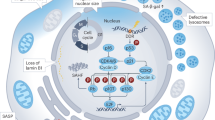
Abstract
The burden of diabetes mellitus (DM) and associated complications is increasing worldwide, affecting many organ functionalities including submandibular glands (SMG). The present study aims to investigate the potential ameliorative effect of glycyrrhizic acid (GA) on diabetes-induced SMG damage. Experimental evaluation of GA treatment was conducted on a rat model of type I diabetes. Animals were assigned to three groups; control, diabetic and GA treated diabetic groups. After 8 weeks, the SMG was processed for assessment of oxidative stress markers, autophagy related proteins; LC3, Beclin-1 and P62, vascular regulator ET-1, aquaporins (AQPs 1.4 and 5), SIRT1 protein expressions in addition to LC3 and AQP5 mRNA expressions. Also, parenchymal structures of the SMG were examined. GA alleviated the diabetes-induced SMG damage via restoring the SMG levels of oxidative stress markers and ET-1 almost near to the normal levels most probably via regulation of SIRT1, AQPs and accordingly LC-3, P62 and Beclin-1levels. GA could be a promising candidate for the treatment of diabetes-induced SMG damage via regulating oxidative stress, autophagy and angiogenesis.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by disturbance in insulin secretion and action1. Subsequent diabetic complications that affect many organs and systems including oral tissue and salivary glands were reported2. Salivary glands play crucial role in maintaining oral homeostasis depending largely on salivary secretions3. Parotid gland, sublingual gland, and submandibular gland (SMG) are the major salivary glands that are responsible for synthesis of saliva, which is an enriched milieu composed mainly of water, electrolytes, and biologically active proteins, including growth factors and cytokines in addition to transporting water and electrolytes3. Specifically, SMG is responsible for production of more than 60% saliva and altering its capacity of secretion will lead to a significant disruption of oral health. It has been reported that during diabetic course, salivary glands are negatively affected, resulting in reduced salivary secretions, periodontal destruction, salivary hypofunction and oral mucosal lesions4.
Though controversial, a relationship was established between diabetic complications and salivary dysfunction in which xerostomia (dry mouth due to reduced salivary flow rate) and polydipsia (pathological thirst) were prevalent in diabetic subjects due to the reduced ability of the salivary gland to secrete saliva2. A recent study showed that diabetes was associated with characteristic histopathological alterations as well as decreased secretion of the SMG without affecting either the sublingual gland or parotid gland3,5.
The pathophysiological mechanism of oral diabetic complications is still not fully elucidated, however the implication of reactive oxygen species (ROS) generation was documented and resulted in oxidative damage of the DNA, proteins, and lipids causing cellular dysfunction6. Moreover, the two processes of oxidative stress and autophagy were reported to be interrelated. Autophagy is considered as a major survival mechanism implied by adaptation of the cells to stress as well as a vital cellular consequence to prevent stem cells damage by extrinsic influences7. Additionally, it is proposed that autophagy may play a crucial role in maintaining irradiated salivary glands8,9.
The central regulator of autophagy is the microtubule-associated protein 1 light chain 3 (LC3) which is involved in autophagosomes formation and has been identified as a biomarker of autophagy10. Interestingly, silent information regulator of transcription1 (SIRT1), a member of sirtuin family, can deacetylate LC3 to initiate autophagosomes formation implying a role of SIRT1 in autophagy modulation11. In aging, SIRT1 is recognized as autophagy substrate and is degraded in cytoplasmic autophagosome–lysosome, via LC11. On the other hand, endothelines (ETs) are family of peptides that are composed of 3 identified isoforms and are well-known for their vasoconstrictive action. ET-1 might be present in many body fluids including saliva, thus the determination of salivary ET-1 might be a useful tool in examining the oral pathological conditions12. Moreover, SIRT1 overexpression has been reported to modulate endothelin (ET-1)13 and oxidative stress in diabetic complications14. Based on these reports, autophagy and SIRT1/ET axis represent valuable targets in diabetes associated oral complications.
