Abstract
Numerous studies have demonstrated the key role of the Salmonella Pathogenicity Island 1-encoded type III secretion system (T3SS1) apparatus as well as its associated effectors in the invasion and intracellular fate of Salmonella in the host cell. Several T3SS1 effectors work together to control cytoskeleton networks and induce massive membrane ruffles, allowing pathogen internalization. Salmonella resides in a vacuole whose maturation requires that the activity of T3SS1 subverts early stages of cell signaling. Recently, we identified five cell lines in which Salmonella Typhimurium enters without using its three known invasion factors: T3SS1, Rck and PagN. The present study investigated the intracellular fate of Salmonella Typhimurium in one of these models, the murine hepatocyte cell line AML12. We demonstrated that both wild-type Salmonella and T3SS1-invalidated Salmonella followed a common pathway leading to the formation of a Salmonella containing vacuole (SCV) without classical recruitment of Rho-GTPases. Maturation of the SCV continued through an acidified phase that led to Salmonella multiplication as well as the formation of a tubular network resembling Salmonella induced filaments (SIF). The fact that in the murine AML12 hepatocyte, the T3SS1 mutant induced an intracellular fate resembling to the wild-type strain highlights the fact that Salmonella Typhimurium invasion and intracellular survival can be completely independent of T3SS1.
Similar content being viewed by others
Introduction
Salmonella enterica subsp. enterica serovar Typhimurium (S. Typhimurium) is the causative agent responsible for the second most deadly foodborne infection known as salmonellosis1. In humans, S. Typhimurium induces gastroenteritis characterized by fever, acute intestinal inflammation and diarrhea within 24 h of infection. In animals, S. Typhimurium is commonly isolated from healthy birds and mammals. However, it can also induce a systemic typhoid-like disease or gastroenteritis in mice or calves, respectively2. This facultative intracellular pathogen needs to cross intestinal barriers to be able to infect its hosts. Therefore, it must adapt to invade, survive and replicate within phagocytic and non-phagocytic cells3.
Salmonella has two Salmonella Pathogenicity Islands (SPI) that encode type III secretion systems (T3SSs). They are T3SS1 and T3SS2, encoded by SPI1 and SPI2, respectively. The T3SSs consist of a needle apparatus that injects effector proteins into host target cells. They are the key factors involved in the interaction of bacteria with host cells. Previously, only T3SS1-dependent invasion of non-phagocytic cells had been described. However, several studies agreed that Salmonella with a non-functional T3SS1 was able to induce pathologies in humans or experimentally infected animals4,5. Moreover, in vitro studies have shown that Salmonella invalidated for T3SS1 maintained its invasive ability in fibroblastic cells6, and required kinases that was not necessary in T3SS1-dependent invasion cell model6. In light of these results, two invasins, Rck and PagN, have been identified in the invasion process7,8. We recently published that Salmonella invalidated for the three known invasion factors (T3SS1 apparatus, Rck and PagN) remains able to invade several non-phagocytic cell models as effectively as wild-type Salmonella9. The main difference in the entry step between T3SS1-dependent and T3SS1-independent invasion is that T3SS1 effectors induce cell invasion by means of a trigger mechanism, while invasins mediate invasion using a zipper mechanism characterized by the interaction between a bacterial outer membrane protein and a host receptor10. The intracellular behavior of Salmonella in a vacuole called SCV (Salmonella containing vacuole) after a T3SS1-dependent entry has been well documented. Several effectors translocated by T3SS1 or T3SS2 have been shown to interact directly with host cell partners11 and are involved in SCV formation and maturation. Among the T3SS1 effectors, two play key roles. The first, SipA, polymerizes actin and participates in triggering large membrane ruffles, leading to Salmonella engulfment12. By mimicry of proteins implicated in vesicular transport, it then promotes fusion with early endosomes13. The second, SopB, not only plays a role in actin remodeling, but also induces Rab5 recruitment, required for optimal biogenesis of SCV through its phosphatase activity14. On the other hand, several T3SS2 effectors are implicated especially in SCV maturation, following well-described kinetics dependent on transient SCV interaction with late endosomal proteins such as Rab7. Among these effectors, SopD2 interacts directly with Rab7 and inhibits vesicular transport, thus favoring vacuole maturation15,16. This is characterized by the recruitment of late endosomal proteins, such as the lysosomal-associated membrane protein 1 (LAMP1)17. SifA is then required for the formation of Salmonella-induced filaments (SIFs) and hence SCV maturation18. Moreover, persistent expression of T3SS1 effectors during late vacuole maturation steps highlights the complexity of SCV biogenesis as well as the close communication between Salmonella and its host, allowing multiplication inside the vacuole while maintaining SCV integrity19. However, it has been shown that Salmonella is able to escape from its early vacuole into the cytosol in several cell types as well as in vivo20,21. The host cytosol environment is favorable for bacterial growth and hyper-replication leading to epithelial cell lysis and bacterial release21,22. In addition to its role in Salmonella invasion and in SCV maturation, T3SS1 is also required for vacuolar escape and replication in the cytosol of epithelial cells23. Nevertheless, SCV maturation and cytosolic escape have only been described in cells where Salmonella enters in a T3SS1-dependent manner. Nothing is known about Salmonella behavior after T3SS1-independent cell invasion.
Here, our goal was to depict S. Typhimurium fate inside host cells in a T3SS1-independent invasion cell model and to determine if the T3SS1 remained indispensable in survival of S. Typhimurium and maturation of SCV as observed in T3SS1-dependent invasion models. We showed that a wild-type strain of S. Typhimurium invaded the murine hepatocyte cell line AML12 through a T3SS1-independent process and retained its ability to multiply in a mature vacuole, it had a functional T3SS1 or not.
Results
A Salmonella Typhimurium ΔinvA mutant enters, survives and multiplies in the same manner as the wild-type strain in murine hepatocytes
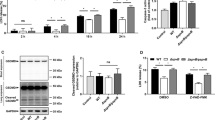
We have recently described that S. Typhimurium 14028, devoid of the three known invasion factors (i.e. T3SS1, Rck and PagN), remained capable of invading several non-phagocytic cell models at a similar rate to the wild-type strain, including murine AML12 hepatocytes9. In order to evaluate the ability of Salmonella to survive and multiply in the AML12 cell line, we first confirmed the similar invasiveness of a non-functional T3SS1 S. Typhimurium 14028 mutant (ST ΔinvA) and its S. Typhimurium 14028 wild-type parent (ST). ST and ST ΔinvA were grown overnight, a condition known to weakly induce the expression of Rck24 and PagN25 (Supplementary Fig. 1). HeLa cells were used as a T3SS1-dependent reference cell model. Adhesion and invasion of HeLa or AML12 cells were evaluated after 60 min of bacterial-cell contact. The level of total bacteria, corresponding to adhered and invaded bacteria, was almost equivalent in both HeLa and AML12 cells. No differences between the wild-type and the ΔinvA mutant were observed (Fig. 1 and Supplementary Fig. 2). As predicted, HeLa cells showed an elevated level of T3SS1-dependent invasion. Indeed, the ΔinvA mutant invaded these cells 50 times less than the wild-type strain (Supplementary Fig. 2). In contrast, no significant difference was observed between the two strains after infection of AML12 cells (Fig. 1). To avoid investigating an artifactual mechanism due to exacerbated AML12 cells’ permissiveness, we quantified the invasion and multiplication ability of two non-invasive E. coli strains: MC1061 and HB101. As expected, MC1061 and HB101 entered AML12 cells respectively 52.03 (p < 0.0001) and 205.4 (p < 0.0001) times less than ST and did not multiply (Supplementary Fig. 2). To evaluate the intracellular multiplication of Salmonella, we next quantified the number of intracellular Salmonella 16 h pi in AML12 and HeLa cells. We observed an increase of Salmonella in AML12 cells of more than 10 times with no significant difference (p = 0.6514) between ST and ST ΔinvA strains (Fig. 1). In contrast, a significant difference between the multiplication rates of ST and ST ΔinvA in HeLa cells was obtained: an increase of 18 times for ST compared to an increase of only 6 times for ST ΔinvA (p = 0.0039). No E coli multiplication was observed in both cell lines. Altogether, these results demonstrate that a ΔinvA mutant of Salmonella Typhimurium invaded, survived and multiplied equally well as the wild-type strain in the non-phagocytic AML12 cells.
