Abstract
We investigated the chemical pressure effects on structural and electronic properties of SnTe-based material using partial substitution of Sn by Ag0.5Bi0.5, which results in lattice shrinkage. For Sn1−2x(AgBi)xTe, single-phase polycrystalline samples were obtained with a wide range of x. On the basis of band calculations, we confirmed that the Sn1−2x(AgBi)xTe system is basically possessing band inversion and topologically preserved electronic states. To explore new superconducting phases related to the topological electronic states, we investigated the In-doping effects on structural and superconducting properties for x = 0.33 (AgSnBiTe3). For (AgSnBi)(1−y)/3InyTe, single-phase polycrystalline samples were obtained for y = 0–0.5 by high-pressure synthesis. Superconductivity was observed for y = 0.2–0.5. For y = 0.4, the transition temperature estimated from zero-resistivity state was 2.4 K, and the specific heat investigation confirmed the emergence of bulk superconductivity. Because the presence of band inversion was theoretically predicted, and the parameters obtained from specific heat analyses were comparable to In-doped SnTe, we expect that the (AgSnBi)(1−y)/3InyTe and other (Ag, In, Sn, Bi)Te phases are candidate systems for studying topological superconductivity.
Similar content being viewed by others
Introduction
Metal tellurides (MTe) with a NaCl-type structure have been extensively studied due to their physical properties as topological materials1,2,3,4, thermoelectric materials5,6,7, and superconductors8,9,10,11,12. Among them, In-doped SnTe superconductors have been drawing attention as a candidate system of a topological superconductor4,13,14,15,16,17. SnTe is a topological crystalline insulator, and superconductivity is typically induced by In-doping at the Sn site. The superconducting transition temperature (Tc) of (Sn, In)Te increases by In doping. Although a simple picture proposes that doped In acts as a hole dopant, detailed analyses of carrier characteristics, superconducting properties, and electronic states of (Sn, In)Te revealed that the In doping does not simply act as a dopant of holes, but the superconductivity emerges in a regime where electron carriers are dominant16. Therefore, to understand the nature and the mechanisms of superconductivity in SnTe-based, development of new superconductors based on NaCl-type tellurides is important.
In MTe, the M site can be alloyed flexibly. For example, single crystals (films) of (Sn, Pb)Te can by grown with a wide solution range, and the alloy system has provided a platform to study topological nature of MTe1,18,19. Because the Pb substitution for SnTe expands the lattice, which corresponds to negative chemical pressure at the M–Te bond, contrasting positive chemical pressure in MTe leads the way for further expanding the research field of superconductivity in MTe. In addition, although In-doped systems of (In, Sn, Pb)Te have been studied as a topological superconductor candidate20, there has been no detailed study on superconducting properties and crystal structure of NaCl-type MTe with a lattice constant smaller than SnTe. In this study, we focused on the (Ag, Sn, Bi)Te system that has been studied as a thermoelectric material and found that the (Ag, Sn, Bi)Te system is possible topological material21,22. In SnTe, Sn is divalent, Sn2+. When Ag+0.5Bi3+0.5 substitutes Sn2+, the total valence states (charge neutrality) has been preserved. Therefore, the Ag0.5Bi0.5 substitution for the Sn site is successfully achieved in a wide range up to the end member of AgBiTe2. Here, we show the evolution of the structural and electronic characteristics of Sn1−2x(AgBi)xTe. Then, we report superconductivity induced by In substitution in (AgSnBi)(1−y)/3InyTe.
Results and discussion
Structural and electronic characteristics of Sn1−2x(AgBi)xTe
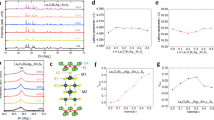
As mentioned above, the (Ag, Sn, Bi)Te system would be an important system to expand the material variety of SnTe-based compounds including new superconductors. We started this work by investigating lattice compression in Sn1−2x(AgBi)xTe, in which x corresponds to the total amount of Ag and Bi substituted for the Sn site in the SnTe structure. Polycrystalline samples of Sn1−2x(AgBi)xTe were synthesised by a melting method. Figure 1a shows the X-ray diffraction (XRD) patterns for Sn1−2x(AgBi)xTe. The XRD peaks correspond to that expected for the NaCl-type structure (Fig. 1b) and systematically shift to higher angles, which indicates lattice shrinkage with increasing x. The lattice constant a was determined by Rietveld refinements and plotted in Fig. 1c. The trend is consistent with previous reports20,21. In the refinements, Ag, Sn, and Bi were assigned to the M site as shown in Fig. 1b, and the nominal composition (fixed) was used. Since we used laboratory XRD in this study, isotropic displacement parameter Biso was fixed to 1 for all sites.