Herbal remedies have been used as an effective treatment for chronic diseases, such as diabetes by traditional medicines or even through advanced pharmacological protocols15. Glycyrrhizic acid (GA) is the major active constituent of a Chinese herbal medicine Glycyrrhiza glabra and possesses a wide spectrum of pharmacological actions as antioxidant, anti-inflammatory, antiviral, anticancer, and antidiabetic activities16. Additionally, our team has recently reported its ameliorating effect on salivary gland toxicity induced by sodium nitrite17. In diabetes, much evidence verified the ameliorative effects of GA on associated complications18,19,20.
Several evaluation protocols should be followed to assess the potentiality of GA in diabetes treatment. Experimental evaluation of GA using various molecular techniques that quantify changes in diabetes-related biological markers is part of these protocols16. Therefore, our goal was to investigate the molecular pathophysiological mechanisms of SMG damage in diabetic rats, as well as the possible molecular protective mechanism of GA activity via detecting oxidative stress, SIRT1, autophagy signaling, water channel proteins aquaporins (AQPs) in addition to ET-1 expressions in SMG tissue.
Results
Histopathological analysis
H&E staining revealed almost normal structure of SMG in control group. Acinar cells with basal nuclei and interlobular ducts with prominent granular convoluted ducts (GCD) were found in control group (Fig. 1A). On the other hand, notable atrophy, and degeneration in the diabetic SMG, including vacuolization of acinar cells, pyknotic nuclei and lysis of entire acini and granular convoluted tubules were observed (Fig. 1B,C). These histopathological changes were markedly reduced and almost reversed by the GA treatment (Fig. 1D). Similarly, in semithin sections of diabetic SMG (Fig. 1F), obvious loss of secretory granules in acinar cells and disruption of acinar and ductal structures were found in addition to vacuolation and nuclear changes. These changes were reduced by GA application (Fig. 1G) that restored almost normal structure resembling control group (Fig. 1E).
Haematoxylin and eosin-stained SMG showing parenchymatous structure with acini (asterisks) and interlobular ducts (arrows) with prominent granular convoluted ducts (GCD) in control group (A). Diabetic group showed vacuolation in both acini and GCD, with stagnation of secretions (curved arrows) (B,C). GA treated group (D) revealed an ameliorative effect by reversing the diabetes impact with minimal atrophy and vacuolations. Similarly, in semithin sections of diabetic SMG (F) obvious loss of secretory granules in acinar cells and disruption of acinar and ductal structures were found in addition to vacuolation and nuclear changes, the changes were reduced by GA application (G) that restored almost normal structure resembling control group (E) (H&E, × 20; Semithin sections TB stain, × 20).
GA ameliorated oxidative stress damage in diabetic SMG
Diabetic group showed an oxidative damage of SMG with statistically significant increase in oxidative stress markers MDA (P < 0.001) (Fig. 2A), and statistically significant reduction in GSH (P < 0.001) and SOD (P < 0.001) compared to the control group (Fig. 2B,C). GA application significantly attenuated MDA (P < 0.001) and restored GSH and SOD (P < 0.001, P < 0.05 respectively) compared to the DM group.
Effect of GA treatment on oxidative stress markers in SMG of diabetic rats. (A) MDA, (B) GSH and (C) SOD. Data were expressed as mean ± SE. P < 0.05 was considered significant. ***Significant compared to control group at P < 0.001, #Significant compared to diabetic group at P < 0.05, ###Significant compared to diabetic group at P < 0.001.
GA restored autophagy related markers LC3, P62 and Beclin-1 levels in diabetic SMG
Compared to control group, diabetic group showed a statistically significant increase in brown dot-like staining, indicating both cytoplasmic and nuclear positive reaction especially in the acini for LC3 (P < 0.0001) (Fig. 3A–D,G). Moreover, diabetic group showed statistically significant increase of P62, both cytoplasmic and nuclear positive reaction especially in the ducts, compared to control group (Fig. 3K,J,M) (P < 0.0001). However, GA application significantly ameliorated LC3 (Fig. 3E,F,G) and P62 expression compared to diabetic group (P < 0.0001) (Fig. 3L,M). Similarly, the gene expression of LC3 in diabetic group showed a statistically significant fold increase (P < 0.0001) compared to control group (Fig. 3H). However, GA application significantly decreased LC3 gene expression compared to diabetic group (P < 0.0001) (Fig. 3H). Moreover, Quantitative results of ELISA showed statistically significant increased LC3II and Beclin-1 expression in DM group compared to control group (P < 0.0001 for both), while GA treated group showed a significant reduction in both LC3II and Beclin-1 compared to DM group (P < 0.001, P < 0.0001 respectively) (Fig. 3I,N).
