of HCV treatment was related to lower incidence of LRC in the 640 patients from the HEPAVIR Spanish cohort during a median follow-up of 31 months19. However, this study exclusively included HIV-HCV coinfected patients with LSM ≥ 9.5 kPa and the authors did not exclude those patients with prior hepatic decompensation. Semmler et al. reported that LSM determined after HCV-eradication and its combination with von Willebrand factor antigen and platelet count) could predict post-SVR hepatic decompensation in patients with c-ACLD20. On the other hand, regression of LSM post-SVR were not associated with an increased risk of hepatic decompensation21. However, this study included patients with previous hepatic decompensation and the impact of LSM regression after SVR was evaluated using a lower threshold compared to our study (≥ 20%).
Hepatic venous pressure gradient (HVPG) remains the most robust predictor of clinical decompensation in patients with cirrhosis22. However, HVPG measurement is an invasive method not available worldwide. The role of LSM regression after SVR remains unclear. Despite a markedly decrease in LSM after SVR, this regression seems not to be correlated with HVPG changes in patients with HCV-related cirrhosis and clinically significant portal hypertension before HCV treatment23. On the other hand, Mandorfer et al. have shown that SVR by DAAs ameliorated portal hypertension and the relative change in LSM (per %; HR = 0.972; 95%CI: 0.945–0.999; p = 0.044) was a predictor of a HVPG decrease ≥ 10%24. Additionally, Thabut et al. analyzed data from 891 patients with HCV with biopsy-proven cirrhosis from the French ANRS CirVir cohort25. The authors reported that unfavorable Baveno VI status at baseline was associated with a significantly lower 5-year overall survival compared to those with favorable Baveno VI at baseline. Moreover, progression of portal hypertension in patients who had HCV suppression at inclusion or during follow-up was more frequent in patients with unfavorable Baveno VI status compared to those with favorable Baveno VI. Our findings reinforced the relationship between the presence of portal hypertension before HCV treatment and clinical outcomes post-SVR: unfavorable Baveno VI status before HCV treatment was associated with a worst prognosis leading to an increased risk of clinical outcomes after SVR [adjusted HR = 4.72 (1.32–16.83)] in a multivariate model adjusted for confounding factors. In the current study, similar results were observed when considering the LSM performed most distantly from the end of treatment [median of 12.5 months (IQR, 10.3–14.9)] instead of the closest TE examination [median of 4.8 months (IQR, 3.4–9.8)] following HCV treatment (Supplementary Table 2).
The main limitation of this study remains the lack of paired liver biopsy to assess the relationship of histological regression of liver fibrosis and lower incidence of clinical outcomes after SVR. However, the accuracy of TE to stage liver fibrosis was extensively validated26 and international guidelines have been recommending liver fibrosis staging using LSM by TE in HCV-infected patients27. Additionally, the performance of liver biopsy in SVR patients should be reserved in cases of known or suspected mixed etiologies (e.g. metabolic syndrome, alcoholism or autoimmunity) and for patients who persist with elevated aminotransferases after SVR. We acknowledge that the retrospective study design might be considered a limitation. However, we performed an analysis of a large real-life cohort of patients with HCV treated by DAAs whose medical records for identification of outcomes were reviewed by two trained investigators (JP and CI). We are aware of the relative short follow-up (median time of 2.3 years) in the current analysis. However, in July 2015, first generations of DAAs became available in Brazil and were made available free of charge by the Public Health System (SUS, Sistema Único de Saúde), but only for patients with advanced liver fibrosis/cirrhosis. Universal access to DAAs was implemented by the Brazilian Ministry of Health in mid-2017. Nevertheless, our median follow-up time was similar to previous studies and our study identified 37 outcomes after SVR in 28 patients with c-ACLD (LSM ≥ 10 kPa) before HCV treatment. Other potential criticisms might be the absence of sensitivity analyses for factors associated with all-cause mortality or liver neoplasm and the fact that TE examinations before and after SVR were not performed in pre-determined time-points by the same operator. The performance of stratified analyses considering all outcomes or sensitivity time-dependent analyses for single events separately were not feasible due to the relatively small number of clinical outcomes during follow-up. The limited number of events might be explained by the exclusion of patients with previous hepatic decompensation and the relatively short follow-up period. We acknowledge that the 14 months median time from baseline LSM to initiation of DAAs was longer than desirable. The HCV care pathway in Brazil remains difficult to navigate due to the need of multiples visits before accessing DAA regimens. This complex process can lead to a relatively long time from liver fibrosis assessment by LSM to start of HCV treatment. However, a period of approximatively 1 year is a relative short time within the natural history of chronic hepatitis C in which significant changes in liver fibrosis might take decades to occur. Additionally, we are aware of the lack of spleen stiffness measurement before and after HCV treatment, the absence of evaluation of portal hypertension progression/regression after SVR and the odds of interobserver variability in liver fibrosis staging using LSM by TE in patients with chronic hepatitis C28.
