Abstract
Glucosamine-6-Phosphate synthase enzyme has been targeted for development of better and safe preservative due to its role in microbial cell wall synthesis. In recent year’s demand of preservatives for the food, cosmetics and pharmaceuticals have increased. Although, the available synthetic preservatives have associated unwanted adverse effects, soa chain of naringin derivatives were schemed synthesized and judged for antioxidant, antimicrobial, preservative efficacy, stability study and topical evaluation. Molecular docking resulted with excellent dock score and binding energy for compound 7, compound 6 and compound 1 as compared to standard drugs. Resultant data of antimicrobial activity revealed compound 7as most potent antimicrobial compound for P. mirabilis, P. aeruginosa, S. aureus, E. coli, C. albicans, and A. niger, respectively, as compared to the standard drugs. The preservative efficacy test of compound 7 in White Lotion USP showed the log cfu/mL value within prescribed limit of USP standard. Compound 7 stabilize the White lotion USP from microbial growth for a period of six months under accelerated storage condition. Compound 7 was further evaluated for toxicity by using the Draize test in rabbits and showed no sign of eye and skin irritation. The outcome demonstrated that synthesized naringin compounds showed glorious antioxidant, antimicrobial, preservative efficacy, stable and safe as compared to standards.
Similar content being viewed by others
Introduction
Foods, cosmetics, and pharmaceutical products containing aqueous base have been reported with a higher risk of microbial growth and undesirable chemical changes. Preservatives like benzoic acid, methylparaben, ethylparaben, sodium benzoate, chlorobutanol, benzyl alcohol, phenyl ethyl alcohol, benzalkonium chloride, etc. have been added to food, pharmaceuticals, biological and other products to improve their shelf life1,2,3,4.
Most of the commercially used preservatives are of synthetic origin and they are associated with harmful side effects viz. cancer, Alzheimer disease, Parkinson disease, type-II diabetes, headache, nausea, weakness, asthma, neurological damage, irritation, allergies, etc.5,6,7,8,9,10,11,12. Hence, there is an urgent need for the search of better, safe and natural preservatives for food, cosmetics and pharmaceutical products. Hence, the various research groups have explored the medicinal plants for their specific antimicrobial and antioxidant along with the preservative efficacy. Among the natural compounds the ferulic acid, gallic acid, caffeic acid, p-coumaric acid, rosemerinic acid etc. have been explored for their preservative potential13,14,15.
Naringin, (7-[[2-O-(6-Deoxy-α-l-mannopyranosyl)-β-d-glucopyranosyl]oxy]-2,3-dihydro-5-hydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) obtained from the grapefruit, lemon, orange juice, pummel, vegetables, Ziziphus spina and Leptospermum scoparium, etc.16,17,18,19,20,21,22. It has been reported to have the potential in the treatment of various human diseases viz. inflammation, cardiovascular disease, antioxidant, diabetes, dyslipidemia, immune system disease, allergy, cancer, neurodegenerative disorder, osteoporosis and neurotrophic effect, etc.23,24,25,26,27,28,29,30,31,32,33,34. Further, the naringin has also been explored in various animal models for anxiety, Parkinson’s disease, sedation, convulsion, neuroprotection, etc.35,36. Naringin and its derivatives viz. naringenin, prunin and alkylprunin esters have also been reported to have potent antibacterial activity against pathogenic bacteria L. monocytogenes, E. coli and S. aureus37,38.
These pharmacological activities of naringin made it a potential candidate for the discovery of novel antimicrobial preservatives.G-6-P synthase is a complex enzyme involved in the formation of UDP-N-acetyl glucosamine and catalyzes the initial step in hexosamine biosynthesis. One of these catalyzed products, N-acetyl glucosamine, is an important part of the peptidoglycan layer of bacterial and fungal cell wall. G-6-P synthase enzyme has been involved in the synthesis of microbial cell wall has been targeted by many researchers including our team for the discovery of new antimicrobials39,40,41. Docking software’s are handy for the screening of thousands of molecules affinity towards a particular disease target and the availability of three-dimensional structure of enzyme G-6-Psynthase (pdb id 1moq) for docking study shall enable the researchers to work in dry lab to develop its inhibitors42,43,44,45. In the present work the in-silico studies for proposed naringin derivatives, their synthesis, evaluation for their antioxidant, antimicrobial, preservative efficacy, stability study, skin membrane permeation study as well as the in-vivo topical and ocular toxicity has been reported.
Experimental
Material and methods
All the analytical grade chemicals were used in the present study and purchased from Loba Chemie (Mumbai, India), Sigma Aldrich (Germany) and SRL (Mumbai, India). Microbiological media like nutrient agar, nutrient broth, and sabouraud dextrose agar and sabouraud dextrose broth were obtained from Hi-media laboratories (Mumbai, India). Standard antimicrobials like streptomycin, ciprofloxacin, ampicillin and fluconazole were obtained from Belco Pharma (Bahadurgarh, India). The standard microbial strains S. aureus MTCC 3160, P. aeruginosa MTCC 1934, E. coli MTCC 45, C. albicans MTCC 183 and A. niger MTCC 282 in lyophilized form were purchased from MTCC, Chandigarh, India. Melting point of naringin derivatives were recorded by Sonar melting point apparatus. Infrared (IR) spectra were recorded on Perkin Elmer FTIR spectrophotometer.1H NMR and 13C NMR spectra were recorded on Bruker Avance II 400 NMR spectrometer. Mass spectra were recorded on Waters Micromass Q-ToF Micro instrument. Elemental analysis was checked on Perkin-Elmer 2400 CHN analyzer. Institutional Animal Ethical Committee of M.D. University, Rohtak, India has approved the experimental protocol for use of rabbits vide letter no. 1767/GO/Re/S/14/CPCSEA, dated- 31/08/2017. Young adult albino rabbits were procured from Lala Lajpat Rai University of Veterinary and Animal Sciences, (Hisar, India). All experiments were performed in accordance with relevant guidelines and regulations.
In silico molecular docking studies
The Schrodinger, Inc. software platform Maestro 10 was used for docking calculations. Laboratory for Preservation Technology and Enzyme Inhibition Studies, Department of Pharmaceutical Sciences, M.D. University, Rohtak, India was used for computational work. The receptor-grid files were generated by grid-receptor generation program (Glide). A conjugate gradient minimization protocol was used in all calculations46.
The energy differences were calculated using the equation:
Protein preparation
Pdb id 1moq (with minimum resolution 1.57 Å) was selected and downloaded from Protein Data Bank. Protein structure was prepared with the protein preparation wizard Prepwiz. During the protein preparation all the water molecules except those coordinated to metals as well between ligand and protein were removed. The energy restrained structure of targeted protein was constructed by using OPLS-2005 force field.
Ligand preparation
The three-dimensional structural of naringin derivatives were constructed by using the Chemdraw ultra 8 and were further preceded for energy minimization by using the LigPrep tool to gain the appropriate conformation through the addition or removal of hydrogen bonds. The partial charges were computed according to the OPLS-2005 force field at biological pH.
ADME studies
In silico prediction for ADME properties of the synthesized compounds were calculated by quick prop from Schrodinger. Various ADME parameters such as Log P, number of rotatable bonds, Log BB, number of hydrogen acceptor and donor atoms were calculated. Lipinski’s rule of five was used for the prediction of drug-likeness properties of synthesized naringin derivatives.
General procedure for the synthesis of naringin derivatives
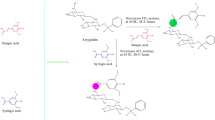
The naringin derivatives were synthesized as per the procedure of Yang et al. and Saini et al. with slide modifications and are outlined in Scheme 147,48. Substituted aniline (0.01 mol) was taken in a round bottom flask and concentrated hydrochloric acid drop wise was added. Equimolar concentration of naringin (0.01 mol) was dissolved in ethanol (50 mL) in equimolar concentration and was refluxed. Completion of the reaction was confirmed by single spot TLC. After the completion of reaction the concentrated reaction mixture was concentrated and the formed precipitated were filtered off desiccated. The crude products were recrystallized using alcohol yielded compound 1–8. The confirmation of the final compounds was made by physicochemical and spectral methods like FTIR, 1H NMR and 13C NMR spectra, Mass spectroscopy and elemental analysis.