LC3 immunostained SMG sections showing positive nuclear and cytoplasmic reaction in intralobular ducts and GCD (arrows) in control group (A,B), while diabetic group showed strong positive cytoplasmic and nuclear reaction in both acini and ducts (arrows) (C,D). GA treated group (E,F) revealed a positive reaction in ducts only. (LC3, × 10, inserts × 20). Quantitative analysis of LC3 immunostaining (%area) (G) and fold change of LC3 gene expression (H). Quantitative analysis of ELISA results, LC3 II expression (I). Data were expressed as mean ± SE. P < 0.05 was considered significant. ****Significant compared to control group at P < 0.0001, ###Significant compared to diabetic group at P < 0.001and ####Significant compared to diabetic group at P < 0.0001. P62 immunostained SMG sections showing both cytoplasmic and nuclear positive reaction especially in the ducts in DM group (K). While, little mild cytoplasmic reaction and nuclear ductal reaction in both control (J) and GA group (L) (arrows) (P62 IHC, × 20). (M) Quantitative analysis of P62 immunostaining (%area). (N) Effect of GA treatment on Beclin-1expression. Data were expressed as mean ± SE. P < 0.05 was considered significant. ****Significant compared to control group at P < 0.0001, ####Significant compared to diabetic group at P < 0.0001.
GA ameliorated SIRT1 expression and its associated vascular marker
There was a nuclear positive expression of SIRT1 in SMG ducts of both control group and GA treated group (P < 0.0001) (Fig. 4A,C,D), which was not the case in DM group where there was a statistically significant reduction in SIRT1 expression (P < 0.0001) (Fig. 4B,D).
Immunostained SMG sections with SIRT1 showing positive nuclear expression of SIRT1 in both control and GA group (arrows) (A,C). Diabetic group (B) showed reduction of ductal expression (arrow) (SIRT1, IHC, × 20). (D) Quantitative analysis of SIRT1 immunostaining (%area). Data were expressed in mean ± SE. P < 0.05 is considered significant. ****Significant compared to control group at P < 0.0001, ####Significant compared to diabetic group at P < 0.0001.
Moreover, our results revealed ET-1 expression around interlobular ducts and blood vessel in control group (Fig. 5A). DM group showed a statistically significant upregulation in ET-1 expression compared to control group (P < 0.001) (Fig. 5A,B,J), while GA significantly downregulated the ET-1 expression compared to DM group (P < 0.0001) (Fig. 5C,J). Interestingly, AQP1 showed a similar pattern of expression. DM showed increased expression of AQP1 (P < 0.0001) compared to control group (Fig. 5D,E,K), while its expression was significantly downregulated in GA group compared to DM group (P < 0.0001) (Fig. 5F,K). However, the expression of AQP4 showed different pattern, where DM group revealed the least expression (Fig. 5H,L) and both control and GA treated group revealed a higher expression related to the basal part of acini and blood vessels (P value < 0.001) (Fig. 5G,I,L).
ET1 immunostained sections of SMG shows positive brown reaction around interlobular ducts and blood vessels in control group (A), DM group shows mucous changes in the acini with marked expression of ET1 in vascular and acinar components of the parenchyma (B). GA application reduced ET-1 expression (C). AQP1 immunoreaction was localized in the parenchymal vasculature of SMG in control group (D) and GA treated group (F), while this reaction was markedly increased in DM group (E). GA restored AQP4 expression levels in diabetic SMG tissue (I) where diabetic group showed a marked reduction in AQP4 expression (H) compared to the control group (G). (ET-1, AQP1, AQP4 IHC, × 20). Quantitative analysis of immunostaining %area of ET-1 (J), AQP1 (K) and AQP4 (L). Data were expressed in mean ± SE. P < 0.05 is considered significant. ***Significant compared to control group at P < 0.001, ****Significant compared to control group at P < 0.0001, ###Significant compared to diabetic group at P < 0.001. ####Significant compared to diabetic group at P < 0.0001.