S. Typhimurium 14028 inactivated for T3SS1 activity by invA mutation remained as invasive as the wild-type strain in the AML12 cell model. In repeated gentamicin protection assays, adhered, internalized and multiplied ST (black triangles) or ST ΔinvA (black squares) were monitored on AML12 cells. Cells were exposed to each strain (MOI = 50) for 60 min (adhesion/invasion) followed by the addition of gentamicin (100 μg/mL) for 60 min (Invasion), followed by a further 15 h with gentamicin (10 µg/mL) (multiplication). The results correspond to the mean ± standard deviation of at least four independent experiments performed in quadruplicate and expressed in log CFU/well. Results were compared using a Mann–Whitney test. No significant differences were observed between the two strains.
In AML12 cells, the wild-type strain of Salmonella Typhimurium 14028 rarely translocates T3SS1 effectors and preferentially invades cells using a zipper mechanism
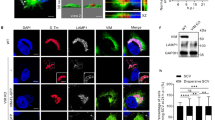
The fact that a ΔinvA mutant strain was as invasive as the wild-type strain in AML12 cells raised the question of the putative role of T3SS1 effectors in the invasion and in the early maturation of the SCV, considering these cells were infected with the wild-type strain. Was the T3SS1 apparatus used or not? The T3SS1 apparatus is a needle complex that allows for the injection of bacterial T3SS1 effector proteins into host cells. Direct interaction of these effectors with host proteins leads to massive actin cytoskeleton recruitment resulting in large membrane rearrangements and engulfment of the pathogen. While invasion-receptor interactions induce weak membrane rearrangement. In order to determine how the wild-type and ΔinvA strains of S. Typhimurium entered AML12 cells, we compared membrane rearrangements induced by ST and ST ΔinvA in AML12 cells using scanning electron microscopy (SEM) (Fig. 2C,D) and transmission electron microscopy (TEM) (Fig. 2E,F). S. Typhimurium SL1344 was chosen as a positive control for the visualization of large membrane rearrangements in HeLa cells (Fig. 2A), because the absence of SopE and a slightly different regulation of SPI1 in S. Typhimurium 14028 does not allow for the visualization of the ruffles even if this strain enters using its T3SS1 in this cell line26. Filopodia were distributed homogeneously at the cell surface of non-infected AML12 cells. (Fig. 2B). Despite numerous acquisitions of AML12 cells infected from 10 to 120 min with ST or ST ΔinvA, we never observed large membranous ruffles on the surface of AML12 cells regardless of which strain was used to infect cells. All captured images showed only weak membrane rearrangements (Fig. 2C,D). One inhibitor of each entry mechanism: zipper or trigger, was then used to confirm our electronic microscopy results. Zipper mediated internalization is known to depend on the PI3kinase pathway6. The PI3 kinase inhibitor wortmannin was used on HeLa and AML12 cells. Significant reduction in invasion for both the ST strain (p = 0.028) and the ST ΔinvA strain (p = 0.083) were observed only in AML12 cells (Fig. 3A). Cytochalasin D is an inhibitor of actin filament function that impacts severely large membranous ruffles. The working solution of Cytochalasin D 1 µg/mL, significantly reduced the entry of the Salmonella wild-type strain into HeLa cells with a ratio of 21.42 (p < 0.0001), but had a weak or insignificant impact on AML12 cell invasion by ST or ST ΔinvA strains; there was a decrease of invasion of 1.93 (p < 0.0311) and of 0.8 (p < 0.8819) respectively (Fig. 3B). These results, in addition to the electron microscopy results favor a zipper-like entry into AML12 cell line. The absence of a trigger mechanism induced by the wild-type strain could have been due to a non-functional T3SS1 after interaction with AML12 cells. To explore this hypothesis, we imaged T3SS1 effector translocation in AML12 cells using a fluorescence-based translocation assay (Fig. 4A–E). Using cytometry, we then quantified the percentage of infected cells in which effector translocation occurred (Fig. 4F). To this end, SopD, a T3SS1 effector, fused with TEM-1 β-lactamase was cloned into a pCX340 vector and transformed in ST or in ST ΔinvA strains expressing DsRed. SopD translocation in AML12 cells was monitored using a CCF4-AM assay. AML12 cells infected with ST or its ΔinvA derivative strain and harboring the empty vector pCX340, were used as negative controls. Using Fluorescence Resonance Energy Transfer (FRET) quantification, it is described that in absence of β-lactamase activity the intact CCF4 molecule results in FRET, emitting a green fluorescent signal. While, in the presence of β-lactamase activity, consistent with SopD translocation, cleavage of CCF4 produces a blue fluorescence signal. All the strains were able to invade this cell line. As predicted, only green fluorescence signals were obtained when AML12 cells were infected with the control strains (Fig. 4A,B,D). Moreover, no SopD translocation was observed when cells were infected with ST ΔinvA (Fig. 4E). By contrast, we observed that ST was able to translocate SopD in some cells (Fig. 4C), but for most cells, ST was able to invade with no apparent SopD translocation. This result was confirmed using flow cytometry analysis. We found that SopD translocation did not occur in 93.1% of the AML12 cells invaded by the ST strain. However, 87.1% of the cells were positive for both intracellular Salmonella and SopD translocation when HeLa cells were used as a control (Fig. 4F).
Weak cell membrane rearrangements observed by electron microscopy during invasion illustrate a zipper mechanism for both ST and ST ΔinvA strains in AML12 cells. (A) HeLa cells infected for 30 min with Salmonella Typhimurium (SL1344) as a trigger reference model. (B) AML12 cells not infected, or (C,E) infected with ST or (D,F) infected with ST ΔinvA for 60 min. (A–D) Infected HeLa cells and non-infected or infected AML12 cells cultured on glass coverslips were used to evaluate membrane ruffling using scanning electron microscopy. (E,F) Infected AML12 cells were trypsinized and used for transmission electron microscopy observation of ultra-thin sections of embedded cells in resin after fixation and impregnation. Scale bars 1 µm.
Salmonella Typhimurium entry into AML12 cells was dependent on the PI3 kinase pathway and required weak actin cytoskeletal rearrangements. HeLa cells and AML12 cells were exposed to (A) wortmannin (100 nM) (Sigma, St-Louis, MO), a PI3 kinase inhibitor, for 30 min or to (B) cytochalasin D (1 µg/mL) for 30 min, followed by an infection and gentamicin protection assay with ST and ST ΔinvA. Data are the means and standard deviations from three independent experiments. Statistical analyses using a Mann Whitney test were performed. Significances were (****) p < 0.0001; (**) p < 0.01; (*) p < 0.05.
T3SS1 effector translocation occurred only in very few infected AML12 cells. AML12 cells were exposed for 30 min to ST or ST ΔinvA strains expressing SopD effector-TEM-1 β-lactamase in pCX340 and DsRed in pGG2, then washed and infection was continued for 60 min. Subsequently, cells were loaded with CCF4-AM for 20 min and visually inspected (A–E) using an inverted confocal microscope equipped with a 100 × oil immersion objective (LEICA TCS SP8, Germany). Images of 1024 × 1024 pixels were acquired using LaserX software (LEICA). Green fluorescence indicates that CCF4-AM was loaded and the presence of blue cells due to CCF4-AM cleavage reveals SopD translocation. White arrowheads indicate Salmonella. (A) non-infected AML12 cells, (B) cells infected with the ST DsRed strain carrying the empty vector pCX340, (C) cells infected with the ST DsRed strain expressing SopD effector-TEM-1 β-lactamase in pCX340, (D) cells infected with the ST ΔinvA DsRed strain carrying the empty vector pCX340 and (E) cells infected with the ST ΔinvA DsRed strain expressing SopD effector-TEM-1 β-lactamase. The cells emitting blue fluorescence were considered as positive for effector translocation. (F) HeLa and AML12 cells were infected as described above with the ST strain expressing SopD effector-TEM-1 β-lactamase and DsRed, CCF4-AM loaded and finally trypsinized. Cell resuspensions were acquired using cytometry (BD LSRFortessa X-20 cell analyzer) and analyzed by BD FACSDiva Software. Green squares represent cells infected without SopD translocation; Blue dots represent infected cells with SopD translocation. Scale bars 10 µm.
In conclusion, the S. Typhimurium 14028 strain enters into AML12 cells independently of T3SS1. Nevertheless, a very low number of bacteria (< 7%) remain able to translocate T3SS1 effectors in this cell line. The T3SS1 effector translocation observed in AML12 cells demonstrated that the T3SS1 apparatus is functional and can inject effectors within the host cells.