Spectral data
Compound 1: 2-(2-(4-(2-chlorophenylimino)-5-hydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-yloxy)-4,5-dihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triol
M.p.: 60–62 °C; TLC (Chloroform:ethanol: 5:1 v/v): Rf = 0.67; Yield = 38.97%; M.Wt. = 690.11; IR (KBr pellets) cm−1: 746 (–Cl– str), 1077 (–C–O–C), 1202 (–C–C–), 1602 (–C=C–), 1638 (–C=N–), 2927 (–C–H–), 3381 (–OH–); 1H NMR (400 MHz, DMSO-d6) δ = 1H NMR (400 MHz, DMSO-d6) δ 8.86 (s, 1H), 8.04 (s, 1H), 7.24–7.17 (m, 3H), 7.15 (dd, J = 7.5, 1.5 Hz, 1H), 6.95 (dd, J = 7.5, 1.5 Hz, 1H), 6.83–6.75 (m, 2H), 6.66 (td, J = 7.5, 1.5 Hz, 1H), 6.27 (dd, J = 14.2, 1.6 Hz, 2H), 5.28 (tt, J = 6.9, 0.7 Hz, 1H), 5.15 (d, J = 6.9 Hz, 1H), 5.02–4.88 (m, 3H), 4.79–4.69 (m, 2H), 4.65 (d, J = 8.6 Hz, 1H), 4.43 (d, J = 8.1 Hz, 1H), 3.90–3.79 (m, 2H), 3.79–3.73 (m, 1H), 3.73–3.65 (m, 2H), 3.65–3.57 (m, 2H), 3.55–3.40 (m, 3H), 3.30–3.11 (m, 3H), 2.63 (dt, J = 12.4, 7.0 Hz, 1H), 2.52 (dt, J = 12.4, 7.0 Hz, 1H), 1.08 (dd, J = 6.7, 1.5 Hz, 3H); 13C NMR (400 MHz, CDCL3) δ = 161.86, 161.84, 158.72, 157.99, 141.99, 133.04, 130.31, 128.53, 127.05, 125.56, 124.66, 120.12, 116.25, 104.88, 103.35, 101.14, 97.13, 96.49, 78.89, 77.04, 75.71, 75.46, 72.64, 71.58, 71.28, 71.10, 70.97, 62.67, 44.27, 36.22, 16.17.; MS ES + (ToF): m/z 690.9 [M+ + 2]; CHNS: Calc (C33H36ClNO13): C, 57.43; H, 5.26; Cl, 5.14; N, 2.03; O, 30.14; Found C, 57.42; H, 5.27; Cl, 5.13; N, 2.02; O, 30.16.
Compound 2: 2-(2-(4-(benzylimino)-5-hydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-Chromen-7-yloxy)-4,5-dihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triol
M.p.: 65–67 °C; TLC (Chloroform:Methanol: 5:1 v/v): Rf = 0.68; Yield = 30.90%; M.Wt. = 669.67; IR (KBr pellets) cm−1: 1073 (–C–O–C), 1171 (–C–C–), 1602 (–C = C–), 1642 (–C=N–), 3029 (–C–H–), 3359 (–OH–); 1H NMR (400 MHz, CDCL3) δ = 1H NMR (400 MHz, DMSO-d6) δ 8.86 (s, 1H), 8.04 (s, 1H), 7.38–7.29 (m, 2H), 7.32–7.23 (m, 1H), 7.27–7.14 (m, 4H), 6.83–6.75 (m, 2H), 6.27 (dd, J = 14.2, 1.6 Hz, 2H), 5.26 (tt, J = 7.0, 0.7 Hz, 1H), 5.15 (d, J = 6.9 Hz, 1H), 4.99–4.88 (m, 2H), 4.76 (d, J = 8.8 Hz, 1H), 4.65 (d, J = 8.6 Hz, 1H), 4.43 (d, J = 8.1 Hz, 1H), 4.32 (dt, J = 8.5, 7.0 Hz, 1H), 3.94–3.79 (m, 4H), 3.83–3.68 (m, 1H), 3.73–3.62 (m, 2H), 3.61 (t, J = 7.0 Hz, 1H), 3.55–3.40 (m, 3H), 3.30–3.11 (m, 3H), 3.08 (q, J = 8.4 Hz, 1H), 2.55 (dt, J = 12.3, 7.0 Hz, 1H), 2.44 (dt, J = 12.3, 7.0 Hz, 1H), 1.08 (dd, J = 6.7, 1.5 Hz, 3H); 13C NMR (400 MHz, CDCL3) δ = 161.59, 161.38, 158.52, 157.99, 139.02, 133.04, 128.53, 128.15, 128.11, 127.42, 116.25, 105.63, 103.35, 100.84, 97.13, 96.63, 78.89, 77.04, 75.71, 75.46, 72.64, 71.58, 71.28, 71.10, 70.97, 62.67, 53.14, 51.29, 37.33, 16.17; MS ES + (ToF): m/z 669.24 [M+ + 2]; CHNS: Calc (C34H39NO13): C, 60.98; H, 5.87; N, 2.09; O, 31.06; Found C, 60.95; H, 5.90; N, 2.08; O, 31.07.
Compound 3: 2-(2-(4-(ethylimino)-5-hydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-yloxy)-4,5-dihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triol
M.p.: 180–182 °C; TLC (Chloroform:Methanol: 5:1 v/v): Rf = 0.61; Yield = 52.78%; M.Wt. = 607.23; IR (KBr pellets) cm−1: 1063 (–C–O–C), 1174 (–C–C–), 1553 (–C=C–), 1687 (–C=N–), 2929 (–C–H–), 3387 (–OH–); 1H NMR (400 MHz, CDCL3) δ = 1H NMR (400 MHz, DMSO-d6) δ 8.86 (s, 1H), 8.04 (s, 1H), 7.24–7.17 (m, 2H), 6.83–6.75 (m, 2H), 6.27 (dd, J = 14.2, 1.6 Hz, 2H), 5.26 (tt, J = 7.1, 0.7 Hz, 1H), 5.15 (d, J = 6.9 Hz, 1H), 4.99–4.88 (m, 2H), 4.76 (d, J = 8.8 Hz, 1H), 4.65 (d, J = 8.6 Hz, 1H), 4.43 (d, J = 8.1 Hz, 1H), 4.29 (dt, J = 8.2, 7.0 Hz, 1H), 3.90–3.79 (m, 2H), 3.79–3.68 (m, 1H), 3.73–3.62 (m, 2H), 3.61 (t, J = 7.0 Hz, 1H), 3.55–3.40 (m, 3H), 3.30–3.11 (m, 3H), 2.91–2.70 (m, 3H), 2.52 (dt, J = 12.3, 7.0 Hz, 1H), 2.41 (dt, J = 12.4, 7.0 Hz, 1H), 1.25–1.15 (m, 3H), 1.08 (dd, J = 6.7, 1.5 Hz, 3H); 13C NMR (400 MHz, CDCL3) δ = 161.59, 161.38, 158.52, 157.99, 133.04, 128.53, 116.25, 105.63, 103.35, 100.84, 97.13, 96.63, 78.89, 77.04, 75.71, 75.46, 72.64, 71.58, 71.28, 71.10, 70.97, 62.67, 52.23, 42.10, 37.33, 16.17, 15.13; MS ES + (ToF): m/z 607.23 [M+ + 2]; CHNS: Calc (C29H37NO13): C, 57.33; H, 6.14; N, 2.31; O, 34.23; Found C, 57.35; H, 6.12; N, 2.30; O, 34.22.