GA restored AQP5 gene and protein expressions
Immunohistochemically, the AQP5 was expressed in the control group on the apicolateral membrane of acinar cells (Fig. 6A). However, a cytoplasmic translocation with a statistically significant reduction of apicolateral AQP5 expression was found in DM group (Fig. 6B,D) (P < 0.0001). GA application restored the expression of acinar protein AQP5 significantly when compared with the diabetic group (P value < 0.0001), (Fig. 6C,D). Similarly, GA administration markedly upregulated SMG AQP5 gene expression in comparison to the diabetic group (P value < 0.0001), (Fig. 6E).
AQP5 immunostained sections of SMG shows, apicolateral localization (arrow) in control (A), and GA (C) groups, with apparent degradation and cytoplasmic translocation in DM (curved arrow) and little apicolateral expression (arrow) (B) (AQP5, IHC, × 40)). (D) Quantitative analysis of AQP5 immunostaining (%area) and fold change of AQP5 gene expression (E), Data were expressed in mean ± SE. P < 0.05 is considered significant. ****Significant compared to control group at P < 0.001, ####Significant compared to diabetic group at P < 0.0001.
Discussion
The present study reported for the first time the potential protective effect of GA on diabetes-induced SMG disorder. Herein, we demonstrated marked structural and functional alterations of the SMG in the experimentally induced diabetes on histopathological examination. In agreement with our results, de Souza et al. reported marked structural, functional, and biochemical alterations in salivary glands including SMG in STZ diabetic rats and theses alterations were reversed by ameliorating the hyperglycemic status21.
Our study showed suppressed SIRT1 immunostaining in diabetic SMG accompanied by triggered oxidative stress as evident by increased MDA and reduced cellular antioxidative moieties represented by GSH and SOD. Concurrently, Lee et al. demonstrated a cytoprotective role of SIRT1 against cytokine induced pancreatic LGR-cells injury22. Naderi et al. reported improvement of STZ-induced pancreatic apoptosis by SIRT1 activation23. Moreover, Huang et al. reported the protective role of SIRT1 in diabetic nephropathy via activation of cellular antioxidative mechanisms24. This might be attributed to SIRT1 ability to prevent endothelial cells senescence and vascular injury in addition to ET-1 activation11,23.
Our results showed a restoration of the SMG levels of SIRT1, oxidative stress, ET-1 near to the normal levels following GA administration to diabetic rat. In line, various studies reported valuable pharmacological effects of GA via activation of SIRT125. In this context, Huo et al. explained the reno-protective effect of GA against high glucose-induced tubular proliferation and oxidative stress via SIRT1 dependent mechanism26. Moreover, in the present study, ET-1 expression was increased in the SMG of diabetic rats, suggesting its role in diabetes-induced SMG injury. Interestingly, Ventimiglia et al. showed existence of an endothelinergic system in the SMG and its participation in central and peripheral regulation of SMG secretion. Moreover, ET-1-induced reduction of blood flow suppress the SMG secretory responses to parasympathetic stimulation27. Of note, a recent study reported a suppressive effect of GA on ET-1 in hepatic ischemia–reperfusion injury20.
Our results also showed implication of autophagy dysregulation in the development of DM related changes in the SMG, and associated decline in AQP5 proteins. Concomitantly, Huang et al. reported activation of autophagy in SMGs of both diabetic patients and mice28. Moreover, Zhou et al. reported increased basal level of autophagy with overexpression of P62 by disrupting the association between Beclin-1 and Bcl-2, resulting in Beclin-1 activation29. Of note, Yang et al. documented an efficient anti-tumor activity of GA against hepatocellular carcinoma though inhibition of autophagy25. Moreover, blocking autophagy was linked to the enhancement of GA anti-tumor action against human sarcoma30.
Aquaporins (AQPs) are transmembrane water channel proteins that permit water passage across membrane. Thus, they are widely distributed among water handling organs and exocrine glands as salivary gland. AQP1 is found in myoepithelial and endothelial cells. Therefore, it is not surprising that it was reported to play a crucial role in angiogenesis31. AQP4 is located in the ductal cells and at the basal region of acinar cells32. AQP5 is located in apicolateral membrane of acinar cells and is important for production of the primary saliva in the acini and is proposed to play a critical role in both development and regeneration32. A previous study attributed the SMG dysfunction in diabetes to autophagy activation and associated degradation of AQP528. Herein, our data demonstrated that diabetes was associated with increased expression of AQP1 as well as suppressed expression of AQP4 and AQP5 in SMG. These effects were reversed by GA administration.