In AML12 cells, Salmonella Typhimurium does not induce passive bacterial entry in a macropinocytotic way
Among endocytic mechanisms, macropinocytosis has been described as a mean of extracellular nutrient uptake. Several pathogens are known to induce this process during their entry into host cells allowing the passive entry of bacteria27. To further investigate the impact of the macropinocytosis in HeLa and AML12 cells, a coinfection experiment was performed. HeLa and AML12 cells were infected independently with ST or ST ΔinvA or coinfected with ST plus E coli HB101 or ST ΔinvA plus E coli HB101; E coli HB101 is non-invasive in HeLa cells and is a weakly invasive strain in AML12 cells (Supplementary Fig. 2). Our coinfection experiments had no impact on Salmonella invasion on either cell model. However, in HeLa cells, E coli HB101 invasion was significantly (p = 0.0002) increased when cells were coinfected with ST but not with ST ΔinvA (Fig. 5A). This result can be explained by the fact that membranous perturbations induced during a trigger mechanism are associated with macropinocytosis inducing entry of a non-invasive strain in cooperation with an invasive strain27. On the other hand, in AML12 cells, E coli invasion was not increased in coinfection assays with ST or ST ΔinvA, (Fig. 5B). Therefore, unlike in HeLa cells, this demonstrates that Salmonella invasion in AML12 cells is not associated with macropinocytosis.
Salmonella Typhimurium vacuole maturation was mostly macropinosome independent in murine AML12 cells. HeLa cells (A) and AML12 cells (B) were grown in 24-well plates. Infection of cells was carried out for 60 min in 300 µL medium without serum at MOI 50 with ST or ST ΔinvA alone or coinfected with the E. coli HB101 strain transformed with pSUP202, allowing for chloramphenicol selection. To quantify invasion, after 60 min of infection, cells were washed, and gentamicin protection assays were performed consisting of three washes with medium followed by 60 min in medium with 100 µg/mL gentamicin. After this, cells were extensively washed and lysed, and serial dilutions were plated on TSA and TSA chloramphenicol. Co-invasion was estimated by subtracting growth observed on TSA chloramphenicol to growth on TSA. The black bar corresponds to ST invasion without coinfection, the white bar ST invasion in coinfection context, the dark grey bar ST ΔinvA invasion without coinfection, the dot bar ST ΔinvA invasion in coinfection context, the light grey bar E. coli HB101 invasion without coinfection, the vertical line bar E. coli HB101 invasion in ST coinfection context, the horizontal line bar E. coli HB101 invasion in ST ΔinvA coinfection context. Data are the means and standard deviations from three independent experiments. Statistical analyses using a Mann Whitney test were performed. Significance was (***) p = 0.0002.
In AML12 cells, Salmonella Typhimurium 14028 resides intracellularly, mainly in a vacuole
We then wished to determine where Salmonella is capable of replicating consecutively in a T3SS1-independent invasion. Indeed, cytosolic Salmonella constitutes a significant proportion of the total bacterial population in several epithelial cell models20. Over time, we followed the replication of S. Typhimurium in AML12 cells with or without chloroquine, as this molecule has been shown to kill intravacuolar Salmonella20. Compared to 1h post-infection (pi), we found that there were 2.76 and 2.62 times more bacteria at 7 h pi for the ST and ST ΔinvA strains, respectively. Our results show that without chloroquine, the ST and ST ΔinvA strains were able to survive and slightly multiply inside AML12 cells during the duration of the experiment. In contrast, a 95% reduction in the number of these two strains was observed when chloroquine was added (Fig. 6A). These results support the idea of an intravacuolar localization of S. Typhimurium in AML12 cells at 7 h pi, leading to a multiplication rate about 10 times for ST and ST ΔinvA at 16 h pi (Supplementary Fig. 2 and Fig. 1). To confirm bacterial localization, we performed a TEM analysis at 7 h pi on AML12 cells infected with ST or ST ΔinvA strains, treated or not with chloroquine. In the absence of chloroquine, we observed the presence of intact bacteria only in vacuolar space (Fig. 6B,C); bacteria appeared as expected damaged or degraded in vacuoles when cells were exposed to chloroquine (Fig. 6D,E). This confirmed the intravacuolar localization of Salmonella in AML12 cells. To further investigate this observation of vacuolar residence of Salmonella in AML12 cells, we compared the localization of Salmonella in HeLa and AML12 cells using a dual color reporter plasmid p4889 (PEM7::DsRed PuhpT::sfGFP) with combined expression of constitutive DsRed and cytosolic GFP28. At each time point imaged, we easily identified Salmonella that was found only in its SCV (red) for AML12 (Fig. C,D) and both cytosolic (red and green) (Fig. 7B) and vacuolar (red) for HeLa cells (Fig. 7A,B). We never observed cytosolic ST (Fig. 7C) or ST ΔinvA (Fig. 7D) in AML12 cells.
Salmonella Typhimurium resided in vacuoles after AML12 cell invasion. After 60 min exposure to ST (black squares) or ST ΔinvA (red triangles) strains, AML12 cells were incubated with gentamicin at 100 µg/mL (solid lines) or gentamicin at 100 µg/mL plus 1.2 mM chloroquine (dashed lines) for 60 min and for 3, 5 and 7 h pi with gentamicin at 10 µg/mL (solid lines) or gentamicin at 10 µg/mL plus 1.2 mM chloroquine 60 min before the end point (A). At the 7 h timepoint, ST and ST ΔinvA infected cells without (respectively B,C) or with chloroquine (respectively D,E) treatment were imaged using transmission electron microscopy (JEOL 1011, Tokyo, Japan). Scale bars 1 µm.
Cytosolic escape did not occur in AML12 cells contrary to Hela cells. HeLa cells (A,B) and AML12 cells (C,D) were exposed to ST p4889 (A–C) and ST ΔinvA p4889 (D) 1 h followed by a gentamicin protection assay, then fixed and stained with mouse Lamp1 or rat Lamp1 antibody, respectively, then revealed with anti-mouse or anti-rat antibody Alexa Fluor 647 (white) imaged at 4, 8, 12, and 24 h pi. (A–D) are organized as a first line of images, allowing to potentially observe cytosolic bacteria (green) second line vacuolar and cytosolic bacteria (red), third line Lamp1 staining (white) and fourth line merged image of the first three lines (cytosolic bacteria appeared yellow to green). Imaging was performed using confocal microscopy with a 100 × oil immersion objective (Leica TCS SP8, Germany). Nuclei were counterstained with DAPI (blue) vacuolar bacteria (red) and cytosolic bacteria (green and weakly red = yellow to orange, mainly green due to hyper-replication). Scale bars 10 µm.
Altogether, these results demonstrate a vacuolar localization of S. Typhimurium in AML12 cells.
Maturation of the Salmonella containing vacuole leads to Salmonella replication with Salmonella-induced filament formation
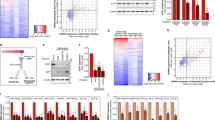
SCV maturation has been deeply scrutinized in T3SS1-dependent invasion models. It is widely accepted that it consists of three stages: early, intermediate and late29. To interpret SCV maturation after T3SS1-dependent invasion, we exposed AML12 cells to ST or ST ΔinvA strains for 1 h. In this way, we investigated the recruitment of several host proteins as markers of the different stages14,17,30, as well as the formation of SIF-like structures and the acidification of the vacuole18,31. Following immunocytochemistry using antibodies raised against Rab5, Rab7, Rab8 and Rab35 at 60 min (Supplementary Fig. 3) and raised against LAMP1 at 2, 4, 6, 12 and 16 h pi (Fig. 8), cells were imaged using confocal microscopy. Neither strain showed a high recruitment or a convincing colocalization of the Rab GTPases tested with intracellular Salmonella. Only a few dots near intracellular Salmonella were observed (Supplementary Fig. 3). However, as early as 4 h pi, we observed a slight recruitment of LAMP1 that became undeniable at 6, 12 and 16 h pi (Fig. 8). Moreover, SIF-like structures were clearly identified at 16 h pi (Fig. 8E2,J2) in 50.33% and 51.67% of ST and ST ΔinvA infected cells, respectively (Supplementary Fig. 4). These results are in accordance with the proportion of SIF observed in HeLa cells for comparable multiplicity of infection (MOI) and cell density32. Having the goal to estimate Rab GTPase recruitment during SCV maturation, AML12 cells were infected with ST or ST ΔinvA, and SCV were purified at 10, 30, 60, 120, 240 min and immunostained using antibodies raised against Rab5, Rab7, Rab8 or Rab35 and analyzed by flow cytometry. The example of Rab8 is demonstrated in Supplementary Fig. 5. At each time point, the percent positive SCV for Rab5 or Rab7 was less than 7% and the percent positive SCV for Rab8 or Rab35 was near 20% decreasing to less than 2% at 4 h pi (Fig. 9), thus confirming a low recruitment of these Rab GTPases to the SCV.