Compound 4: (Z)-2-(4,5-dihydroxy-2-(5-hydroxy-4-(2-hydroxyethylimino)-2-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-yloxy)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triol
M.p. = 130–132 °C; TLC (Chloroform:Methanol: 5:1 v/v):Rf = 0.65; Yield = 32.35%; M.Wt. = 623.6; IR (KBr pellets) cm−1: 1081 (–C–O–C), 1179 (–C–C–), 1628 (–C=C–), 1654 (–C=N–), 2929 (–C–H–), 3257 (–OH–); 1H NMR (400 MHz, CDCL3) δ = 8.86 (s, 1H), 8.04 (s, 1H), 7.50–7.37 (m, 4H), 7.32–7.23 (m, 1H), 7.27–7.17 (m, 2H), 6.83–6.75 (m, 2H), 6.27 (dd, J = 14.2, 1.6 Hz, 2H), 5.27 (tt, J = 6.9, 0.7 Hz, 1H), 5.15 (d, J = 6.9 Hz, 1H), 4.99–4.88 (m, 2H), 4.76 (d, J = 8.8 Hz, 1H), 4.65 (d, J = 8.6 Hz, 1H), 4.43 (d, J = 8.1 Hz, 1H), 4.34–4.21 (m, 2H), 3.90–3.79 (m, 2H), 3.79–3.68 (m, 1H), 3.73–3.62 (m, 2H), 3.61 (t, J = 7.0 Hz, 1H), 3.55–3.40 (m, 3H), 3.30–3.11 (m, 3H), 2.96 (dd, J = 9.1, 8.5 Hz, 1H), 2.55 (dt, J = 12.3, 7.0 Hz, 1H), 2.44 (dt, J = 12.3, 7.0 Hz, 1H), 1.51 (d, J = 6.9 Hz, 3H), 1.08 (dd, J = 6.7, 1.4 Hz, 3H); 13C NMR (400 MHz, CDCL3) δ = 158.62, 157.99, 145.53, 133.04, 128.53, 128.18, 126.65, 126.00, 116.25, 105.27, 103.35, 101.00, 97.13, 96.56, 78.89, 77.04, 75.71, 75.46, 72.64, 71.58, 71.28, 71.10, 70.97, 62.67, 55.92, 51.69, 37.84, 23.74, 16.17; MS ES + (ToF): m/z 623.6 [M+ + 2]; CHNS: Calc (C29H37NO14): C, 56.38; H, 6.36; N, 2.05; O, 35.21; Found C, 56.36; H, 6.38; N, 2.09; O, 35.84.
Compound 5: (Z)-2-(4,5-dihydroxy-2-(5-hydroxy-2-(4-hydroxyphenyl)-4-(isopropylimino)-3,4-dihydro-2H-chromen-7-yloxy)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triol
M.p. = 125–127 °C; TLC (Chloroform:Methanol: 5:1 v/v):Rf = 0.66; Yield = 23.22%; M.Wt. = 621.63; IR (KBr pellets) cm−1: 1054 (–C–O–C), 1156 (–C–C–), 1602 (–C=C–), 1627 (–C=N–), 2950 (–C–H–), 3414 (–OH–); 1H NMR (400 MHz, CDCL3) δ = 8.86 (s, 1H), 8.04 (s, 1H), 7.24–7.17 (m, 2H), 6.83–6.75 (m, 2H), 6.27 (dd, J = 14.2, 1.6 Hz, 2H), 5.27 (tt, J = 6.9, 0.7 Hz, 1H), 5.15 (d, J = 6.9 Hz, 1H), 4.99–4.88 (m, 2H), 4.76 (d, J = 8.8 Hz, 1H), 4.65 (d, J = 8.6 Hz, 1H), 4.43 (d, J = 8.0 Hz, 1H), 4.22 (dt, J = 9.5, 7.0 Hz, 1H), 3.90–3.78 (m, 2H), 3.81–3.67 (m, 1H), 3.71–3.61 (m, 2H), 3.60 (d, J = 6.9 Hz, 1H), 3.57–3.38 (m, 3H), 3.30–3.11 (m, 3H), 2.59–2.43 (m, 2H), 2.48–2.37 (m, 1H), 2.05 (dd, J = 11.2, 9.5 Hz, 1H), 1.53–1.31 (m, 4H), 1.08 (dd, J = 6.7, 1.5 Hz, 3H), 0.90 (t, J = 8.0 Hz, 6H); 13C NMR (400 MHz, CDCL3) δ = 161.71, 161.63, 158.62, 157.99, 133.04, 128.53, 116.25, 105.27, 103.35, 101.00, 97.13, 96.56, 78.89, 77.04, 75.71, 75.46, 72.64, 71.58, 71.28, 71.10, 70.97, 62.67, 61.08, 52.03, 37.84, 28.26, 16.17, 10.17; MS ES + (ToF): m/z 621.61 [M+ + 2]; CHNS: Calc (C30H39NO13): C, 57.96; H, 6.32; N, 2.25; O, 33.46; Found C, 57.95; H, 6.33; N, 2.25; O, 33.45.
Compound 6: 2-(4,5-dihydroxy-2-(5-hydroxy-2-(4-hydroxyphenyl)-4-(3-nitrophenylimino)-3,4-dihydro-2H-chromen-7-yloxy)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triol
M.p.: 140–142 °C; TLC (Chloroform:Methanol: 5:1 v/v): Rf = 0.65; Yield = 60.56%; M.Wt. = 700.64; IR (KBr pellets) cm−1: 990 (–C–O–C), 1082 (–C–C–), 1344 (–NO2), 1518 (–C=C–), 1625 (–C=N–), 2927 (–C–H–), 3324 (–OH–); 1H NMR (400 MHz,CDCL3) δ = 8.86 (s, 1H), 8.04 (s, 1H), 8.03–7.96 (m, 2H), 7.24–7.17 (m, 2H), 6.93–6.85 (m, 2H), 6.83–6.75 (m, 2H), 6.27 (dd, J = 14.2, 1.6 Hz, 2H), 6.14 (d, J = 10.7 Hz, 1H), 5.28 (tt, J = 6.9, 0.7 Hz, 1H), 5.15 (d, J = 6.9 Hz, 1H), 4.99–4.85 (m, 3H), 4.76 (d, J = 8.8 Hz, 1H), 4.65 (d, J = 8.6 Hz, 1H), 4.43 (d, J = 8.1 Hz, 1H), 3.90–3.79 (m, 2H), 3.74 (ddd, J = 12.4, 7.4, 6.8 Hz, 1H), 3.71–3.62 (m, 2H), 3.61 (t, J = 7.0 Hz, 1H), 3.55–3.40 (m, 3H), 3.30–3.11 (m, 3H), 2.62 (dt, J = 12.3, 7.0 Hz, 1H), 2.51 (dt, J = 12.4, 7.0 Hz, 1H), 1.08 (dd, J = 6.7, 1.4 Hz, 3H); 13C NMR (400 MHz, CDCL3) δ = 161.86, 161.84, 158.72, 157.99, 151.03, 141.99, 133.04, 128.53, 125.52, 116.81, 116.25, 104.88, 103.35, 101.14, 97.13, 96.49, 78.89, 77.04, 75.71, 75.46, 72.64, 71.58, 71.28, 71.10, 70.97, 62.67, 44.72, 36.22, 16.17; MS ES + (ToF): m/z 700.21 [M+ + 2]; CHNS: Calc (C33H36N2O15): C, 56.57; H, 5.18; N, 4.00; O, 34.25; Found C, 56.59; H, 5.16; N, 4.01; O, 34.23.