In conclusion, the present study clearly indicated that diabetes affected both parenchymatous (acini and ducts) as well as connective tissue of the SMG. The acini, which are responsible for synthesis of saliva, were mostly affected as depicted with AQP5 degradation and autophagy activation. The vasculature supporting the acini and ducts revealed altered expression of AQP1, AQP4 and ET1. On the other hand, GA treatment exhibited an ameliorative effect against aforementioned features of diabetes-induced SMG dysfunction possibly through SIRT1 activation. Further studies are recommended to validate our findings.
Methodology
Ethical statement, study design and allocation
Ethical statement
Approval was obtained from the ethical committee of Faculty of Medicine, Mansoura University (No. R21.05.1328) in accordance with “principles of laboratory animal care NIH publication revised 1985” (Code number: 2020–107). Reporting of all experimental procedures complied with recommendations in ARRIVE guidelines.
Study design and allocation
Randomized, placebo-controlled, blinded animal study was conducted. The sample size was calculated using G power 3.9.1.4 software, to detect a 0.7 effect size between the null hypothesis and the alternative hypothesis with significance level of 0.05 and a power of 0.85, using a one-way ANOVA F-test. Twenty seven male Wistar rats, 100–120 g, were maintained in a controlled temperature (24–26 °C), relative humidity of 60–80% and on a 12-h light–dark cycle for one week acclimatization. Rats were randomly allocated using list randomizer (https://www.random.org/lists) into 3 groups with 9 rats/group as follow; Group1: served as a control, Group 2: represented diabetic rats, and Group 3: denoted as the treated group in which the diabetic rats received intraperitoneal (IP) injection of 100 mg/kg/3 times a week GA (Sigma-Aldrich, St Louis, MO, USA) for 8 weeks33,34.
Diabetes induction
After overnight fasting, rats assigned to groups 2 and 3 were injected with (50 mg/kg/ip) of freshly prepared streptozotocin (STZ) dissolved in citrate buffer, pH 4.5 (STZ, Sigma Chemical Co., St. Louis, MO, USA) while, the control animals in group 1 were injected by an equal volume of the buffer by the same qualified person35. Three days after the STZ injection, animals with stable fasting blood glucose levels at > 250 mg/dl were considered diabetic.
Euthanasia and biopsy collection
After eight weeks of treatment, all rats were anesthetized with Xylazine (5 mg/kg, ADWIA Co. S.A.E 10 of Ramadan city, Egypt) and Ketamine (40 mg/kg, Segmatec Pharmaceutical Industries Co., Egypt) injection into the peritoneum (IP) and euthanized by decapitation (at 8 am to minimize the circadian effect)36,37 and the SMG tissues were collected. The right halves were processed for the histological analysis, and the left halves were snap frozen in liquid nitrogen and kept at − 80 °C until used for oxidative stress estimation, RT-PCR and ELISA techniques.
Histological analysis
The 4 μm sections of paraformaldehyde-fixed and paraffin-embedded SMG tissues were stained with hematoxylin and eosin (H&E). For the semithin sections, tissue biopsies were dehydrated through an ascending series of ethanol (to 100%) and then washed in dry acetone and embedded in epoxy resin then stained with toluidine blue.
Immunohistochemistry (IHC) and image analysis
The protein expression of SIRT1 (Bioss Antibodies, USA, 1:200), ET-1 (Bioss Antibodies, USA, 1:200), AQP1 (Scervicebio Co., USA, 1:1000), AQP4 (Scervicebio Co., USA, 1:1500), AQP5 (ABclonal, USA, 1:200) and autophagy biomarkers LC3 (Abcam, USA, 1:1200), P62 (ABclonal, USA, 1:200) were determined in each group by incubating tissue sections in primary antibodies overnight followed by incubation with secondary antibodies to perform IHC. The visualization of slides was detected using 3,3-Diaminobenzidine (DAB, Abcam, USA), and counterstained with hematoxylin. Then, the sections were analyzed and photographed using an Olympus microscope (Japan) with installed camera. The positive reaction was thresholded and calculated in relation to the surface area using Image J. The data were then decoded and statistically analyzed.