Figure 2 shows the calculated band structure for SnTe with various lattice constants of 6.6, 6.4, 6.3 (close to the lattice constant of SnTe), and 6.1 Å. By calculating the lowest energy of the Sn-p orbital and the highest energy of the Te-p orbital at the L point in reciprocal space, we confirmed that a band inversion transition occurs at around a = 6.35 Å (Fig. 2e). Note that the contributions of Sn-p orbitals are represented by the size of the circle symbols in Fig. 2a–d. Furthermore, calculation results with a smaller lattice constant show that SnTe-based materials with a small lattice constant of 6.1 Å also shows a band inversion. The observed trend is consistent with a previous theoretical work on SnTe and PbTe systems2. Therefore, we consider that the current system of Sn1−2x(AgBi)xTe basically possesses a topologically-preserved band structure with a wide range of x. Although calculations in this work have been performed on SnTe with different lattice constants, no obvious modification of the band structure is expected when Sn was partially replaced by Ag0.5Bi0.5 in real materials because the difference in spin–orbit interactions expected from those elements is not large. Since the orbital characteristics in the band structure does not largely change between SnTe and Sn1−2x(AgBi)xTe, we consider that the topological invariant in this system is mirror Chern number from the analogy to SnTe, which suggests that the Sn1−2x(AgBi)xTe system is a potential topological crystalline insulator. On the basis of the investigations of lattice constant and band structure for the SnTe-based system Sn1−2x(AgBi)xTe, we selected AgSnBiTe3 (x = 0.33) for a parent phase in which In-substitution effects are examined in this study.
Electronic structure for SnTe with various lattice constants. (a–d) Calculated band structure. Contribution of Sn-p orbitals is represented by the size of pink-circle symbol. According to the calculations, a band inversion occurs with lattice constants smaller than 6.35 Å as plotted in (e). Experimental data in Ref.23 is plotted. Gap energy was calculated from the lowest energy of the Sn-p orbital and the highest energy of the Te-p orbital at the L point.
Superconducting properties of (AgSnBi)(1−y)/3InyTe
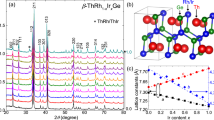
For the In-doped AgSnBiTe3 system (see Fig. 3c for crystal structure), we used a chemical formula, (AgSnBi)(1−y)/3InyTe, because the In amount doped to the parent phase of AgSnBiTe3 can be easily understood. Figure 3a shows the powder XRD patterns of (AgSnBi)(1−y)/3InyTe; the In-doped samples were synthesised by high-pressure annealing. As shown in Fig. 3b, Rietveld refinement reveals that tiny (shoulder) anomaly was observed. Such a shoulder structure would be due to the presence of inhomogeneous regime with a slightly different lattice constant and was observed in NaCl-type tellurides containing multiple M-site elements24,25. In particular, we tested several annealing conditions for y = 0.4, and the condition described in the “Methods” section was found to be the best. As shown in Fig. 3d, the lattice constant decreases with increasing y, which is a trend similar to other In-doped M–Te systems8,12. The actual element concentrations of the samples were examined by energy-dispersive X-ray spectroscopy (EDX), and the results are shown in Fig. S1 (“Supplementary information”). Basically, the actual compositions were close to the nominal values.