The major strength of our study relies on the large sample size of a real-world cohort of patients with HCV treated by DAAs with reliable paired LSM by TE before HCV treatment and after SVR. To the best of our knowledge, this is the first study which evaluated the impact of LSM regression in the incidence of severe clinical outcomes after HCV eradication in Latin America. Additionally, we included patients with c-ACLD before HCV treatment but without previous hepatic decompensation and the analysis of paired TE examinations were performed considering the same probe to avoid issues related to technical differences between M and XL probes. We acknowledge that TE is not recommended by the current guidelines to monitor fibrosis changes after SVR because the cutoffs and the interval for identifying cirrhosis regression are yet to be defined29. However, this study suggested that the comparison of LSM performed after SVR [at median times of 4.8 and 12.5 months after end of treatment] with that performed before HCV treatment may provide important information regarding the risk of severe outcomes in the following years following SVR.
In conclusion, the findings of this study showed that low albumin and unfavorable Baveno VI status (LSM ≥ 20 kPa or platelet count < 150 × 109/mm3) before HCV treatment were associated with an increased risk of clinical outcome during follow-up of patients with c-ACLD. Additionally, our results also suggested a significant decrease in the incidence of LRC or death associated with regression of LSM after SVR in this population. Non-invasive methods are low-cost alternative for monitoring patients with advanced fibrosis/cirrhosis. The use of simple parameters before HCV treatment and repetition of LSM post-SVR can help to identify patients with different risks of developing severe outcome after HCV eradication.
References
Jakobsen, J. C. et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst. Rev. 6, 12143. https://doi.org/10.1002/14651858.CD012143.pub2 (2017).
Pecoraro, V. et al. New direct-acting antivirals for the treatment of patients with hepatitis C virus infection: A systematic review of randomized controlled trials. J. Clin. Exp. Hepatol. 9(4), 522–538. https://doi.org/10.1016/j.jceh.2018.07.004 (2019).
Falade-Nwulia, O. et al. Oral direct-acting agent therapy for hepatitis c virus infection: A systematic review. Ann. Intern. Med. 166(9), 637–648. https://doi.org/10.7326/M16-2575 (2017).
European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 69(2), 461–511 https://doi.org/10.1016/j.jhep.2018.03.026 (2018).
Ghany, M. G., Morgan, T. R. & Panel A-IHCG. Hepatitis C guidance 2019 update: American Association for the study of liver diseases-infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 71(2), 686–721. https://doi.org/10.1002/hep.31060 (2020).
Carrat, F. et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 393(10179), 1453–1464. https://doi.org/10.1016/s0140-6736(18)32111-1 (2019).
Ioannou, G. N. et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology 157(5), 1264–1278. https://doi.org/10.1053/j.gastro.2019.07.033 (2019).
Erman, A. et al. Estimating chronic hepatitis C prognosis using transient elastography-based liver stiffness: A systematic review and meta-analysis. J. Viral Hepat. 25(5), 502–513. https://doi.org/10.1111/jvh.12846 (2018).
Vergniol, J. et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 140(7), 1970–1979. https://doi.org/10.1053/j.gastro.2011.02.058 (2011).
Stafylidou, M. et al. Performance of Baveno VI and expanded Baveno VI criteria for excluding high-risk varices in patients with chronic liver diseases: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 17(9), 1744–1755. https://doi.org/10.1016/j.cgh.2019.04.062 (2019).
Fernandes, F. F. et al. Effectiveness of direct-acting agents for hepatitis C and liver stiffness changing after sustained virological response. J. Gastroenterol. Hepatol. 34(12), 2187–2195. https://doi.org/10.1111/jgh.14707 (2019).
Singh, S., Facciorusso, A., Loomba, R. & Falck-Ytter, Y. T. Magnitude and kinetics of decrease in liver stiffness after antiviral therapy in patients with chronic hepatitis C: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 16(1), 27–38. https://doi.org/10.1016/j.cgh.2017.04.038 (2018).
de Franchis, R. & Baveno, V. I. F. Expanding consensus in portal hypertension: Report of the Baveno VI consensus workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 63(3), 743–752. https://doi.org/10.1016/j.jhep.2015.05.022 (2015).