Compound 7: 2-(2-(4-(4-chloro-2-nitrophenylimino)-5-hydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-yloxy)-4,5-dihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triol
M.p. = 130–132 °C; TLC(Chloroform:Methanol: 5:1 v/v): Rf = 0.71; Yield = 75.55%; M.Wt. = 734.17; IR (KBr pellets) cm−1: 764 (–Cl– Str), 1090 (–C–O–C), 1250 (–C–C–), 1341 (–NO2), 1601 (–C=C–), 1639 (–C=N–), 2925 (–C–H–), 3474 (–OH–); 1H NMR (400 MHz, CDCL3) δ = 8.86 (s, 1H), 8.14 (d, J = 1.6 Hz, 1H), 8.07–7.99 (m, 2H), 7.24–7.17 (m, 2H), 6.90 (d, J = 7.4 Hz, 1H), 6.83–6.75 (m, 2H), 6.27 (dd, J = 14.2, 1.6 Hz, 2H), 5.78 (d, J = 10.5 Hz, 1H), 5.28 (tt, J = 6.9, 0.7 Hz, 1H), 5.15 (d, J = 6.9 Hz, 1H), 5.02–4.88 (m, 3H), 4.76 (d, J = 8.8 Hz, 1H), 4.65 (d, J = 8.6 Hz, 1H), 4.43 (d, J = 8.1 Hz, 1H), 3.90–3.79 (m, 2H), 3.74 (ddd, J = 12.4, 7.4, 6.8 Hz, 1H), 3.71–3.62 (m, 2H), 3.61 (t, J = 7.0 Hz, 1H), 3.55–3.40 (m, 3H), 3.30–3.11 (m, 3H), 2.63 (dt, J = 12.4, 7.0 Hz, 1H), 2.52 (dt, J = 12.4, 7.0 Hz, 1H), 1.08 (dd, J = 6.7, 1.4 Hz, 3H); 13C NMR (400 MHz, CDCL3) δ = 161.86, 161.84, 158.72, 157.99, 148.39, 140.87, 133.04, 128.53, 126.05, 125.57, 121.70, 119.23, 116.25, 104.88, 103.35, 101.14, 97.13, 96.49, 78.89, 77.04, 75.71, 75.46, 72.64, 71.58, 71.28, 71.10, 70.97, 62.67, 44.27, 36.22, 16.17; MS ES + (ToF): m/z 734.17 [M+ + 2]; CHNS: Calc (C33H35ClN2O15): C, 53.92; H, 4.80; Cl, 4.82; N, 3.81; O, 32.65; Found C, 53.91; H, 4.81; Cl, 4.80; N, 3.80; O, 32.66.
Compound 8: 2-(2-(4-(benzylimino)-5-hydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-yloxy)-4,5-dihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triol
M.p.: 128–130 °C; TLC (Chloroform:Methanol: 5:1 v/v); Rf = 0.64; Yield = 55.43%; M.Wt. = 683.7; IR (KBr pellets) cm−1: 1029 (–C–O–C), 1170 (–C–C–), 1602 (–C=C–), 1642 (–C=N–), 2875 (–C–H–), 3371 (–OH–); 1H NMR (400 MHz, CDCL3) δ = 8.86 (s, 1H), 8.04 (s, 1H), 7.50–7.37 (m, 4H), 7.32–7.23 (m, 1H), 7.27–7.17 (m, 2H), 6.83–6.75 (m, 2H), 6.27 (dd, J = 14.2, 1.6 Hz, 2H), 5.27 (tt, J = 6.9, 0.7 Hz, 1H), 5.15 (d, J = 6.9 Hz, 1H), 4.99–4.88 (m, 2H), 4.76 (d, J = 8.8 Hz, 1H), 4.65 (d, J = 8.6 Hz, 1H), 4.43 (d, J = 8.1 Hz, 1H), 4.34–4.21 (m, 2H), 3.90–3.79 (m, 2H), 3.79–3.68 (m, 1H), 3.73–3.62 (m, 2H), 3.61 (t, J = 7.0 Hz, 1H), 3.55–3.40 (m, 3H), 3.30–3.11 (m, 3H), 2.96 (dd, J = 9.1, 8.5 Hz, 1H), 2.55 (dt, J = 12.3, 7.0 Hz, 1H), 2.44 (dt, J = 12.3, 7.0 Hz, 1H), 1.51 (d, J = 6.9 Hz, 3H), 1.08 (dd, J = 6.7, 1.4 Hz, 3H); 13C NMR (400 MHz, CDCL3) δ = 158.62, 157.99, 145.53, 133.04, 128.53, 128.18, 126.65, 126.00, 116.25, 105.27, 103.35, 101.00, 97.13, 96.56, 78.89, 77.04, 75.71, 75.46, 72.64, 71.58, 71.28, 71.10, 70.97, 62.67, 55.92, 51.69, 37.84, 23.74, 16.17; MS ES + (ToF): m/z 683.26 [M+ + 2]; CHNS: Calc (C35H41NO13): C, 61.49; H, 6.04; N, 2.05; O, 30.42; Found C, 61.48; H, 6.02; N, 2.05; O, 30.40.
Antioxidant activity
DPPH radical scavenging assay
Antioxidant activity of the synthesized was evaluated by photocolorimetric assay by using DPPH (2,2-diphenyl-1-pycrilhydrazil hydrate) free radical scavenging method. Briefly, 0.1 mM solution of DPPH was prepared in methyl alcohol and 1 mL of this solution was added in to 1 mL of sample or standard. Discolorations were measured at 517 nm after incubation for 30 min at 30 °C in the dark. Lesser absorbance of the reaction mixture indicates the higher free radical scavenging potential. The test was performed in triplicate and the % inhibition values of all the synthesized compounds were calculated by using the formula:
Here, Ac (absorbance of the control) and As (absorbance of the sample)49,50.
Antimicrobial activity
Minimum inhibitory concentrations
Antimicrobial activity of different synthesized compounds was determined against S. aureusMTCC3160, P. aeruginosaMTCC1934, E. coliMTCC45, P. mirabilisMTCC3310, C. albicansMTCC183 and A. nigerMTCC282 by using tube dilution method. For determining the antimicrobial potential dilutions of test and standard compounds were prepared in nutrient broth I.P. (bacteria) and sabouraud dextrose broth I.P. (fungi). After the incubation period the sterilized 0.9% NaCl solution was used to harvest the bacterial and fungal cultures from agar slant through proper shaking and then the suspensions of microorganisms were diluted with the sterile 0.9% NaCl solution to CFU count was adjusted by adjusting the density of microorganism suspension to that of 0.5 McFarland standards by adding distilled water. The number of CFU was determined by dilution pour-plate method. A serial dilution of 50 µg/mL, 25 µg/mL, 12.5 µg/mL, 6.25 µg/mL, 3.12 µg/mL and 1.56 µg/mL was used for determination of MIC. The samples tubes were incubated at 37 °C for 24 h (bacteria), at 25 °C for 7 days (A. niger), and at 37 °C for 48 h (C. albicans), and the results were recorded in pMIC51,52,53.
Preservative effectiveness
White lotion USP was used for the evaluation of preservative efficacy of naringin derivatives. Selected derivatives of naringin were used as preservatives in amount equivalent to the standard taken in White lotion USP prepared as per the method of Khatkar et al. The synthesized compounds 1–8 in equimolar amount (0.0013 mol of methyl paraben) were used as novel preservatives by replacing standard preservatives sodium benzoate, methyl paraben and propyl paraben in both the preparations13.
Challenge microorganisms
Standard microbial strains of S. aureus MTCC 3160, P. aeruginosa MTCC 1934, E. coli MTCC 45, C. albicans MTCC 183 and A. niger MTCC 282 were used as common contaminants as per USP criteria for preservative efficacy testing in the pharmaceutical preparations.
Preparation of inoculums
The slants of E. coli, P. aeruginosa, and S. aureus were incubated at the 37 °C for 24 h. The slants of C. albicans were incubated at 37 °C for 48 h, whereas; the slants of A. niger were incubated at 25 °C for 7 days54.
Test procedure
White lotions USP in final containers was used in the challenge test for preservative efficacy. The preparation was inoculated with a 0.5–1% volume of microbial inoculum having a concentration of 1 × 105–1 × 106 CFU/mL. Inoculated samples were made homogeneous after adding microorganism and incubated. The CFU/mL of White lotion USP was determined at interval of 0 days, 7 days, 14 days, 21 days, and 28 days in agar plates. Log CFU/mL of white lotion USP was calculated as not less than 2.0 log reductions from initial count on 14th day of incubation and no increase in CFU from 14th day count to 28th day in case of bacteria and no increase from the initial calculated count on 14th day and 28th day in case of fungi55,56.