In Fig. 4, the temperature dependence of electrical resistivity of AgSnBiTe3 (x = 0.33, y = 0) is displayed. Resistivity slightly decreases with decreasing temperature, and an increase in resistivity was observed at low temperatures. The result is consistent with the band calculations, based on SnTe lattice, in Fig. 2, and hence, the parent phase AgSnBiTe3 would possess a narrow band gap. Since the calculated gap energy (Fig. 2e) indicates that a band inversion is expected for a metal telluride with a = 6.2 Å, AgSnBiTe3 with a = 6.20217(9) Å is expected to have topologically preserved electronic states near the Fermi energy (EF). Thus, the In-substitution effects on physical properties of AgSnBiTe3 are of interest because of the analogy to (Sn, In)Te, in which topological superconductivity is expected to emerge. As expected, In-doped AgSnBiTe3 shows superconductivity as displayed in Fig. 4. The resistivity data for y = 0.4 shows a metallic behavior and zero resistivity was observed at Tczero = 2.4 K. For the other samples with different In concentration, the temperature dependence of resistivity are shown in Fig. S2 (“Supplementary information”). The sample with y = 0.1 shows almost no temperature dependence in resistivity, but other samples (y = 0.2–0.5) show metallic conductivity.
To investigate upper critical field (Bc2), temperature dependences of resistivity were measured under various magnetic fields up to 1.5 T as plotted in Fig. 5a. For the estimation of Bc2, the transition temperature was determined as a temperature where resistivity drops to 50% of the normal-state resistivity. Using the WHH model (Werthamer–Helfand–Hohenberg model)26, which is applicable for a dirty-limit type-II superconductor, the Bc2(0) was estimated as 1.2 T, as shown in Fig. 5b.
Estimation of upper critical field Bc2 for y = 0.4. (a) Temperature dependences of resistivity under magnetic fields up to 1.5 T. With increasing field, the Tc systematically decreases. (b) Temperature dependence of Bc2. For the plot of Bc2, middle-point temperatures, where resistivity became 50% of normal-state resistivity at 3 K, were estimated in (a). The dotted line shows WHH fitting.
Figure 6 shows the analysis results of specific heat measurements. In Fig. 6a, specific heat data under 0 and 5 T in a form of C/T are plotted as a function of T2. From the data under 5 T, the electronic specific heat parameter (γ) and the Debye temperature (θD) were estimated as 2.35(5) mJ mol−1 K−2 and 186 K, respectively. The low-temperature specific-heat formula with anharmonic term was used for the analysis: C = γT + βT3 + δT5, where βT3 is the lattice contribution to the specific heat, and the δT5 term accounts for anharmonicity of the lattice. The θD was calculated from β = (12/5)π4(2N)kBθD−3, where N and kB are the Avogadro constant and Boltzmann constant, respectively. To characterize the superconducting properties, the electronic contribution (Cel) under 0 T, which was calculated by subtracting lattice contributions from the specific heat data under 0 T, is plotted in the form of Cel/T as a function of temperature in Fig. 6b. The clear jump of Cel/T and decrease in Cel/T at low temperatures suggest the emergence of bulk superconductivity. The superconducting jump in Cel (ΔCel) estimated with Tc = 2.16 K is 1.34γTc, which is comparable to the value expected from a full-gap superconductivity based on the BCS model (ΔCel = 1.43γTc)27. Those values obtained from specific heat are similar to those obtained for In-doped SnTe and Ag-doped SnTe superconductors with 20% In (or Ag) doping8,28.
Specific heat data for y = 0.4. (a) Specific heat (C/T) data under magnetic fields (B) of 0 and 5 T plotted as a function of T2. By fitting the data under B = 5 T, electronic specific heat parameter (γ) and Debye temperature (θD) were estimated. (b) Temperature dependence of the electronic contribution (Cel/T) under B = 0 T. The electronic contribution was calculated by subtracting the lattice contribution. Tc and the superconducting jump at Tc (ΔC) were estimated by considering the entropy balance, as guided by the red dashed lines. ΔC/γTc = 1.34, which is close to BCS value of 1.43.
To investigate the In-doping dependence of superconducting states, temperature dependences of magnetic susceptibility (4πχ) was measured for (AgSnBi)(1−y)/3InyTe. For, y = 0 and 0.1, no superconducting transition was observed at temperatures above 1.8 K. Superconducting diamagnetic signals were observed for y = 0.2–0.5, as displayed in Fig. 7a. Particularly, samples with y = 0.3 and 0.4 showed a large shielding fraction. Note that the data has not been corrected by demagnetisation effect. The observation of the largest shielding volume fraction is consistent with the bulk superconductivity confirmed in specific heat measurements. As shown in Fig. 7b, Tc estimated from magnetic susceptibility (Tcmag) becomes the highest at y = 0.4 and decreases with further In doping (y = 0.5).