Kamarudin, A. N., Cox, T. & Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: Current methods and applications. BMC Med. Res. Methodol. 17(1), 53. https://doi.org/10.1186/s12874-017-0332-6 (2017).
Flynn, R. Survival analysis. J. Clin. Nurs. 21(19–20), 2789–2797. https://doi.org/10.1111/j.1365-2702.2011.04023.x (2012).
Li, D. K. et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology 67(6), 2244–2253. https://doi.org/10.1002/hep.29707 (2018).
Kronfli, N. et al. Liver fibrosis in human immunodeficiency virus (HIV)-hepatitis C virus (HCV) coinfection before and after sustained virologic response: What is the best noninvasive marker for monitoring regression?. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa702 (2020).
Pons, M. et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J. Hepatol. 72(3), 472–480. https://doi.org/10.1016/j.jhep.2019.10.005 (2020).
Corma-Gómez, A. et al. Liver stiffness at the time of sustained virological response predicts the clinical outcome in people living with human immunodeficiency virus and hepatitis C virus with advanced fibrosis treated with direct-acting antivirals. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciz1140 (2019).
Semmler, G. et al. Non-invasive risk stratification after HCV-eradication in patients with advanced chronic liver disease. Hepatology https://doi.org/10.1002/hep.31462 (2020).
Dajti, E. et al. Spleen stiffness measurements predict the risk of hepatic decompensation after direct-acting antivirals in HCV cirrhotic patients. Ultraschall Med. https://doi.org/10.1055/a-1205-0367 (2020).
Ripoll, C. et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 133(2), 481–488. https://doi.org/10.1053/j.gastro.2007.05.024 (2007).
Lens, S. et al. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J. Hepatol. 73(6), 1415–1424. https://doi.org/10.1016/j.jhep.2020.05.050 (2020).
Mandorfer, M. et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J. Hepatol. 65(4), 692–699. https://doi.org/10.1016/j.jhep.2016.05.027 (2016).
Thabut, D. et al. Validation of Baveno VI criteria for screening and surveillance of esophageal varices in patients with compensated cirrhosis and a sustained response to antiviral therapy. Gastroenterology 156(4), 997–1009. https://doi.org/10.1053/j.gastro.2018.11.053 (2019).
Bota, S. et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 33(8), 1138–1147. https://doi.org/10.1111/liv.12240 (2013).
European Association for Study of L & Asociacion Latinoamericana para el Estudio del H. Asociacion Latinoamericana para el Estudio del H EASL-ALEH clinical practice guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 63(1), 237–264. https://doi.org/10.1016/j.jhep.2015.04.006 (2015).
Perazzo, H. et al. Interobserver variability in transient elastography analysis of patients with chronic hepatitis C. Liver Int. 35(5), 1533–1539. https://doi.org/10.1111/liv.12551 (2015).
Ferraioli, G. Review of liver elastography guidelines. J. Ultrasound Med. 38(1), 9–14. https://doi.org/10.1002/jum.14856 (2019).
Funding
This work was supported by funding from INI-FIOCRUZ [Programa de Incentivo à Jovens Pesquisadores for HP grant number INI-003-FIO-19–2-5] and from Estacio de Sa University (UNESA) [Programa Pesquisa e Produtividade for GP]. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.P.: data collection, drafting, interpretation of data and critical revision of the manuscript; G.P.: study concept and design; analysis and interpretation of data and critical revision of the manuscript; L.G., J.D., L.V., C.B.: interpretation of data and critical revision of the manuscript; C.I., R.S., U.C.: data collection; E.P.N., B.G., V.G.V.: interpretation of data and critical revision of the manuscript; F.F.: study concept and design; study supervision, interpretation of data and critical revision of the manuscript; H.P.: study concept and design; study supervision; interpretation of data; statistical analysis; critical revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piedade, J., Pereira, G., Guimarães, L. et al. Liver stiffness regression after sustained virological response by direct-acting antivirals reduces the risk of outcomes. Sci Rep 11, 11681 (2021). https://doi.org/10.1038/s41598-021-91099-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91099-1
This article is cited by
-
Prognostic Effects of Liver Fibrosis and Steatosis Determined Using Transient Elastography in Patients with Chronic Hepatitis B or C
Digestive Diseases and Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.