Stability studies of the selected preservatives
From the results of preservative efficacy study, selected compound was further evaluated for its stability as per the protocol provided in ICH guidelines. The selected compound was added in the final containers containing White Lotion USP. The preparation having standard preservative and test compound was stored at 40 ± 2 °C at 75% RH ± 5% RH (as per ICH guidelines) and was analyzed for the change in pH and cfu/ml at the time interval of 0, 1, 2, 3, 4, 5 and 6 months.
Biological evaluation of selected preservatives
Finally, the selected most active compounds from preservative efficacy and stability study were evaluated for in vitro skin permeation, skin and eye irritation study. Naringin compound 7 was selected for their biological evaluation as per the following procedures:
In vitro skin permeation study using Franz diffusion cell
The skin permeation study of selected derivative through transdermal was evaluated by using modified Franz diffusion cell apparatus with a glass diffusion cell along with a receptor and donor cell. The receptor cell has an internal volume of 10 ml and side arm allowed sampling of receptor fluid. The donor cell was clamped on to the top of the receptor cell. Water at 37 °C was circulated through the water jacket surrounding the receptor cell. Strat-M (a synthetic human skin) membrane was used for transdermal diffusion testing. Membrane was kept in freshly prepared solution of phosphate buffer (pH 7.4) and mounted between donor and receptor cell. The receptor was filled with phosphate buffer solution. A volume of 1 ml selected compound 0.1% solution was placed in donor cell, while the receptor cell fluid was kept under stirring and permeation for 120 min. At appropriate time, 1 ml sample was withdrawn from receptor cell at 5, 10, 15, 30, 60 and 120 min., replacing the withdrawn sample with fresh buffer solution. Samples were analyzed by measuring the absorbance at 291 nm. Concentration of different compounds at different time interval was calculated by standard plot57,58.
Skin irritation test in rabbits
Draize test has based on the principle of skin damage caused by direct toxic action of irritant substances. The Draize 24-h patch test in rabbits has been utilized as the most widely used animal test for testing of primary irritant substances59. Test substance (0.5 g) was smoothly applied over the previously shaved rabbit’s skin on 6 cm2 area and covered with gauze patch. If, no irritation has been observed in initial test, then confirmatory test will be conducted in another two rabbits, patch was removed after 4 h of contact and observations were made after 1, 24, 48 and 72 h after patch removal. The dermal irritation scores were recorded on the basis of type and severity of lesions, and graded as per standard Draize test scoring criteria60.
Eye irritation test rabbits
Test substance (100 mg/0.1 ml) was smoothly applied in rabbit’s eyes. No irritation was observed in initial test, (after the 1 h of administration), then confirmatory test was conducted and observations were noted. The grades of ocular reaction (conjunctivae, cornea and iris) will be observed as per OECD guidelines and recorded at an interval of 1, 24, 48, and 72 h after the application of test substance. Different grades for eye irritation severity were recorded as per standard criteria.
Statistical analyses
All the data was represented as mean ± standard deviation (SD) for three triplicates of each sample. One-way ANOVA test at a significance level of 0.05 (p < 0.05) using MS excel statistical tool was used to analyze the experimental data.
Ethics approval and consent to participate
Institutional Animal Ethical Committee of M.D. University, Rohtak, India has approved the experimental protocol for use of rabbits vide letter no. 1767/GO/Re/S/14/CPCSEA, dated- 31/08/2017. Authors also confirming that all experiments were performed in accordance with relevant guidelines and regulations.
Results and discussion
Molecular docking
On the basis of molecular docking and ADMET parameters of proposed naringin derivatives as compound 1 to 8 were selected for further synthesis and biological evaluation. Compound 6 and 7 exhibited dock score (− 7.98 and − 8.45, respectively) and binding energy (− 65.35 kJ/mol and − 69.22 kJ/mol, respectively) as compared to the dock scores (− 5.18, − 5.06, − 5.12) and binding energies (− 37.16 kJ/mol, − 25.41 kJ/mol and − 23.15 kJ/mol) of standard drugs ciprofloxacin, ampicillin, and fluconazole, respectively. Docking results of compound 7, showed the formation of four hydrogen bonds between residues Ala 602, Val 399, Cys 300 and Thr 302 with hydroxyl and oxygen atom of synthesized ligands. Hydrophobic interactions were seen among residues Ser 303 and Glu 488. Compound 6 has been attached to Gln 348, Thr 302 and Val 602 with hydroxyl as well oxygen atom of synthesized compounds by four hydrogen bonds and was also found to interact hydrophobically with Glu 488 and Leu 601 residues. The results of molecular docking for different ligands within G-6-P synthase pocket and their interaction with different amino acid residues have been shown in Table 1. Here, the inhibition of G-6-P synthase enzyme further evaluated by the outcomes of the inhibition likes antimicrobial activity. This further made the clearance behind the inhibition of G-6-P synthase enzyme by different proposed molecules.
ADME study
Different ADMET parameters of proposed aesculin derivatives were determined in silico by QikProp application (Schrodinger LLC) and PreADMET software. The parameters were analyzed and were compared with the standard values. Here, QPPCaco descriptor determined the Caco-2 cell permeability and predicted value defined the barrier between gut and blood system. QPlogBB was the blood/brain partition coefficients determined to be used as a parameter contributing the entry of drugs to the central nervous system. QPPMDCK was used for the estimation of oral absorption. QPlogKp was the descriptor that determined the dermal penetration. The lower values logKp was considered to have low skin permeation and all the selected aesculin derivatives (marked yellow) were having the best suited values range and were to be selected as preservative for topical formulation. QPlogKhsa descriptor determined the binding of drugs to plasma proteins61,62,63,64,65. The different ADME parameters of proposed naringin derivatives have been represented in Table 2. All the results were expressed as mean ± standard deviation (n = 3) and results were found significant p < 0.05.
Chemistry
Scheme 1 was used for the synthesis of selected naringin derivatives. The FTIR data revealed the formation of compound 1 to 8, which was confirmed by peak shifted from 1730 cm−1 (–C=0) to 1633 cm−1, − 1690 cm−1 (–C=N–) and appearance of 750 cm−1 (–Cl–) for compound 1, 1690 cm−1 (–C=N–) for compound 2, 1632 cm−1 (–C=N–) for compound 3, 1629 cm−1 (–C=N–) for compound 4, 1632 cm−1 (–C=N–) for compound 5, 1633 cm−1 (–C=N–) for compound 6, 1690 cm−1 (–C=N–), for compound 7 and 1693 cm−1 (–C=N–) for compound 8 respectively. The change in chemical shift value, coupling constant and multiplicities were analyzed by 1HNMR and 13C NMR signals of synthesized compounds. The FTIR, 1H NMR, 13C NMR data, mass spectroscopy, and elemental analysis confirmed the chemical structures of synthesized naringin derivatives.
Antioxidant activity
DPPH radical scavenging activity
DPPH free radical scavenging assay confirmed that compounds 7, 6 and 1 possessed good antioxidant potential with IC50 6.23 ± 0.03 µM, 7.03 ± 0.03 µM, 7.31 ± 0.06 µM, respectively as compared to standard l-ascorbic acid IC508.11 ± 0.06 µM. Antioxidant potential (IC50) of other synthesized naringin derivatives Compound 2, 3, 4, 5, 8 and naringin itself has been found as 14.52 ± 0.40 µM, 10.62 ± 0.01 µM, 18.77 ± 0.06 µM, 11.32 ± 0.16 µM, 20.9 ± 0.26 µM and 6.36 ± 0.36 µM, respectively. Here, the better antioxidant property of amygdalin derivative shall be useful in the preservation of food, cosmetics, and pharmaceuticals66.
Antimicrobial activity
Minimum inhibitory concentrations
The recorded pMIC values, revealed compound 7 as most effective antimicrobial (pMIC 2.07, 2.37, 2.07, 2.37, 1.77, and 1.77 µM/mL for P. mirabilis, P. aeruginosa, S. aureus, E. coli, C. albicans, and A. niger, respectively), as compared to the standard drugs ciprofloxacin (pMIC 1.12, 1.42, 1.12, and 1.42 µM/mL for P. mirabilis, P. aeruginosa, S. aureus, and E. coli, respectively), Ampicillin (pMIC 1.14, 0.84, 0.84, and 1.74 µM/mL for P. mirabilis, P. aeruginosa, S. aureus, and E. coli, respectively) and fluconazole (pMIC 1.08, and 1.38 µM/mL for C. albicans, and A. niger, respectively). The results of antimicrobial activity revealed that the synthesized compounds have antimicrobial potential as compared to standard drugs as shown in Table 3. The probable mechanism of antimicrobial activity of naringin derivatives may be due to the better inhibition of G-6-Psynthase.