Salmonella containing vacuole maturation occurred in the presence of LAMP1 and led to SIF formation. The kinetics of LAMP1 recruitment was imaged for AML12 cells infected with ST DsRed and ST ΔinvA DsRed at (A,F) 2 h, (B,G) 4 h, (C,H) 6 h, (D,I) 12 h and (E,J) 16 h pi. At each time point, cells were fixed and labelled with rat LAMP1 antibody, revealed with anti-rat Alexa Fluor 488 (green) and imaged using confocal microscopy with a 100 × oil immersion objective (Leica TCS SP8, Germany). Nuclei were counterstained with DAPI (blue). Squares outlined in white are a 2 × manual magnification of selected regions to visualize progressive recruitment of LAMP1. White outlined squares E1, J1 focus on Salmonella stuck in LAMP1 positive vacuoles and E2, J2 focus on SIF-like structures. Scale bars 10 µm.
Salmonella containing vacuole associated with Rab GTPase markers. The localization of Rab GTPase to SCV from AML12 cells infected by ST DsRed at 10 min, 30 min, 60 min, 120 min and 240 min was estimated by labelling purified SCV with (black bar) mouse Rab5 antibody, (grey bar) mouse Rab7 antibody, (horizontal line bar) rabbit Rab8 antibody or (vertical line bar) rabbit Rab35 antibody revealed with anti-mouse Alexa Fluor 488 and anti-rabbit Alexa Fluor 488, respectively. Acquisition of SCV events were discriminated from PNS based on DsRed fluorescence using cytometry (BD LSRFortessa X-20 cell analyzer) and analyzed using FCS Express 4 Flow. Data are the means and standard deviations from three independent triplicates experiments.
Previous studies have demonstrated that recruitment of LAMP1 and the presence of SIF-like structures depend on T3SS2 effectors when Salmonella enters host cells using its T3SS133. Since T3SS2 is expressed in an environment with low pH as well as a low concentration of divalent cations, we tested whether or not the SCVs were acidified in the AML12 cells. Cresyl violet was used, as it is a sensitive and convenient acidotrophic fluorescent stain for acidified cell compartments34. To validate this tool for use in the study of SCV acidification, murine macrophages RAW 264.7 were infected with ST for 10, 30, 60, 120, 240 min and loaded with cresyl violet (Supplementary Fig. 6A to E, respectively). As expected, as early as 30 min pi, 35% of SCVs were acidified (Supplementary Fig. 6F) and this percentage continued to increase until reaching 89% 4 h pi, demonstrating that cresyl violet can be used to monitor SCV acidification. In parallel, AML12 cells were infected with ST and ST ΔinvA for 2 h to 16 h pi and loaded with cresyl violet in order to determine if SCV acidified during AML12 infection (Fig. 10A–J and Supplementary Fig. 7A–C). At 4 h pi, 50% of the wild-type strains were embedded in an acidified vacuole. In contrast, at 16 h, there were over 90% (Supplementary Fig. 7A and C). Moreover, we observed that the vacuole acidification followed the same time-course as LAMP1 recruitment. Similar results were obtained when AML12 cells were infected with the invA mutant strain (Fig. 10F–J and Supplementary Fig. 7B and C).
Salmonella Typhimurium progressed in acidified organelles in AML12 cells. The kinetics of vacuole acidification was processed for AML12 cells infected with ST GFP and ST ΔinvA GFP at (A,F) 2 h, (B,G) 4 h, (C,H) 6 h, (D,I) 12 h and (E,J) 16 h pi. At each time point after 5 min exposure to 1 µM cresyl violet (cv) (red), cells were directly imaged using confocal microscopy with a 100 × oil immersion objective (Leica TCS SP8, Germany). Squares outlined in white are a 2 × manual magnification of select regions so as to visualize progressive organelle acidification. Scale bars 10 µm.
Finally, we exposed AML12 cells to ST ΔssaJ (lacking T3SS2) or to ST ΔinvA ΔssaJ (lacking T3SS1 and T3SS2) strains for 1 h and observed the recruitment of LAMP1 at 4, 6, 12, 16 and 20 h pi, as well as SIF formation. Unsurprisingly, for both strains, recruitment of LAMP1 in the SCV was detected between 4 and 12 h pi. However, at 16 h pi or longer, no SIF formation was seen (Fig. 11A–J). These results support the fact that there was no translocation of T3SS2 effectors due to ssaJ invalidation and also confirm that SIF formation observed with the wild-type strain was due to T3SS2 effectors.
T3SS2-invalidated Salmonella Typhimurium resided in a vacuole in the presence of LAMP1 without SIF formation. The kinetics of LAMP1 recruitment was imaged for AML12 cells infected with ST ΔssaJ DsRed and ST ΔinvA ΔssaJ DsRed at (A,F) 4 h, (B,G) 6 h, (C,H) 12 h, (D,I)16 h and (E,J) 20 h pi. At each time point, cells were fixed and labelled with rat LAMP1 antibody, revealed with anti-rat Alexa Fluor 488 (green) and imaged using confocal microscopy with a 100 × oil immersion objective (Leica TCS SP8, Germany). Nuclei were counterstained with DAPI (blue). Scale bars 10 µm.
Overall, these results demonstrate that after a T3SS1-independent entry in AML12 cells, Salmonella resides in a vacuole whose early maturation does not require the strong recruitment of Rab GTPase markers, as observed during a T3SS1-dependent entry14,30. Indeed, few SCVs were positive for Rab5 and Rab7 during the first 4 h of infection. After 2 h of infection, the percentage of SCV positive for Rab8 or Rab35 decreased to less than 5%. In contrast, the maturation of the vacuole appears later, similar to the two entry mechanisms with an acidification of the vacuole, LAMP1 recruitment and SIF-like structures formation. These observations were valid for both ST and ST ΔinvA strains. In the absence of a T3SS1-mediated entry, Salmonella succeeded in retaining an SCV which favored its multiplication.
Discussion
The interaction between S. Typhimurium and host cells has been explored in depth. Extensive knowledge exists about the crucial role of T3SS1 effector proteins in Salmonella invasion and intracellular behavior. These bacterial effectors interact with host cytoskeleton proteins allowing rearrangements in the host cell membrane. This leads to SCV formation, the maturation of which requires controlling of endocytic pathways using T3SS1 and T3SS2 effectors. This paradigm appears to be true for the majority of cell lines because among the non-phagocytic cell models that were previously tested, most were invaded by Salmonella via the T3SS1-dependent pathway9,35,36. This is the case for HeLa cells, which are often used to interpret Salmonella behavior37, but also for human colonic epithelial cell lines T84, and HT2935 as well as the alveolar murine epithelial cell MLE-12 line38. However, Salmonella can also enter non-phagocytic cell lines without its T3SS1. This is the case for fibroblastic cells6 or HT29 cells when these cells are cultured in 3D39. Similarly, Caco-2 cells, a partially T3SS1-independent cell model, become clearly T3SS1-independent in a co-culture model mimicking M cells40. In addition, the culture conditions of Salmonella also influence Salmonella-host cell interaction in vitro41. Consistent data illustrate the ability of Salmonella to use bacterial factors other than the T3SS1 to conserve their invasive power. Currently, Rck and PagN two membrane proteins of Salmonella, which are only slightly expressed in classic culture conditions, have been shown to be involved in the host cell invasion process8,42. Moreover, in a recently published study, we identified a panel of cells that could be invaded by a mutant devoid of all three known invasion factors at the same level as its wild-type counterpart9. This result clearly shows that further research is required in identifying new invasion factors.