The estimated Tc for y = 0.4 is lower than that for (Sn, In)Te (Tc ~ 4.5 K)16. The difference may be caused by three possible reasons: (1) carrier concentration, (2) the effect of disorder, and (3) lattice constant. On (1) carrier concentration, we performed measurements of Seebeck coefficient (at room temperature) and Hall coefficient (at 5 K for y = 0.4). Figure 8 shows the y dependence of Seebeck coefficient (S). For y = 0, a large positive value of S was observed. This is consistent with the band calculation, indicating that the parent phase of y = 0 is a semiconductor. With increasing y, S becomes negative, and the absolute value for y > 0.1 becomes less than 10 μV K−1, which is a typical value of metals. In Fig. S3 (“Supplementary information”), the magnetic field dependence of Hall resistance at 5 K is plotted. By linear fitting of the data and assuming a single-band model, carrier concentration was calculated as 7.4 × 1021 cm−3. These results on the evolution of carrier concentration by In substitution in (AgSnBi)(1−y)/3InyTe would suggest that electrons are doped by In3+ substitution, and the doping situation in the present system is clearly different from that observed in (Sn, In)Te16. Therefore, to investigate the relationship between superconducting properties and carrier concentration in (AgSnBi)(1−y)/3InyTe, further investigation with various probes is needed. On the effect of (2) disorder, it is a fact that high configurational entropy of mixing is present at the M site as described in the following discussion. However, comparison of Tc between y = 0.4 (M = Ag, In, Sn, Bi) and other MTe superconductors, for example, M = In (Tc ~ 3 K)9,12 and M = Sn0.8Ag0.2 (Tc = 2.3 K)11, suggests that the higher disorder in y = 0.4 (M = Ag, In, Sn, Bi) is not highly affecting Tc. On the effect of (3) lattice constant, the electronic structure of SnTe-based materials is modified by lattice constant as shown above. In addition, in Ref.25, we showed that Tc of MTe shows a positive relation to lattice constant. Therefore, the Tc obtained for y = 0.4 would be reasonable to the trend of Tc-lattice constant for MTe. According to those facts, we consider that the difference in Tc between y = 0.4 and (Sn, In)Te is caused by the difference in the electronic states (carrier concentration and/or lattice constant).
Solubility limit and phase stabilisation by configurational entropy of mixing
The doping phase diagram for superconductivity of (AgSnBi)(1−y)/3InyTe is compared with that for the (Sn, In)Te and (Pb, In)Te systems in the following discussion. Having looked at the phase diagrams of (Sn, In)Te and (Pb, In)Te8,12, superconductivity is observed in a wide range of In concentration. As shown in Fig. 7b, however, the superconducting properties (Tc and shielding volume fraction) becomes the highest for y = 0.4, and superconductivity seems to be suppressed for further In doping. Therefore, the trend of In-doping effect on superconductivity in the AgSnBiTe3 system is different from that in the SnTe and PbTe systems. We consider that the suppression of superconductivity is caused by the solution limit of In rather than the changes in electronic states.