Biochemical analysis of oxidative stress markers
The SMG tissue was homogenized with sodium phosphate buffer, centrifuged, and the supernatant was used for the biochemical analysis. Oxidative stress markers; reduced glutathione (GSH), superoxide dismutase (SOD) and malondialdehyde (MDA) were measured spectrophotometrically38,39.
Assessment of SMG levels of Beclin-1and LC3II
Rat Beclin-1 ELISA Kit (MBS733192) and Rat LC3II ELISA kit (MBS169564) were used for quantitative measurement of Beclin-1and LC3II protein levels in the SMG homogenate according to the manufacturers’ instructions.
Quantitative Assay of LC3 and AQP5 gene expression using RT-PCR
Total RNA was extracted from SMG samples, and then RNA quality and purity were assured. Then cDNA was synthesized from RNA. The cDNA was amplified and used in SYBR Green Based Quantitative Real-Time PCR. For Relative Quantification (RQ) of LC3 gene expression, a primer with Gene Bank Accession No. NM_022867.2, Forward sequence: 5-ACG-GCT-TCC-TGT-ACA-TGG-TC-3 and Reverse sequence: 5-GTG-GGT-GCC-TAC-GTT-CTG-AT was used. And for AQP5, a primer with Gene Bank Accession No. NM_012779.2 was used. The forward primer sequence was 5-GGGCCATCTTGTGGGGATCT-3 and the reverse primer sequence was 5-CCAGTGAGAGGGGCTGAACC-3. The RQ of both genes expression was performed using comparative 2−ΔΔCt method, where the amount of the target genes mRNA were normalized to an endogenous reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and relative to a control40.
Statistical analysis
Data were tested for normal distribution by Shapiro–Wilk test. Quantitative data were analyzed using Graph Prism 8 (GraphPad Software, Inc., CA, USA) to test the significance between different groups using analysis of variance (ANOVA) followed by Tukey’s test. Data were presented as mean ± standard error (SE). Significance was inferred at P < 0.05.
Change history
03 January 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-023-49826-3
References
Regnell, S. E. & Lernmark, Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 60, 1370–1381. https://doi.org/10.1007/s00125-017-4308-1 (2017).
Mauri-Obradors, E., Estrugo-Devesa, A., Jané-Salas, E., Viñas, M. & López-López, J. Oral manifestations of diabetes mellitus. A systematic review. Med. Oral Patol. Oral Cir. Bucal 22, 586–594. https://doi.org/10.4317/medoral.21655 (2017).
Yasser, S. & Shon, A. Histomorphometric and immunohistochemical study comparing the effect of diabetes mellitus on the acini of the sublingual and submandibular salivary glands of albino rats. Open Access Maced. J. Med. Sci. 8, 49–54. https://doi.org/10.3889/oamjms.2020.3722 (2020).
Peralta, I. et al. Larrea divaricata Cav. aqueous extract and nordihydroguariaretic acid modulate oxidative stress in submandibular glands of diabetic rats: A buccal protective in diabetes. BMC Complement. Altern. Med. 19, 227. https://doi.org/10.1186/s12906-019-2636-z (2019).
Chen, S. Y., Wang, Y., Zhang, C. L. & Yang, Z. M. Decreased basal and stimulated salivary parameters by histopathological lesions and secretory dysfunction of parotid and submandibular glands in rats with type 2 diabetes. Exp. Ther. Med. 19, 2707–2719. https://doi.org/10.3892/etm.2020.8505 (2020).
Knaś, M. et al. Oxidative damage to the salivary glands of rats with streptozotocin-induced diabetes-temporal study: Oxidative stress and diabetic salivary glands. J. Diabetes Res. 2016, 4583742. https://doi.org/10.1155/2016/4583742 (2016).
Zhuang, H., Ali, K., Ardu, S., Tredwin, C. & Hu, B. Autophagy in dental tissues: A double-edged sword. Cell Death Dis. 7, e2192. https://doi.org/10.1038/cddis.2016.103 (2016).