In this context of multiple Salmonella invasion factors, we aimed to investigate whether the T3SS1 was required for intracellular Salmonella survival in the cell models described as T3SS1-independent. To determine this, we selected the AML12 murine hepatocyte cell line described as a T3SS1-independent cell model9. In all these experiments we decided to use ST and ST ΔinvA grown overnight, a culture condition that weakly induces the expression of Rck and PagN (Supplementary Fig. 1). Firstly, we confirmed that ST and ST ΔinvA were able to invade, survive and multiply at the same level in the AML12 cell line. Electronic imaging analysis revealed only weak membrane rearrangements consistent with the zipper mechanism (Fig. 2) and a low level of cells (5.5%) containing translocated T3SS1 effectors (Fig. 4). This supporting the fact that the T3SS1 apparatus was functional but not used in the invasion mechanisms of the murine AML12 hepatocytes in contrast to human HeLa adenocarcinoma epithelial cells. In addition to being involved in invasion process, T3SS1 is required for escape, survival and hyper-replication into the cytosol of cells where entry is T3SS1-dependent43,44. In the present study, using AML12 cells Salmonella resided in a SCV following invasion and, ST as ST ΔinvA behaved in the same manner. Compared to results from the hyper-replicative model HeLa, C2BBe1, HuTu80 or HCT116 cells, no cytosolic replication occurred in AML12 cells20. The absence of Salmonella hyper-replication in the AML12 cytosol may explain the low rate of Salmonella at 7 h pi (Fig. 6). However, this low multiplication rate of 7 h pi is offset by the multiplication of Salmonella in the SCV observed at 16 h pi (Supplementary Fig. 2). Nevertheless, it would be interesting to explore whether or not the AML12 cellular context is in favor of non-growing intracellular bacteria, already described as persisters in macrophages45,46. This would be another way to explain the low rate of Salmonella multiplication observed at 7 h pi in AML12 cells. In fact, our work highlights an aspect of an epithelial cell type that promotes bacterial invasion and survival regardless of T3SS1 effectors. The rare translocation of T3SS1 effectors in host cells was not due to Salmonella culture conditions; the same inoculum had a marked opposite effect on HeLa and AML12 cells.
The steps of SCV maturation in T3SS1-dependent cell models have been well defined and can be detailed in early, intermediary and late vacuoles. T3SS1 effectors contribute with T3SS2 effectors in recruiting molecular cell partners, including Rab GTPases, that enrich the vacuole and allow for its maturation. In contrast, after a T3SS1-independent entry process the intracellular behavior of Salmonella, the nature of the vacuole and the requirements for vacuole maturation needed to be explored. Our chloroquine assays showed that ST resided only in the vacuoles of AML12 cells. Survival in this vacuole is T3SS1-independent. Indeed, we observed the same intravacuolar localization (Fig. 8) and the same multiplication rate for ST and ST ΔinvA in AML12 cells, which is about 10 times. Moreover, we demonstrated the absence of recruitment of endocytic markers such as Rab5 and Rab7 in AML12 cells, which are used to characterize the SCV in HeLa cells14,47. This result was in accordance with the results obtained using the invA mutant as the recruitment of endosomal markers depends on their direct or indirect interaction with T3SS1 effectors14,48. This is not the first time that a SCV without Rab5 or Rab7 recruitment has been observed. Indeed, in HeLa cells infected with ST ΔinvA strain expressing the invasin Inv of Yersinia allowing Salmonella invasion, it has been shown that Rab GTPase recruitment on the SCV is drastically different from that observed for the ST strain. For example, these vacuoles are not enriched with Rab5 and are poorly enriched with Rab7, but seem more enriched with Rab8 and Rab3530. In contrast, we could clearly observe vacuole recruitment of the late endosomal marker LAMP1 with both ST and ST ΔinvA strains. Similarly, whichever the strain considered, we observed the formation of SIF-like structures, but later (16 h) than for T3SS1-dependent models (8–12 h). These results confirm the maturation of the SCV even though early endosomal markers were not highly recruited. One can either hypothesize that Salmonella in the vacuole waits for the natural acidification of the phagosome to initiate the T3SS2 dialogue, or that a signaling cascade requiring other bacterial and cellular actors, allowing Salmonella to survive in a mature vacuole, is involved and remains to be identified. In actual fact, it was reported that vacuolar constraints stimulate the PhoP-PhoQ system and influence gene transcription like pagN, potentially implicated in Salmonella intracellular fate49.
In conclusion, Salmonella can enter, survive and multiply in a SCV without using its T3SS1, as demonstrated in this paper using the AML12 cell line. This interaction with host cells does not lead to hyper-replication in the cytosol, and the maturation of the SCV follows a different pathway than that described to date. We are currently investigating whether or not this process is universal for other cell lines (e.g. for which a T3SS1-independent invasion has already been observed). Moreover, host cell proteins and Salmonella partners implicated in the SCV maturation in this context remain to be identified.
Materials and methods
Generation and maintenance of Salmonella strains
Salmonella enterica serovar Typhimurium ST 14028 (ST) for which we previously demonstrated the T3SS1 independent entry into AML-12 cells was the wild-type strain used in this study. The T3SS1 deficient strain S. Typhimurium ΔinvA (ST ΔinvA) and the T3SS2 deficient strain S. Typhimurium ΔssaJ were obtained by deleting the invA or the ssaJ open reading frame respectively, using the λ Red recombinase method50 previously described10. Briefly, the open reading frame was stably replaced by a kanamycin marker, and successful deletion/insertion of the target gene was confirmed using PCR analyses, and the kanamycin cassette was removed. DsRed and GFP expressing strains used in this study were obtained by bacterial electroporation of plasmid pGG2-DsRed51 and pFPV25.1 GFP52. For infection, bacteria expressing pGG2-DsRed or pFVP25.1 GFP were grown in TSB with 10 µg/mL carbenicillin 8 h at 37 °C with shaking, diluted 1:100 in fresh TSB, and incubated overnight at 37 °C without shaking. Bacteria were diluted 1:5 and absorbance was measured. Subsequently, bacteria were added to the cells at the required multiplicity of infection (MOI) in medium without fetal calf serum. Salmonella enterica serovar Typhimurium SL1344 grown using the same conditions as for ST, was used as control known to induce a trigger mechanism imaged by MEB.
Invasins expression profiles
For the Rck and PagN invasins expression study, Salmonella Typhimurium 14028 BamB::3xFLAG or PagN::3xFLAG were obtained (as already described for Salmonella Typhimurium 14028 Rck::3xFLAG24) and were grown overnight in TSB. Then, 10 µl of a 1010 bacteria/mL suspension in Laemmli buffer were loaded after heat denaturation at 100 °C on 4–15% Mini-PROTEAN® TGX™ Precast Protein Gels. Transfer was performed using a nitrocellulose membrane, and blots were stained with rabbit anti-Flag M2 or homemade rabbit anti-BamB53. This was followed by incubation with anti-rabbit HRP revealed with SuperSignal West Dura from Thermofisher and acquired using FX Fusion (Vilber Lourmat).
Cell culture
The murine AML12 hepatocytes (ATCC CRL-2254) are a spontaneously immortalized cell line from mice transgenic for transforming growth factor alpha. AML12 was identified as a cell model of T3SS1-independent invasion. Cells were routinely grown in 75 cm2 plastic tissue culture flasks at 37 °C under 5% CO2 in DMEM-F12 cell culture media with 0.005 mg/mL insulin, 0.005 mg/mL transferrin, 5 ng/mL selenium, 40 ng/mL dexamethasone and 10% fetal bovine serum, without antimicrobial compounds. HeLa cells (ATCC CRM-CCL-2), used as a reference for a T3SS1-dependent cell model, were grown in DMEM with 2 mM L-Glutamine and 5% fetal bovine serum9.
Adhesion, invasion, multiplication assays
Salmonella adhesion, invasion and multiplication assays were performed with sub-confluent cell monolayers seeded in 24-well plates. Infection of cells was carried out for 60 min in 300 µL of medium without serum at MOI 50. For the adhesion/invasion assay, five washes in buffered saline water were followed by cell lysis in 4 °C H2O using serial dilution plated on Tryptic Soy Agar (TSA-DIFCO). To quantify for invasion only, after 60 min of infection, cells were washed, and gentamicin protection assays were performed consisting of three washes with medium, followed by 60 min in medium with 100 µg/mL gentamicin. After this, as for the adhesion/invasion assay, cells were extensively washed and lysed, and serial dilutions were plated on TSA. Following the gentamicin protection assays, gentamicin multiplication assays were performed. Cells were washed three times with medium followed by a further 16 h in medium with 10 µg/mL. Subsequently, as for the adhesion/invasion assay, cells were extensively washed and lysed, and serial dilutions were plated on TSA.