Preservative efficacy study
The highly active antimicrobial compounds 6 and 7 in the series were selected for the evaluation of preservative efficacy. The results of preservative efficacy testing were performed in triplicate and have been reported as mean values in Table 4. Compound 7 showed the values of log CFU/mL reduction within the prescribed limit and the results were comparable to that of the standard preservatives sodium benzoate, propyl paraben and methyl paraben. Result of compound 6 showed a less than 2.0 log reductions from initial count on 14 days and number of CFU/ml values of preservative efficacy against some microbial strains increased on the 14th day to 28th day as compare to standard preservatives sodium benzoate, propyl paraben and methyl Paraben and found significant with p < 0.05.Preservative efficacy of compound 7 in White lotion USP and degree of microbial log reduction have been represented in Fig. 1.
Stability study
Results of stability study revealed that the pH of White lotion USP samples were in range of 5.5–6.0.The results of the microbial study indicated that no microbial growth was observed in samples containing compound 7 over a period of six months period as per ICH guidelines. These results indicated that the product was stable as compared to standard preservative with added naringin compound 7 as preservative. The results of stability study were performed in triplicate and were reported as mean values. Results for microbial growth and pH changes also found to be significant at p < 0.05.
Biological evaluation of selected preservatives
In vitro skin permeation study using Franz diffusion cell
In vitro skin permeation study of selected compound 7 was performed by using Franz diffusion cell having receptor and donor cell. Concentration of compound 7 at different time interval was calculated by standard plot. It was observed from the skin permeation data with different time intervals that maximum amount of drug was released within 120 min. It was concluded from the skin permeation data as shown in Table 5 that the maximum amount of drug released within first 120 min i.e. 45.05%. The total amount of selected compound 7 that was permeated through selected skin graft was found to be 4.27 mg (0.0013 mol of sample taken).
Skin irritation test of naringin derivative in rabbits
The dermal irritation scores were evaluated by the type and severity of the lesions produced. All the rabbits were checked for the reversibility of any skin reaction up to 14 days, by evaluating skin irritation responses i.e. alopecia, hyperplasia, hyperkeratosis and scaling. Scoring of the dermal reaction was done with the standard OECD guideline-404. The test compound 7 did not showed any type of dermal reaction and was considered as non-irritant. No skin erythema and edema formation was observed during test. The irritation score for erythema and edema in all the rabbits were found have Score 0. Skin area of rabbit used for testing of compound 7 before and after application have been shown in Fig. 2.
Eye irritation test of naringin derivative in rabbits
Compound 7 was also evaluated for eye irritation test in rabbits. The observations were checked for reversibility of any eye irritation reaction up to 21 days of test compound application. The grades of ocular toxicity reactions were observed and recorded at 1, 24, 48, and 72 h following test substance application as per standard OECD guideline-405. The test compound compound 7 did not showed any type of eye irritation reaction and was considered as non irritant for eyes. No ulceration, conjunctivae redness and chemosis were observed in eye irritation test. The irritation score for cornea chemosis, conjunctivae and iris in all the rabbits were found to have 0. Changes in produced in the eyes of the rabbits used for testing of compound 7 before and after application have been shown in Fig. 3.
Structure activity relationship (SAR) studies
Design approach of naringin derivative for G-6-P inhibition and antioxidant activity has been represented in Fig. 4. The structure activity relationship of the synthesized naringin derivatives with their antioxidant activity results have been summarized as:
-
1.
The substitution of carbonyl group of naringin with aliphatic aliphatic amines decreased the biological activity i.e. compounds 3, 4, 5 shows lower activity as compared to compounds 1, 2 and 7.
-
2.
The substitution of carbonyl group of naringin with aromatic amide ring attached directely to Naringin enhanced the biological activity.
-
3.
The presence of electronegative halides on ortho position of aromatic amide ringj enhanced the biological activity (compound 1 and 7).
-
4.
The presence of nitro group on para position of aromatic amide ringj enhanced the biological activity (compound 6 and 7).
Conclusion
The above mentioned wet and dry laboratory study results clarify the mechanism of enzyme G-6-Psynthase inhibition. The most active compound 7 i.e. 2-(4,5-dihydroxy-2-(5-hydroxy-2-(4-hydroxyphenyl)-4-(4-nitrophenyl amino)-3,4-dihydro-2H-chromen-7-yloxy)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3-yloxy)-6-methyl-tetrahydro-2H-pyran-3,4,5-triolshowed antioxidant, antimicrobial, better preservative efficacy and prevent the change in pH as well microbial count of formulation for food as well as pharmaceutical products, which were in agreement with the results of molecular docking and highlight the mechanism of their preservative activity. Therefore, the synthesized amygdalin derivatives can be used as novel food and pharmaceutical preservatives to prevent them from microbial degradation.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADMET:
-
Absorption, distribution, metabolism, excretion and toxicity
- G-6-Psynthase:
-
Glucosamine-6-phosphate synthase
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- UDP-N-acetyl glucosamine:
-
Uridine diphosphate N-acetylglucosamine
- FTIR:
-
Fourier-transform infrared spectroscopy
- 1H NMR:
-
Proton nuclear magnetic resonance
- 13C NMR:
-
Carbon 13 nuclear magnetic resonance
- UV:
-
Ultra violet
- TLC:
-
Thin layer chromatography
- IC50 :
-
Inhibitory concentration
- MIC:
-
Minimum inhibitory concentrations
- CFU:
-
Colony forming unit
- HBA:
-
Hydrogen bond acceptor
- HBD:
-
Hydrogen bond donor
- MW:
-
Molecular weight
- MTCC:
-
Microbial type culture collection
- DMSO:
-
Dimethyl sulfoxide
- BOD:
-
Biological oxygen demand
- USP:
-
Unites state pharmacopoeia
- PDB ID:
-
Protein data bank identification
- OPLS:
-
Optimized potential for liquid simulations
References
Hugo, W.B. & Russell, A.D. Pharmaceutical Microbiology, 7th edn (Blackwell Science, Hoboken, 2004) http://hdl.handle.net/123456789/434.
Kulkarni, C., Deshpande, A. & More, S. Assessment of microbial contamination in commercial herbal oral medicinal liquids. Int. J. Pharm. Res. Dev. 2(9), 191–193. https://doi.org/10.13040/IJPSR.0975-8232.4(7).24960-01 (2010).
Seetaramaiah, K., Anton, S. A., Murali, R. & Manavalan, R. Preservatives in food products—review. Int. J. Pharm. Biol. Arch. 2, 583–599 (2011).
Shaikh, S. M., Doijad, R. C., Shete, A. S. & Sankpal, P. S. A review on: Preservatives used in pharmaceuticals and impacts on health. Pharma Tutor 4(5), 25–34 (2016).
Rowe, R.C., Sheskey, P.J. & Quinn, M.E. A Handbook of Pharmaceutical Excipients, 6th edn, 190–195 (Marcel-Deckker, New York, 2009). https://ptabdata.blob.core.windows.net/files/2017/PGR201700022/v47_Grun%20Exh.%201062.pdf.
Golightly, L. K., Smolinske, S. S., Bennett, M. L., Sutherland, E. W. & Rumack, B. H. Pharmaceutical excipients: Adverse effects associated with inactive ingredients in drug products Part I. Med. Toxicol. Adverse Drug Exp. 3, 128–165. https://doi.org/10.1007/bf03259883 (1988).
Furrer, P., Mayer, J. M. & Gurny, R. Ocular tolerance of preservatives and alternatives. Eur. J. Pharm. Biopharm. 53, 263–280. https://doi.org/10.1016/s09396411(01)00246-6 (2002).