We tried to synthesise samples with y > 0.5, but a high-purity sample was not obtained. In addition, the Bi amount for y = 0.5 deviates from the nominal value with a large error, which means that the sample with y = 0.5 also contains inhomogeneity larger than those in y < 0.5. Seeing lattice constants for In-doped samples, we find that the lattice constant is smaller than the end member of InTe with a ~ 6.175 Å8. Therefore, we consider that the suppression of superconductivity observed for y = 0.5 is due to the increase in inhomogeneity in the sample, and the solution limit of In for AgSnBiTe3 is around y = 0.4 under pressures up to 2 GPa. As a fact, y = 0.4 samples show degradation of superconducting properties by passing time. Thus, in this study, investigations of superconducting properties of y = 0.4 have been performed in 24 h after the high-pressure synthesis. Because the (Ag,In,Sn,Bi)Te system is a kind of disordered system with high configurational entropy of mixing (ΔSmix), we briefly discuss the possible explanation of the solution limit of In in (Ag, In, Sn, Bi)Te. As established in a field of high-entropy alloys, the phase of multiple-element system can be stabilized owing to high ΔSmix29,30, which decreases Gibbs free energy, ΔG = ΔH − TΔS, at high temperatures, where H is enthalpy. ΔSmix values for Sn1−2x(AgBi)xTe and (AgSnBi)(1−y)/3InyTe were calculated using ΔSmix = − RΣicilnci, where ci and R are the atomic fraction of component i and the gas constant, respectively. As shown in Fig. 9a, for Sn1−2x(AgBi)xTe, ΔSmix is relatively high with a wide range of x, which would be the reason why the phases could be obtained with a wide range of x without the use of high-pressure synthesis. For In doping, we selected AgSnBiTe3 (x = 0.33) as a parent phase in this study. For (AgSnBi)(1−y)/3InyTe, ΔSmix becomes the highest at y = 0.3, and it decreases with further In substitution, as shown in Fig. 9b. Therefore, the possible explanation of the solution limit of In for AgSnBiTe3 is as follows. Due to the small lattice constant of ~ 6.2 Å for AgSnBiTe3, In substitution was challenging, but the phase was stabilized up to y = 0.4 by the use of high pressure and high ΔSmix. If we could get a phase with a higher In concentration, the superconducting phase diagram would be expanded from that shown in Fig. 7b.
Summary
We have synthesised polycrystalline samples of Sn1−2x(AgBi)xTe and (AgSnBi)(1−y)/3InyTe to explore a new candidate phase of topological superconductor. For Sn1−2x(AgBi)xTe, single-phase samples were obtained with a wide range of x. According to band calculations, we confirmed that the Sn1−2x(AgBi)xTe system is basically possessing band inversion and topologically preserved electronic states. To investigate the effects of In substitution, we selected x = 0.33 (AgSnBiTe3) as a parent phase. For (AgSnBi)(1−y)/3InyTe, In-doped samples were obtained for y = 0–0.5 by high-pressure synthesis, and superconductivity was observed for y = 0.2–0.5. For y = 0.4, specific heat investigation confirmed the emergence of bulk superconductivity. Although the current study is the material exploration with polycrystalline samples, we expect that the single crystals of (Ag,In,Sn,Bi)Te are grown in the next step, and characteristics including surface states, which are expected for a topological superconductor, are experimentally examined by surface-sensitive probes like angle-resolved photoemission spectroscopy.
Methods
Polycrystalline samples of Sn1−2x(AgBi)xTe (x = 0.00, 0.05, 0.10, 0.15, 0.20, 0.25, 0.30, 0.33, 0.35, 0.40, 0.45) and (AgSnBi)(1−y)/3InyTe (y = 0.0, 0.1, 0.2, 0.3, 0.4, 0.5) were prepared by a melting method in an evacuated quartz tube. Powders or grains of Ag (99.9% up), Sn (99.99%), Bi (99.999%), In (99.99%), and Te (99.999%) were mixed and melted in an evacuated quartz tube at 900 °C for 10 h, followed by furnace cooling to room temperature. For (AgSnBi)(1−y)/3InyTe, the obtained samples were powdered and annealed in a high-pressure-synthesis instrument under 2 GPa at 500 °C for 30 min. A cubic-anvil-type 180-ton press was used, and the sample sealed in a BN crucible was heated by carbon heater.
The phase purity and the crystal structure of Sn1−2x(AgBi)xTe and (AgSnBi)(1−y)/3InyTe were examined by laboratory X-ray diffraction (XRD) by the θ–2θ method with a Cu–Kα radiation on a MiniFlex600 (RIGAKU) diffractometer equipped with a high-resolution detector D/tex Ultra. The schematic images of crystal structures were drawn by VESTA31 using a structural data refined by Rietveld refinement using RIETAN-FP32. The actual compositions of the examined samples were analysed using an energy dispersive X-ray spectroscopy (EDX) on TM-3030 (Hitachi).