Scanning and transmission electron microscopy
HeLa and AML12 cells were cultured at 106 cells per well 48 h before infection on glass coverslips (diameter 12 mm) in 24-well plates for scanning electron microscopy, and then directly in wells for transmission electron microscopy. Cells were infected with Salmonella strains at MOI 50 for 30 min in HeLa cells, trigger mechanism control and for 15, 30, 45 or 60 min in AML12 cells.
Scanning electron microscopy samples were carried out using infected cells fixed after incubation in 4% paraformaldehyde, 1% glutaraldehyde (SIGMA, St-Louis, MO) in 0.1 M phosphate buffer (pH 7.2) for 24 h. After washing in PBS, samples were fixed with 2% osmium tetroxide for 1 h. A graded series of ethanol solutions allowed for full dehydratation of cells, then samples were dried in hexamethyldisilazane (HMDS, SIGMA). Samples were coated with 40 Å platinum, using a GATAN PECS 682 apparatus (Pleasanton, CA). Observations were performed using a Zeiss Ultra plus FEG-SEM scanning-electron microscope (Oberkochen, Germany).
Transmission electron microscopy were performed using infected cells resuspended after trypsinization, washed in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde, 1% glutaraldehyde (SIGMA, St-Louis, MO) in 0.1 M phosphate buffer (pH 7.2) for 24 h. Samples were washed in phosphate-buffered saline (PBS) and post-fixed with 2% osmium tetroxide (AGAR SCIENTIFIC, Stansted, UK) for 1 h. Then, complete dehydration of samples was carried out in a graded series of ethanol solutions followed by propylene oxide.This was followed by an impregnation step with a mixture of (1∶1) propylene oxide/Epon resin (SIGMA). Samples were kept overnight in pure resin. Ultra-thin sections (90 nm) of embedded cells in Epon resin (SIGMA) polymerized 48 h at 60 °C, were collected using a Leica EM UC7 ultramicrotome (Wetzlar, Germany). Sections were stained with 5% uranyl acetate (AGAR SCIENTIFIC), 5% lead citrate (SIGMA) and were observed using a transmission electron microscope (JEOL 1011, Tokyo, Japan).
T3SS1 effector translocation assays
β-lactamase reporter assays were performed as previously described9,54. SopD fused with β-lactamase TEM-1 was cloned in pCX340 plasmid. Recombinant plasmid was introduced either in ST DsRed or ST ΔinvA DsRed. The resulting strains were used to infect AML12 cells for 30 min as described above, and 60 min in the presence of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to induce SopD-β-lactamase expression and in the presence of 1 mM probenecid. Post-infection, a small molecule substrate of β-lactamase (LiveBLAzer FRET-B/G Loading Kit with CCF4-AM, THERMOFISHER SCIENTIFIC) was added into the cell culture medium and incubated for 20 min at room temperature in the presence of 1 mM probenecid. Translocation of effectors fused to β-lactamase TEM-1 would result in the cleavage of CCF4 and emission of blue instead of green fluorescence using 405 nm laser excitation. The infected cells were visually inspected using an inverted confocal microscope equipped with a 100 × oil immersion objective (LEICA TCS SP8, Germany). Images of 1024 × 1024 pixels were acquired using LaserX software (LEICA). The cells emitting blue fluorescence were considered as positive for effector translocation. Flow cytometry analysis was performed with trypsinized cells from β-lactamase reporter assays described above. Analysis was carried out using 405 nm violet and 488 nm blue lasers to estimate the cleavage of CCF4-AM and 561 nm yellow/green laser for Salmonella pGG2-DsRed infected cells with a BD LSRFortessa X-20 cell analyzer. Twenty thousand infected cells were gated based on green/red (cells infected without cleaved CCF4) or blue/red (cells infected with cleaved CCF4).
Invasion inhibition by drug assay
HeLa and AML12 cells were grown in 24-well plates preincubated with medium containing DMSO, wortmannin (100 nM) (SIGMA, St-Louis, MO) for 30 min, or with cytochalasin D (1 µg/mL) (SIGMA, St-Louis, MO) for 30 min followed by an infection and gentamicin protection assay with ST and ST ΔinvA contining drugs.
Macropinocytosis analysis through non-invasive Escherichia coli strain uptake
HeLa cells and AML12 cells were grown in 24-well plates. Infection of cells was carried out for 60 min in medium without serum at a MOI of 50 with either a Salmonella strain alone or with a Salmonella strain and also with E. coli HB101 or MC1061 strain transformed with pSUP202 allowing for chloramphenicol selection. To quantify invasion, after 60 min of infection, cells were washed and gentamicin protection assays were performed as described above. Serial dilutions were plated on TSA and TSA chloramphenicol 30 µg/mL. The latter medium allowed counting of E. coli while the number of Salmonella was obtained by substracting the number of colonies on TSA with the number of colonies on TSA chloramphenicol.
Assessment of bacterial survival: vacuolar versus cytosolic
To evaluate whether or not multiplication occurred in a vacuole or in the cytosol, chloroquine resistance assays were performed. First, a serial dilution of chloroquine was carried out. The concentration of 1.2 mM chloroquine was estimated to be the most suitable to obtain the best vacuolar bacterial mortality without affecting AML12 cell survival and this was confirmed through a cell survival assay (Tetrazolium reduction assay (MTT) (Data not shown). After 60 min of contact between bacteria and cells, cells were incubated in growth media with gentamicin alone at 100 µg/mL or with the addition of chloroquine at 1.2 mM for 60 min. This was followed by lysis and plating, corresponding to 1 h pi. Afterwards, the same procedure was repeated at 3 h, 5 h and 7 h pi with gentamicin alone at 10 µg/mL or with the addition of chloroquine at 1.2 mM 60 min before the end point. In addition, at the 7 h timepoint, ST and ST ΔinvA infected cells, chloroquine treated or not, were imaged using transmission electron microscopy (JEOL 1011, Tokyo, Japan).
Salmonella cytosolic escape
To visualize bacterial escape from vacuoles, a dual color reporter plasmid p4889 (PEM7::DsRed PuhpT::sfGFP) was used28. PEM7::DsRed, allows for the expression of constitutive DsRed corresponding to vacuolar and cytosolic bacteria (red), PuhpT::sfGFP allows for inducible GFP expression in cytosol corresponding to cytosolic localization of bacteria (green). In confocal imaging, green and weakly red bacteria appear yellow to orange, mainly green due to Salmonella hyper-replication in the cytosol. HeLa cells were exposed to ST p4889 for 1 h, followed by a gentamicin protection assay. They were then fixed and stained with mouse Lamp1 antibody (Clone H4A3 from DSHB) and revealed with anti-mouse Alexa Fluor 647 (Thermofisher). AML12 cells were exposed to ST p4889 and ST ΔinvA p4889 for 1 h followed by a gentamicin protection assay, fixed and stained with rat Lamp1 antibody (Clone 1D4B from DSHB), and then revealed with anti-rat Alexa Fluor 647 (Thermofisher). Images were obtained at 4, 8, 12, and 24 h pi. Imaging was performed using confocal microscopy (LEICA TCS SP8, Germany).
Flow cytometry analysis of SCV
AML12 cells grown in P6 plates were infected with ST DsRed (MOI 100) 10 min, 30 min, 60 min in DMEM without SVF, 120 min (corresponding to an additional 60 min in complete medium gentamicin 100 µg/mL) and 240 min (corresponding to an additional 120 min in complete medium gentamicin 10 µg/mL). After infection, cells were washed in PBS 0.2% BSA and scraped in 3 mL of homogenization buffer HB (Sucrose 250 mM (SIGMA), Imidazole 3 mM pH7.4 (SIGMA), 0.1% gelatin (SIGMA), 0.5 mM EGTA (SIGMA), and Protease inhibitor cocktail (ROCHE). After centrifugation 5 min at 1800 g, pellets were resuspended in 400 µL of HB and gently homogenized by six passages through 22G syringe. After three centrifugations for 5 min at 100 g, 100 µL of supernatant containing post-nuclear supernatant (PNS) were labelled with mouse Rab5 antibody (SANTACRUZBIOTECH), mouse Rab7 antibody (SANTACRUZBIOTECH), rabbit Rab8 antibody (PROTEIN TECH) or rabbit Rab35 antibody (INVITROGEN) and revealed with anti-mouse or anti-rabbit Alexa Fluor 488 (INVITROGEN). Isotypic controls were done.