Rowe, R.C., Sheskey, P.J., Cook, W.G. & Fenton, M.E. Handbook of Pharmaceutical Excipients, 7th edn, 784–790 (Pharmaceutical Press, London, 2012). https://doi.org/10.12691/ajps-3-5-3.
Graf, P., Hallen, H. & Juto, J. E. Benzalkonium chloride in a decongestant nasal spray aggravates rhinitis medicamentosa in healthy human volunteers. Clin. Exp. Allergy. 25, 395–400. https://doi.org/10.1111/j.1365-2222.1995.tb01069.x (1995).
Cooper, S. M. & Shaw, S. Allergic contact dermatitis from parabens in a tar shampoo. Contact Dermatitis. 39, 140. https://doi.org/10.1111/j.1600-0536.1998.tb05871.x (1998).
Soni, M. G., Carabin, I. G. & Burdock, G. A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 43, 985–1015. https://doi.org/10.1016/j.fct.2005.01.020 (2005).
Vilaplana, J. & Romaguera, C. Contact dermatitis from paraben used as preservatives in eye drops. Contact Dermatitis. 43, 248–258. https://doi.org/10.1097/DER.0000000000000061 (2000).
Khatkar, A., Nanda, A. & Narasimhan, B. Evaluation of preservative effectiveness of p-coumaric acid derivatives in aluminium hydroxide gel-USP. Chron. Young Sci. 4, 144–147. https://doi.org/10.4103/2229-5186.115554 (2013).
Khatkar, A., Nanda, A., Kumar, P. & Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab. J. Chem. 10, S3804–S3815. https://doi.org/10.1016/j.arabjc.2014.05.018 (2017).
Balasubramanian, N., Khatkar, A. & Nanda, A. Evaluation of preservative effectiveness of ferulic acid derivatives in aluminium hydroxide gel- USP. Int. J. Pharm. Sci. Res. 4(7), 1000–1004. https://doi.org/10.13040/IJPSR.0975-8232.4(7).2721-25 (2013).
Ahmed, B. H., Obbed, A. Y., Wabaidur, M. S., Al Othman, S. M. & Al-Shaalan, Z. A. High-performance liquid chromatography analysis of phenolic acid, flavonoid, and phenol contents in various natural Yemeni honeys using multi-walled carbon nanotubes as a solid-phase extraction adsorbent. J. Agric. Food Chem. 62, 5443–5450. https://doi.org/10.1021/jf5011758 (2014).
Berhow, M., Tisserat, B., Kanes, K. & Vandercook, C. Survey of phenolic compounds produced in citrus. U.S.D.A. A.R.S. Tech. Bull. 1856, 1–154 (1998).
Berhow, M. A. & Vandercook, C. E. Biosynthesis of naringin and prunin in detached grapefruit. Phytochemistry 28, 1627–1630. https://doi.org/10.1016/S0176-1617(11)80266-X (1989).
Yusof, S., Ghazali, H. M. & King, G. S. Naringin content in local citrus fruits. Food Chem. 37, 113–121. https://doi.org/10.1016/j.jfca.2005.12.006 (1990).
Horowitz, R. M. Relations between the taste and structure of some phenolic glycosides. In Biochemistry of Phenolic Compounds (ed. Harbone, J. B.) 545–571 (Academic Press, New York, 1964). https://doi.org/10.2307/2440572.
Habib, H. M., Al Meqbali, F. T., Kamal, H., Souka, U. D. & Ibrahim, W. H. Bioactive components, antioxidant and DNA damage inhibitory activities of honeys from arid regions. Food Chem. 153, 28–34. https://doi.org/10.1016/j.foodchem.2013.12.044 (2014).
Wabaidur, S. M., Ahmed, Y. B. H., Alothman, Z. A., Obbed, M. S. & Al-Harbi, N. M. Ultra high-performance liquid-chromatography with mass spectrometry method for the simultaneous determination of phenolic constituents in honey from various floral sources using multi walled carbon nanotubes as extraction sorbents. J. Sep. Sci. 38, 2597–2606. https://doi.org/10.1002/jssc.201500407 (2015).
Arts, I. C. W. & Hollman, P. C. H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 81, 317–325. https://doi.org/10.1093/ajcn/81.1.317S (2005).
Curro, M., Risitano, R., Ferlazzo, N., Cirmi, S. & Gangemi, C. Citrus bergamiajuice extract attenuates β-amyloid-induced pro-inflammatory activation of thp-1 cells through mapk and ap-1 pathways. Sci. Rep. 6, 208–209. https://doi.org/10.1007/s00726-016-2366-1 (2016).
Graf, B. A., Milbury, P. E. & Blumberg, J. B. Flavonols, flavonones, flavanones and human health: Epidemological evidence. J. Med. Food. 8, 281–290. https://doi.org/10.1089/jmf.2005.8.281 (2005).
Marder, M. & Paladini, A. C. GABA(A) receptor ligands of flavonoid structure. Curr. Top. Med. Chem. 2(8), 853–867. https://doi.org/10.2174/1568026023393462 (2006).
Medina, J. H., Paladini, A. C., Wolfman, C., Levi, M. & Calvo, D. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand form benzodiazepine receptors, with anticonvulsant properties. Biochem. Pharmacol. 40(10), 2227–2231. https://doi.org/10.1016/0006-2952(90)90716-x (1990).
Johnston, G. A. GABA (A) receptor channel pharmacology. Curr. Pharm. Des. 11(15), 1867–1885. https://doi.org/10.2174/1381612054021024 (2005).
Ferlazzo, N., Cirmi, S., Russo, M., Trapasso, E. & Ursino, M. R. NF-κB mediates the antiproliferative and proapoptotic effects of bergamot juice in HepG2 cells. Life Sci. 146, 81–91. https://doi.org/10.1016/j.lfs.2015.12.040 (2016).
Ferlazzo, N., Visalli, G., Smeriglio, A., Cirmi, S. & Lombardo, G. E. Flavonoid fraction of orange and bergamot juices protect human lung epithelial cells from hydrogen peroxide-induced oxidative stress. Evid. Based Complement. Altern. Med. https://doi.org/10.1155/2015/957031 (2015).
Filocamo, A., Bisignano, C., Ferlazzo, N., Cirmi, S. & Mandalari, G. In vitro effect of bergamot (Citrus bergamia) juice against caga-positive and negative clinical isolates of helicobacter pylori. BMC Complement. Altern. Med. 15, 256. https://doi.org/10.1186/s12906-015-0769-2 (2015).
Impellizzeri, D., Bruschetta, G., Paola, R., Ahmad, A. & Campolo, M. The anti-inflammatory and antioxidant effects of bergamot juice extract (BJe) in an experimental model of inflammatory bowel disease. Clin. Nutr. 34, 1146–1154. https://doi.org/10.1186/s12906-015-0769-2 (2015).
Adams, M., Gmunder, F. & Hamburger, M. Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. J. Ethnopharmacol. 113, 363–381. https://doi.org/10.1016/j.jep.2007.07.016 (2007).
Guzman-Gutierrez, S. L. & Navarrete, A. Pharmacological exploration of the sedative mechanism of hesperidin identified as the active principle of Citrus sinensis flowers. Planta Med. 75(4), 295–301. https://doi.org/10.1055/s-0029-1185306 (2009).
Fernandez, S. P., Nguyen, M., Yow, T. T., Chu, C. & Johnston, G. A. R. The flavonoid glycosides, Myricitrin, Gossypin and Naringin exert anxiolytic action in mice. Neurochem. Res. 34(10), 1867–1875. https://doi.org/10.1007/s11064-009-9969-9 (2009).
Fernandez, S. P., Wasowski, C., Loscalzo, L. M., Granger, R. E. & Johnston, G. A. Central nervous system depressant action of flavonoid glycosides. Eur. J. Pharmacol. 539(3), 168–176. https://doi.org/10.1016/j.ejphar.2006.04.004 (2006).
Celiz, G., Daz, M. & Audisio, M. C. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microbiol. 111(3), 731–738. https://doi.org/10.1111/j.1365-2672.2011.05070.x (2011).