The temperature dependence of magnetic susceptibility was measured using a superconducting quantum interference devise (SQUID) on MPMS-3 (Quantum Design) after zero-field cooling (ZFC) with an applied field of 10 Oe. The temperature dependence of electrical resistivity was measured by a four-probe method with an applied DC current of 1 mA on PPMS (Quantum Design) under magnetic fields. We used Ag paste and Au wires (25 μm in diameter) for the four-probe setup. The temperature dependence of specific heat was measured under 0 and 5 T by a relaxation method on PPMS. The resistivity and specific heat measurements were performed using a 3He probe system (Quantum Design). Hall coefficient was measured by four-probe setup on PPMS at low temperatures. Hall coefficient was estimated from the slope in the magnetic field dependence of Hall voltage. Seebeck coefficient at room temperature was measured under steady-state, where the thermo-electromotiveforce (∆V) and the temperature difference (∆T) were simultaneously measured, and the S was determined from the slope of ∆V/∆T.
First principles band calculations were performed using WIEN2k package33. The electronic density of PbTe and SnTe was self-consistently calculated within the modified Becke–Johnson potential34 using a 12 × 12 × 12 k-mesh and RKmax = 9 with the spin–orbit coupling included.
References
Tanaka, Y. et al. Experimental realization of a topological crystalline insulator in SnTe. Nat. Phys. 8, 800 (2012).
Hsieh, T. H. et al. Topological crystalline insulators in the SnTe material class. Nat. Commun. 3, 982 (2012).
Ando, Y. Topological insulator materials. J. Phys. Soc. Jpn. 82, 102001 (2013).
Sasaki, S. et al. Odd-parity pairing and topological superconductivity in a strongly spin–orbit coupled semiconductor. Phys. Rev. Lett. 109, 217004 (2012).
Zhong, Y. et al. Optimized strategies for advancing n-type PbTe thermoelectrics: A review. ACS Appl. Mater. Interfaces 12, 49323 (2020).
Zhang, Q. et al. High thermoelectric performance by resonant dopant indium in nanostructured SnTe. Proc. Natl. Acad. Sci. 110, 13261 (2013).
Chen, Y. et al. SnTe–AgSbTe2 thermoelectric alloys. Adv. Energy Mater. 2, 58 (2012).
Kobayashi, K., Ai, Y., Jeschke, H. O. & Akimitsu, J. Enhanced superconducting transition temperatures in the rocksalt-type superconductors In1−xSnxTe (x ≤ 0.5). Phys. Rev. B 97, 104511 (2018).
Kriener, M. et al. Tailoring band structure and band filling in a simple cubic (IV, III)-VI superconductor. Phys. Rev. Mater. 2, 044802 (2018).
Erickson, A. S. et al. Enhanced superconducting pairing interaction in indium-doped tin telluride. Phys. Rev. B 79, 024520 (2009).
Mizuguchi, Y. & Miura, O. High-pressure synthesis and superconductivity of Ag-doped topological crystalline insulator SnTe (Sn1−xAgxTe with x = 0–0.5). J. Phys. Soc. Jpn. 85, 053702 (2016).
Katsuno, M. et al. High-Pressure Synthesis and Superconducting Properties of NaCl-Type In1−xPbxTe (x = 0–0.8). Condens. Matter 5, 14 (2020).
Balakrishnan, G., Bawden, L., Cavendish, S. & Lees, M. R. Superconducting properties of the In-substituted topological crystalline insulator SnTe. Phys. Rev. B 87, 140507 (2013).
Novak, M. et al. Unusual nature of fully gapped superconductivity in In-doped SnTe. Phys. Rev. B 88, 140502 (2013).
Zhong, R. D. et al. Optimizing the superconducting transition temperature and upper critical field of Sn1−xInxTe. Phys. Rev. B 88, 020505 (2013).
Haldolaarachchige, N. et al. Anomalous composition dependence of the superconductivity in In-doped SnTe. Phys. Rev. B 93, 024520 (2016).
Ando, Y. & Fu, L. Topological crystalline insulators and topological superconductors: from concepts to materials. Annu. Rev. Condens. Matter Phys. 6, 361 (2015).