Vacuolar acidification
For identification of acidified vacuoles, 300 µL of 2.105 cell/mL suspension of RAW 264.7 cells or AML12 cells were seeded per well using 8 well chamber slide (Ibidi) 24 h before infection in complete medium. RAW264.7 cells were infected at MOI10 in complete medium with ST GFP 10, 30, 60, 120 and 240 min and incubated for 5 min with 1 µM cresyl violet at 37 °C. AML12 cells were infected with ST GFP or ST ΔinvA GFP at MOI 50 and incubated for 5 min with 1 µM cresyl violet at 37 °C before image capture at 2, 4, 6, 12 and 16 h pi. Acidified SCVs were imaged. Three independent experiments were performed, and 100 infected cells were visually counted at each time point. Images were acquired using an inverted confocal microscope equipped with a 100 × oil immersion objective (LEICA TCS SP8, Germany), 488 nm and 585 nm laser excitation. Image detectors were set to collect signals emitted between 525/575 nm and 600/650 nm for bacteria and for cresyl violet in acidified cell vacuoles, respectively.
Immunofluorescence and confocal microscopy
HeLa and AML12 cells were seeded in 24-well plates with 12 mm diameter glass coverslips at a density of 105 cells per well, 24 h before infection, as previously described9. Cell infection was carried out using ST DsRed or ST ΔinvA DsRed for 1 h at 37 °C in 5% CO2 at MOI 50. Cells were washed five times and exposed to 100 µg/mL gentamicin for 60 min, and when needed, to 10 µg/mL gentamicin for 4, 6, 12 16 and 24 h. After infection, cells were washed in PBS and fixed for 10 min with 4% formaldehyde in PBS at room temperature until selected end points: 2, 4, 6, 12, 16 and 24 h. Fixed cells were permeabilized with 0.1% saponin in PBS. Cells were immunostained with primary antibody anti-LAMP1 diluted 1/20 in PBS plus goat serum with 0.1% saponin (Supernatant culture from Clone 1D4B rat origin for AML12 cells and Clone H4A3 mouse for HeLa cells obtained from the DSHB, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52,242), washed in PBS with 0.1% saponin and immunostained with secondary antibody goat anti-Rat or anti-Mouse Alexa Fluor 488 or Alexa Fluor 647 diluted 1/400 in PBS plus goat serum 0.1% with 0.1% saponin. Cell nuclei were counterstained with DAPI. Glass slides were then coverslipped using in Fluoromount mounting medium9. Cells were observed using a SP8 confocal laser-scanning microscope equipped with a 100 × oil immersion objective (LEICA). Images of 1024 × 1024 pixels were acquired using LaserX software (LEICA). Sections 0.28 µm thick (38 per image) were assembled into Z stacks using Las AF lite 2.6.3 build 8173 software (LEICA). Figure 2 shows one representative section of the Z stacks.
Statistical analysis
Experiments were repeated two to four times in triplicates or quadruplicates. Statistical analyses using a Mann Whitney test were performed using GraphPad Prism version 6.07 for Windows, GraphPad Software, La Jolla California USA, http://www.graphpad.com.
References
European Food Safety, A., European Centre for Disease, P. & Control. The European Union one health 2019 zoonoses report. EFSA J. 19, e06406. https://doi.org/10.2903/j.efsa.2021.6406 (2021).
Baumler, A. & Fang, F. C. Host specificity of bacterial pathogens. Cold Spring Harb. Perspect. Med. 3, a010041. https://doi.org/10.1101/cshperspect.a010041 (2013).
Ribet, D. & Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 17, 173–183. https://doi.org/10.1016/j.micinf.2015.01.004 (2015).
Hu, Q. et al. Salmonella enterica serovar Senftenberg human clinical isolates lacking SPI-1. J. Clin. Microbiol. 46, 1330–1336. https://doi.org/10.1128/JCM.01255-07 (2008).
Coombes, B. K. et al. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect. Immun. 73, 7161–7169. https://doi.org/10.1128/IAI.73.11.7161-7169.2005 (2005).
Aiastui, A., Pucciarelli, M. G. & Garcia-del Portillo, F. Salmonella enterica serovar typhimurium invades fibroblasts by multiple routes differing from the entry into epithelial cells. Infect. Immun. 78, 2700–2713. https://doi.org/10.1128/IAI.01389-09 (2010).
Cirillo, D. M. et al. Identification of a domain in Rck, a product of the Salmonella typhimurium virulence plasmid, required for both serum resistance and cell invasion. Infect. Immun. 64, 2019–2023 (1996).
Lambert, M. A. & Smith, S. G. The PagN protein of Salmonella enterica serovar Typhimurium is an adhesin and invasin. BMC Microbiol. 8, 142. https://doi.org/10.1186/1471-2180-8-142 (2008).
Roche, S. M. et al. Salmonella Typhimurium invalidated for the three currently known invasion factors keeps its ability to invade several cell models. Front. Cell Infect. Microbiol. 8, 273. https://doi.org/10.3389/fcimb.2018.00273 (2018).
Rosselin, M. et al. Rck of Salmonella enterica, subspecies enterica serovar enteritidis, mediates zipper-like internalization. Cell Res. 20, 647–664. https://doi.org/10.1038/cr.2010.45 (2010).
Schleker, S. et al. The current Salmonella-host interactome. Proteomics Clin. Appl. 6, 117–133. https://doi.org/10.1002/prca.201100083 (2012).
Zhou, D., Mooseker, M. S. & Galan, J. E. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. Proc. Natl. Acad. Sci. USA 96, 10176–10181. https://doi.org/10.1073/pnas.96.18.10176 (1999).
Singh, P. K. et al. Salmonella SipA mimics a cognate SNARE for host Syntaxin8 to promote fusion with early endosomes. J. Cell Biol. 217, 4199–4214. https://doi.org/10.1083/jcb.201802155 (2018).
Mallo, G. V. et al. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J. Cell Biol. 182, 741–752. https://doi.org/10.1083/jcb.200804131 (2008).
Brown, N. F. et al. Mutational analysis of Salmonella translocated effector members SifA and SopD2 reveals domains implicated in translocation, subcellular localization and function. Microbiology 152, 2323–2343. https://doi.org/10.1099/mic.0.28995-0 (2006).
Schroeder, N. et al. The virulence protein SopD2 regulates membrane dynamics of Salmonella-containing vacuoles. PLoS Pathog. 6, e1001002. https://doi.org/10.1371/journal.ppat.1001002 (2010).
Meresse, S., Steele-Mortimer, O., Finlay, B. B. & Gorvel, J. P. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 18, 4394–4403. https://doi.org/10.1093/emboj/18.16.4394 (1999).
Brumell, J. H., Goosney, D. L. & Finlay, B. B. SifA, a type III secreted effector of Salmonella typhimurium, directs Salmonella-induced filament (Sif) formation along microtubules. Traffic 3, 407–415 (2002).
Brawn, L. C., Hayward, R. D. & Koronakis, V. Salmonella SPI1 effector SipA persists after entry and cooperates with a SPI2 effector to regulate phagosome maturation and intracellular replication. Cell Host Microbe 1, 63–75. https://doi.org/10.1016/j.chom.2007.02.001 (2007).
Knodler, L. A., Nair, V. & Steele-Mortimer, O. Quantitative assessment of cytosolic Salmonella in epithelial cells. PLoS ONE 9, e84681. https://doi.org/10.1371/journal.pone.0084681 (2014).
Chong, A. et al. Cytosolic replication in epithelial cells fuels intestinal expansion and chronic fecal shedding of Salmonella Typhimurium. Cell Host Microbe 29, 1177-1185.e1176. https://doi.org/10.1016/j.chom.2021.04.017 (2021).
Knodler, L. A. et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. USA 107, 17733–17738. https://doi.org/10.1073/pnas.1006098107 (2010).
Powers, T. R. et al. Intracellular niche-specific profiling reveals transcriptional adaptations required for the cytosolic lifestyle of Salmonella enterica. PLoS Pathog. 17, e1009280. https://doi.org/10.1371/journal.ppat.1009280 (2021).