Thorburn, A. Autophagy and its effects: Making sense of double-edged swords. PLoS Biol. 12, e1001967. https://doi.org/10.1371/journal.pbio.1001967 (2014).
Morgan-Bathke, M. et al. Autophagy correlates with maintenance of salivary gland function following radiation. Sci. Rep. 4, 5206. https://doi.org/10.1038/srep05206 (2014).
Huang, R. & Liu, W. Identifying an essential role of nuclear LC3 for autophagy. Autophagy 11, 852–853. https://doi.org/10.1080/15548627.2015.1038016 (2015).
Xu, C. et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 22, 1170–1179. https://doi.org/10.1038/s41556-020-00579-5 (2020).
Hoffmann, R. R., Yurgel, L. S. & Campos, M. M. Evaluation of salivary endothelin-1 levels in oral squamous cell carcinoma and oral leukoplakia. Regul. Pept. 166, 55–58. https://doi.org/10.1016/j.regpep.2010.08.006 (2011).
Mortuza, R., Feng, B. & Chakrabarti, S. SIRT1 reduction causes renal and retinal injury in diabetes through endothelin 1 and transforming growth factor β1. J. Cell. Mol. Med. 19, 1857–1867. https://doi.org/10.1111/jcmm.12557 (2015).
Tang, Q., Len, Q., Liu, Z. & Wang, W. Overexpression of miR-22 attenuates oxidative stress injury in diabetic cardiomyopathy via Sirt 1. Cardiovas. Ther. https://doi.org/10.1111/1755-5922.12318 (2018).
Hussan, F., Yahaya, M. F., Teoh, S. L. & Das, S. Herbs for effective treatment of diabetes mellitus wounds: Medicinal chemistry and future therapeutic options. Mini Rev. Med. Chem. 18, 697–710. https://doi.org/10.2174/1389557517666170927155707 (2018).
Wang, Z. H., Hsieh, C. H., Liu, W. H. & Yin, M. C. Glycyrrhizic acid attenuated glycative stress in kidney of diabetic mice through enhancing glyoxalase pathway. Mol. Nutr. Food Res. 58, 1426–1435. https://doi.org/10.1002/mnfr.201300910 (2014).
Elsherbini, A. M., Maysarah, N. M., El-Sherbiny, M., Al-Gayyar, M. M. & Elsherbiny, N. M. Glycyrrhizic acid ameliorates sodium nitrite-induced lung and salivary gland toxicity: Impact on oxidative stress, inflammation and fibrosis. Hum. Exp. Toxicol. 40, 707–721. https://doi.org/10.1177/0960327120964555 (2021).
Hou, S., Zhang, T., Li, Y., Guo, F. & Jin, X. Glycyrrhizic acid prevents diabetic nephropathy by activating AMPK/SIRT1/PGC-1α signaling in db/db mice. J. Diabetes Res. 2017, 2865912. https://doi.org/10.1155/2017/2865912 (2017).
Li, Y. et al. Ability of post-treatment glycyrrhizic acid to mitigate cerebral ischemia/reperfusion injury in diabetic mice. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 26, e926551. https://doi.org/10.12659/msm.926551 (2020).
Kou, X., Zhu, J., Xie, X., Hao, M. & Zhao, Y. The protective effect of glycyrrhizin on hepatic ischemia-reperfusion injury in rats and possible related signal pathway. Iran. J. Basic Med. Sci. 23, 1232–1238. https://doi.org/10.22038/ijbms.2020.44101.10334 (2020).
de Souza, D. N. et al. Effect of tungstate administration on the lipid peroxidation and antioxidant parameters in salivary glands of STZ-induced diabetic rats. Biol. Trace Ele. Res. 199, 1525–1533. https://doi.org/10.1007/s12011-020-02273-x (2021).
Lee, J. H. et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes 58, 344–351. https://doi.org/10.2337/db07-1795 (2009).
Naderi, R., Shirpoor, A., Samadi, M., Pourheydar, B. & Moslehi, A. Tropisetron attenuates pancreas apoptosis in the STZ-induced diabetic rats: Involvement of SIRT1/NF-κB signaling. Pharmacol. Rep. PR 72, 1657–1665. https://doi.org/10.1007/s43440-020-00146-7 (2020).
Huang, K. et al. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic. Biol. Med. 65, 528–540. https://doi.org/10.1016/j.freeradbiomed.2013.07.029 (2013).