Yan, C. et al. Experimental observation of Dirac-like surface states and topological phase transition in Pb1−xSnxTe(111) films. Phys. Rev. Lett. 112, 186801 (2014).
Zhang, C. L. et al. Highly tunable topological system based on PbTe–SnTe binary alloy. Phys. Rev. Mater. 4, 091201 (2020).
Du, G. et al. Fully gapped superconductivity in In-doped topological crystalline insulator Pb0.5Sn0.5Te. Phys. Rev. B 92, 020512 (2015).
Tan, G. et al. SnTe–AgBiTe2 as an efficient thermoelectric material with low thermal conductivity. J. Mater. Chem. A 2, 20849 (2014).
Mashadieva, L. F. et al. The Ag2Te–SnTe–Bi2Te3 system and thermodynamic properties of the (2SnTe)1–x(AgBiTe2)x solid solutions series. J. Alloy Compd. 219, 310 (1995).
Springholz, G. Molecular beam epitaxy of IV-VI semiconductors: multilayers, quantum dots and device applications. in Molecular Beam Epitaxy: From Research to Mass Production (ed. Henini, M.) (Elsevier, 2013).
Mizuguchi, Y. Superconductivity in high-entropy-alloy telluride AgInSnPbBiTe5. J. Phys. Soc. Jpn. 88, 124708 (2019).
Kasem, M. R. et al. Superconducting properties of high-entropy-alloy tellurides M–Te (M: Ag, In, Cd, Sn, Sb, Pb, Bi) with a NaCl-type structure. Appl. Phys. Express 13, 033001 (2020).
Werthamer, N. R., Helfand, E. & Hohenberg, P. C. Temperature and purity dependence of the superconducting critical field, Hc2. III. Electron spin and spin–orbit effects. Phys Rev. 147, 295 (1966).
Bardeen, J., Cooper, L. N. & Schrieffer, J. R. Microscopic theory of superconductivity. Phys. Rev. 106, 162 (1957).
Mizuguchi, Y. et al. Specific heat and electrical transport properties of Sn0.8Ag0.2Te superconductor. J. Phys. Soc. Jpn. 85, 103701 (2016).
MacDonald, B. E. et al. Recent progress in high entropy alloy research. JOM 69, 2024 (2017).
Ng, C. et al. Entropy-driven phase stability and slow diffusion kinetics in an Al0.5CoCrCuFeNi high entropy alloy. Intermetallics 31, 165 (2012).
Momma, K. & Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41, 653–658 (2008).
Izumi, F. & Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 130, 15–20 (2007).
Blaha, P. et al. WIEN2k: An APW+lo program for calculating the properties of solids. J. Chem. Phys. 152, 074101 (2020).
Tran, F. & Blaha, P. Accurate band gaps of semiconductors and insulators with a semilocal exchange-correlation potential. Phys. Rev. Lett. 102, 226401 (2009).
Acknowledgements
The authors thank O. Miura for experimental supports. This work was partly supported by JSPS KAKENHI (Grant nos. 18KK0076, 21H00151, and 21K18834) and Advanced Research Program under the Human Resources Funds of Tokyo (Grant number: H31-1).
Author information
Authors and Affiliations
Contributions
Y.M. and Y.G. led the project. T.M. and Y.M. synthesised the samples. T.M. and Y.M. characterised the samples using XRD and EDX. T.M. performed the magnetization measurements. T.M., K.H., A.Y., R.K., R.H., T.D.M., Y.A., and Y.M. performed electrical resistivity and Hall measurements. T.M., M.R.K., A.Y., R.H., T.D.M., Y.A., and Y.M. performed specific heat measurements. M.R.K., T.K., and Y.M. performed Seebeck coefficient measurements. Theoretical calculations were carried out by H.U. This work was supervised in whole by Y.M., A.Y., and Y.G. The manuscript was written by H.U. and Y.M. with input from all coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitobe, T., Hoshi, K., Kasem, M.R. et al. Superconductivity in In-doped AgSnBiTe3 with possible band inversion. Sci Rep 11, 22885 (2021). https://doi.org/10.1038/s41598-021-02341-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02341-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.