Abed, N. et al. Direct regulation of the pefI-srgC operon encoding the Rck invasin by the quorum-sensing regulator SdiA in Salmonella Typhimurium. Mol. Microbiol. 94, 254–271. https://doi.org/10.1111/mmi.12738 (2014).
Kroger, C. et al. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe 14, 683–695. https://doi.org/10.1016/j.chom.2013.11.010 (2013).
Clark, L. et al. Differences in Salmonella enterica serovar Typhimurium strain invasiveness are associated with heterogeneity in SPI-1 gene expression. Microbiology (Reading) 157, 2072–2083. https://doi.org/10.1099/mic.0.048496-0 (2011).
Francis, C. L., Ryan, T. A., Jones, B. D., Smith, S. J. & Falkow, S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364, 639–642. https://doi.org/10.1038/364639a0 (1993).
Schulte, M., Olschewski, K. & Hensel, M. Fluorescent protein-based reporters reveal stress response of intracellular Salmonella enterica at level of single bacterial cells. Cell Microbiol. 23, e13293. https://doi.org/10.1111/cmi.13293 (2021).
Steele-Mortimer, O. The Salmonella-containing vacuole: moving with the times. Curr. Opin. Microbiol. 11, 38–45. https://doi.org/10.1016/j.mib.2008.01.002 (2008).
Smith, A. C. et al. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J. Cell Biol. 176, 263–268. https://doi.org/10.1083/jcb.200611056 (2007).
Drecktrah, D., Knodler, L. A., Ireland, R. & Steele-Mortimer, O. The mechanism of Salmonella entry determines the vacuolar environment and intracellular gene expression. Traffic 7, 39–51. https://doi.org/10.1111/j.1600-0854.2005.00360.x (2006).
Birmingham, C. L., Jiang, X., Ohlson, M. B., Miller, S. I. & Brumell, J. H. Salmonella-induced filament formation is a dynamic phenotype induced by rapidly replicating Salmonella enterica serovar typhimurium in epithelial cells. Infect. Immun. 73, 1204–1208. https://doi.org/10.1128/IAI.73.2.1204-1208.2005 (2005).
Knuff, K. & Finlay, B. B. What the SIF is happening-the role of intracellular Salmonella-induced filaments. Front. Cell. Infect. Microbiol. 7, 335. https://doi.org/10.3389/fcimb.2017.00335 (2017).
Ostrowski, P. P., Fairn, G. D., Grinstein, S. & Johnson, D. E. Cresyl violet: a superior fluorescent lysosomal marker. Traffic 17, 1313–1321. https://doi.org/10.1111/tra.12447 (2016).
Raffatellu, M. et al. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype typhimurium invasion of epithelial cells. Infect. Immun. 73, 146–154. https://doi.org/10.1128/IAI.73.1.146-154.2005 (2005).
Bakowski, M. A., Cirulis, J. T., Brown, N. F., Finlay, B. B. & Brumell, J. H. SopD acts cooperatively with SopB during Salmonella enterica serovar Typhimurium invasion. Cell Microbiol. 9, 2839–2855. https://doi.org/10.1111/j.1462-5822.2007.01000.x (2007).
Giannella, R. A., Washington, O., Gemski, P. & Formal, S. B. Invasion of HeLa cells by Salmonella typhimurium: A model for study of invasiveness of Salmonella. J. Infect. Dis. 128, 69–75. https://doi.org/10.1093/infdis/128.1.69 (1973).
Bueno, S. M. et al. Salmonella pathogenicity island 1 differentially modulates bacterial entry to dendritic and non-phagocytic cells. Immunology 130, 273–287. https://doi.org/10.1111/j.1365-2567.2009.03233.x (2010).
Radtke, A. L., Wilson, J. W., Sarker, S. & Nickerson, C. A. Analysis of interactions of Salmonella type three secretion mutants with 3-D intestinal epithelial cells. PLoS ONE 5, e15750. https://doi.org/10.1371/journal.pone.0015750 (2010).
Martinez-Argudo, I. & Jepson, M. A. Salmonella translocates across an in vitro M cell model independently of SPI-1 and SPI-2. Microbiology 154, 3887–3894. https://doi.org/10.1099/mic.0.2008/021162-0 (2008).
Ibarra, J. A. et al. Induction of Salmonella pathogenicity island 1 under different growth conditions can affect Salmonella-host cell interactions in vitro. Microbiology 156, 1120–1133. https://doi.org/10.1099/mic.0.032896-0 (2010).
Barilleau, E. et al. Investigation of the invasion mechanism mediated by the outer membrane protein PagN of Salmonella Typhimurium. BMC Microbiol. 21, 153. https://doi.org/10.1186/s12866-021-02187-1 (2021).
Chong, A., Starr, T., Finn, C. E. & Steele-Mortimer, O. A role for the Salmonella type III secretion system 1 in bacterial adaptation to the cytosol of epithelial cells. Mol Microbiol https://doi.org/10.1111/mmi.14361 (2019).
Finn, C. E., Chong, A., Cooper, K. G., Starr, T. & Steele-Mortimer, O. A second wave of Salmonella T3SS1 activity prolongs the lifespan of infected epithelial cells. PLoS Pathog. 13, e1006354. https://doi.org/10.1371/journal.ppat.1006354 (2017).
Helaine, S. et al. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343, 204–208. https://doi.org/10.1126/science.1244705 (2014).
Schulte, M., Olschewski, K. & Hensel, M. The protected physiological state of intracellular Salmonella enterica persisters reduces host cell-imposed stress. Commun. Biol. 4, 520. https://doi.org/10.1038/s42003-021-02049-6 (2021).
D’Costa, V. M. et al. Salmonella disrupts host endocytic trafficking by SopD2-mediated inhibition of Rab7. Cell Rep. 12, 1508–1518. https://doi.org/10.1016/j.celrep.2015.07.063 (2015).
Liebl, D., Qi, X., Zhe, Y., Barnett, T. C. & Teasdale, R. D. SopB-mediated recruitment of SNX18 facilitates Salmonella Typhimurium internalization by the host cell. Front. Cell. Infect. Microbiol. 7, 257. https://doi.org/10.3389/fcimb.2017.00257 (2017).
Nunez-Hernandez, C. et al. Genome expression analysis of nonproliferating intracellular Salmonella enterica serovar Typhimurium unravels an acid pH-dependent PhoP-PhoQ response essential for dormancy. Infect. Immun. 81, 154–165. https://doi.org/10.1128/IAI.01080-12 (2013).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–6645. https://doi.org/10.1073/pnas.120163297 (2000).
Lelouard, H. et al. Pathogenic bacteria and dead cells are internalized by a unique subset of Peyer’s patch dendritic cells that express lysozyme. Gastroenterology 138(173–184), e171-173. https://doi.org/10.1053/j.gastro.2009.09.051 (2010).
Valdivia, R. H. & Falkow, S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22, 367–378 (1996).
Namdari, F. et al. Deciphering the roles of BamB and its interaction with BamA in outer membrane biogenesis, T3SS expression and virulence in Salmonella. PLoS ONE 7, e46050. https://doi.org/10.1371/journal.pone.0046050 (2012).
Cheng, S. et al. Identification of a novel Salmonella type III effector by quantitative secretome profiling. Mol. Cell. Proteomics 16, 2219–2228. https://doi.org/10.1074/mcp.RA117.000230 (2017).
Acknowledgements
We thank C. Rossignol for her technical support in confocal imagery and Y. Le Vern for his advice on cytometry, both are from the Imagery and Infectiology team ISP, INRA, University of Tours, Nouzilly, France. We are also grateful to M. Hensel from Osnabrück university for providing the plasmid p4889.
Author information
Authors and Affiliations
Contributions
S.H. designed, performed research and wrote the paper; E.B. performed the adhesion invasion assay, S.C.V. purification and staining, drug assay, controled and helped in different technical aspects; J.T. constructed Salmonella mutant strains and β-lactamase reporter strains; S.R., A.W., I.V.P. and P.V. contributed in discussion and read and corrected the paper, S.G. and J.B.G. performed the electronic microscopy and SM introduced SH to the world of intracellular behavior of Salmonella, as well as reading and correcting the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holbert, S., Barilleau, E., Roche, S.M. et al. Murine AML12 hepatocytes allow Salmonella Typhimurium T3SS1-independent invasion and intracellular fate. Sci Rep 11, 22803 (2021). https://doi.org/10.1038/s41598-021-02054-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02054-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.