Chen, J. et al. 18β-Glycyrrhetinic-acid-mediated unfolded protein response induces autophagy and apoptosis in hepatocellular carcinoma. Sci. Rep. 8, 9365. https://doi.org/10.1038/s41598-018-27142-5 (2018).
Hou, S., Zheng, F., Li, Y., Gao, L. & Zhang, J. The protective effect of glycyrrhizic acid on renal tubular epithelial cell injury induced by high glucose. Int. J. Mol. Sci. 15, 15026–15043. https://doi.org/10.3390/ijms150915026 (2014).
Harrison, A. P., Cunningham, M. E. & Edwards, A. V. Effects of endothelin on submandibular salivary responses to parasympathetic stimulation in anaesthetized sheep. Auton. Neurosci. Basic Clin. 99, 47–53. https://doi.org/10.1016/s1566-0702(02)00062-0 (2002).
Huang, Y. et al. Aquaporin 5 is degraded by autophagy in diabetic submandibular gland. Science China. Life sciences 61, 1049–1059. https://doi.org/10.1007/s11427-018-9318-8 (2018).
Zhou, L. et al. Bcl-2-dependent upregulation of autophagy by sequestosome 1/p62 in vitro. Acta Pharmacol. Sin. 34, 651–656. https://doi.org/10.1038/aps.2013.12 (2013).
Shen, S. et al. Blocking autophagy enhances the apoptotic effect of 18β-glycyrrhetinic acid on human sarcoma cells via endoplasmic reticulum stress and JNK activation. Cell Death Dis. 8, e3055. https://doi.org/10.1038/cddis.2017.441 (2017).
Yin, T. et al. Correlation between the expression of aquaporin 1 and hypoxia-inducible factor 1 in breast cancer tissues. J. Huazhong Univ. Sci. Technol. Med. Sci. 28, 346–348. https://doi.org/10.1007/s11596-008-0327-y (2008).
D’Agostino, C. et al. Insight into salivary gland aquaporins. Cells https://doi.org/10.3390/cells9061547 (2020).
Zeece, M. In Introduction to the Chemistry of Food (ed. Zeece, M.) 213–250 (Academic Press, 2020).
Fernando, H. A. et al. Glycyrrhizic acid can attenuate metabolic deviations caused by a high-sucrose diet without causing water retention in male Sprague-Dawley rats. Nutrients 6, 4856–4871 (2014).
Furman, B. L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 70, 54741–454720. https://doi.org/10.1002/0471141755.ph0547s70 (2015).
Leary, S. AVMA Guidelines for the Euthanasia of Animals, 2013 edn. Journal of the American Veterinary Medical Association. https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (2013).
Ko, M. J., Mulia, G. E. & van Rijn, R. M. Commonly used anesthesia/euthanasia methods for brain collection differentially impact MAPK activity in male and female C57BL/6 mice. Front. Cell. Neurosci. 13, 96–96. https://doi.org/10.3389/fncel.2019.00096 (2019).
Rendra, E. et al. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 224, 242–253. https://doi.org/10.1016/j.imbio.2018.11.010 (2019).
Timasheva, Y. R. et al. Multilocus associations of inflammatory genes with the risk of type 1 diabetes. Gene 707, 1–8. https://doi.org/10.1016/j.gene.2019.04.085 (2019).
Wang, J. et al. Aberrant expression of beclin-1 and LC3 correlates with poor prognosis of human hypopharyngeal squamous cell carcinoma. PLoS ONE 8, e69038. https://doi.org/10.1371/journal.pone.0069038 (2013).
Acknowledgements
The authors deeply acknowledge the Researchers Supporting program (TUMA-Project-2021-3), Almaarefa University, Riyadh, Saudi Arabia for supporting steps of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this research. Maysarah was added for her major contribution in revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-023-49826-3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asseri, S.M., Elsherbiny, N.M., El-Sherbiny, M. et al. RETRACTED ARTICLE: Glycyrrhizic acid ameliorates submandibular gland oxidative stress, autophagy and vascular dysfunction in rat model of type 1 diabetes. Sci Rep 12, 725 (2022). https://doi.org/10.1038/s41598-021-04594-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04594-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.