Pereira, R. M., Andrades, N. E., Paulino, N., Sawaya, A. C. & Eberlin, M. N. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, anti-inflammatory and tumor cell cytotoxicity. Molecules 12, 1352–1366. https://doi.org/10.3390/12071352 (2007).
Lather, A., Sharma, S. & Khatkar, A. Virtual screening of novel glucosamine-6-phosphate synthase inhibitors. C.C.H.T.S. 21, 1–12. https://doi.org/10.2174/1386207321666180330114457 (2018).
Floquet, N., Richez, C., Durand, P., Maigret, B. & Badeta, B. Discovering new inhibitors of bacterial glucosamine-6P synthase GlmS) by docking simulations. Bioorganic Med. Chem. Lett. 17, 1966–1970. https://doi.org/10.1016/j.bmcl.2007.01.052 (2007).
Sarojini, B. K., Krishna, B. G., Darshanraj, C. G., Bharath, B. R. & Manjunatha, H. Synthesis, characterization, in vitro and molecular docking studies of new 2,5-dichloro thienyl substituted thiazole derivatives for antimicrobial properties. Eur. J. Med. Chem. 45, 3490–3496. https://doi.org/10.1016/j.ejmech.2010.03.039 (2010).
Black, M. T. Evidence that the catalytic activity of prokaryote leader peptidase depends upon the operation of a serine-lysine catalytic dyad. J. Bacteriol. 175, 4957–4961. https://doi.org/10.1128/jb.175.16.4957-4961.1993 (1993).
Black, M. T. & Hodgson, J. Novel target sites in bacteria for overcoming antibiotic resistance. Adv. Drug Deliv. Rev. 57, 1528–1538. https://doi.org/10.1016/j.addr.2005.04.006 (2005).
Bockstael, K. & Aerschot, A. V. Antimicrobial resistance in bacteria. Central Eur. J. Med. 4(2), 141–155. https://doi.org/10.2478/s11536-008-0088-9 (2009).
Soper, T. S. & Manning, J. M. Different modes of action of inhibitors of bacterial d-amino acid transaminase. A target enzyme for the design of new antibacterial agents. J. Biol. Chem. 256, 4263–4268. https://doi.org/10.1021/acschembio.7b00142 (1981).
Friesner, R. A., Murphy, R. B., Repasky, M. P., Frye, L. L. & Greenwood, J. R. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 49, 6177–6196. https://doi.org/10.1021/jm051256o (2006).
Yangn, Z. & Sun, P. Campare of three ways of synthesis of simple Schiff base. Molbank. https://doi.org/10.3390/M514 (2006).
Saini, M. et al. Synthesis, in vitro antimicrobial, anticancer evaluation and QSAR studies of N′-(substituted)-4-(butan-2-lideneamino) benzohydrazides. Arab. J. Chem. 7, 448–460. https://doi.org/10.1016/j.arabjc.2013.05.010 (2014).
Blois, M. S. Antioxidant determinations by the use of a stable free radical. Nature 181(4617), 1199–1200. https://doi.org/10.1038/1811199a0 (1958).
Mohamed, S. K., Ahmed, A. A. A., Yagi, S. M. & Alla, A. E. W. H. A. Antioxidant and antibacterial activities of total polyphenols isolated from pigmented sorghum (Sorghum bicolor) Lines. J. Genet. Eng. Biotechnol. 7(1), 51–58. https://doi.org/10.30574/gscbps.2020.10.1.0241 (2009).
Cappucino, J.G. & Sherman, N. Microbiology—A Laboratory Manual, 263; https://sacmicro.files.wordpress.com/2017/02/cappuccino-jamessherman-natalie-microbiology-a-laboratory-manual-pearson-education-2014.pdf (Addison Wesley, Boston, 1999).
Indian Pharmacopoeia, Vol-I. Indian Pharmacopoeia Commission. The Controller of Publications, New Delhi, 37. http://pharmacentral.in/wp-content/uploads/2018/05/INDIAN%20PHARMACOPOEIA%202007.pdf (2007).
Kowser, M. M. & Fatema, N. Determination of MIC and MBC of selected azithromycin capsule commercially available in Bangladesh. ORION Med. J. 32(1), 619–620. https://doi.org/10.4236/aim.2014.412091 (2009).
Indian Pharmacopoeia, 27–28 https://igmpiindia.org/ipc.pdf (Indian Pharmacopoeia Commission, Ghaziabad, 2010).
Dafale, N. A., Semwal, U. P., Agarwal, P. K., Sharma, P. & Singh, G. N. Valuation of preservative effectiveness in antacid, cough syrup and ophthalmic solution by microbial challenge test. Int. J. Pharm. 1(3), 193–199. https://doi.org/10.13040/IJPSR.09758232.1(3).193-99 (2014).
The United States Pharmacopoeia. Antimicrobial effectiveness testing. 214850; (United States Pharmacopoeial Conventon Inc., Rockville, 2004). https://www.drugfuture.com/Pharmacopoeia/usp35/PDF/00520054%20%5B51%5D%20ANTIMICROBIA%20EFFECTIVENESS%20TESTING.pdf.
Liebenberg, W., Engelbrecht, E., Wessels, A., Devarakonda, B. & Yang, W. A comparative study of the release of active ingredients from semisolid cosmaceuticals measured with Franz, Enhancer or Flow through cell diffusion apparatus. J. Food Drug Anal. 12(1), 19–28 (2012).
Prabha, K. S., Ramakrishna, C., Srivani, M., Priyanka, V. N. & Priya, Y. B. Comparative invitro release of diclofenac sodium gel from different marketed products. Int. J. Life Sci. Pharm. Res. 2(3), 88–93 (2012).
Beuro of Indian Standards. Indian Standard (4011:1997). Methods of Test for Safety Evaluation of Cosmetics, 2nd revision; http://bwcindia.org/Web/Info&Action/Legislation/TestingofCosmetics.pdf (2008).
Yadav, N. P., Meher, J. G., Pandey, N., Luqman, S. & Yadav, K. S. Enrichment, development, and assessment of Indian basil oil based antiseptic cream formulation utilizing hydrophilic-lipophilic balance approach. Biomed. Res. Int. https://doi.org/10.1155/2013/410686 (2013).
Hopkins, A. L. & Groom, C. R. The drug gable genome. Nat. Rev. Drug Discov. 1, 27–733. https://doi.org/10.1038/nrd892 (2002).
Irvine, J. D., Takahashi, L., Lockhart, K., Cheong, J. & Tolan, J. W. MDCK (Madin Darby Canine Kidney) cells: A tool for membrane permeability screening. J. Pharm. Sci. 88(1), 28–33. https://doi.org/10.1021/js9803205 (1999).
Kulkarni, A., Han, Y. & Hopfinger, A. J. Predicting Caco-2 cell permeation coefficients of organic molecules using membrane-interaction QSAR analysis. J. Chem. Inf. Comput. Sci. 42(2), 331–342. https://doi.org/10.1021/ci010108d (2002).
Teague, S. J., Davis, A. M., Leeson, P. D. & Opera, T. A. The design of lead like combinatorial libraries. Chem. Int. Ed. Eng. 38, 3743–3748 (1999).
Veber, D. F., Johnson, S. R., Cheng, H. Y., Smith, B. R. & Ward, K. W. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623. https://doi.org/10.1021/jm020017n (2002).
Basanta, M. F. Antioxidant Japanese plum (Prunussalicina) microparticles with potential for food preservation. J. Funct. Foods. 24, 287–296. https://doi.org/10.1016/j.jff.2016.04.015 (2016).
Acknowledgements
Authors are grateful to the Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, Haryana (India) for providing the necessary facilities to carry out this research work.
Funding
No funding received for this research work from outside sources.
Author information
Authors and Affiliations
Contributions
The authors A.L., S.S. and A.K. have designed, synthesized and carried out the work in equal contribution. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lather, A., Sharma, S. & Khatkar, A. Naringin derivatives as glucosamine-6-phosphate synthase inhibitors based preservatives and their biological evaluation. Sci Rep 10, 20477 (2020). https://doi.org/10.1038/s41598-020-77511-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77511-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.