Abstract
Secondary spontaneous pneumothorax (SSP) occurs in the context of underlying pulmonary disease. Our objectives were to estimate the relationship between SSP and short term air pollution exposure with nitrogen dioxide (NO2), ozone (O3) and particulate matter with a diameter ≤ 10 μm (PM10). Patients with SSP were included between June 1, 2009 and May 31, 2013, in 14 Emergency Departments in France. In this case–crossover design study, PM10, NO2, and O3 data were collected hourly from monitoring stations. Quantitative values, fast increase in air pollutant concentration, and air quality threshold exceedance were retained. These assessments were calculated for each of the 4 days prior to the event (Lag 1–Lag 4) for all case and control period, and for the entire exposure period. A total of 135 patients with SSP were included, with a mean age of 55.56 (SD 18.54) years. For short term exposure of PM10, NO2 and O3, no differences were observed between case and control periods in terms of quantitative values of air pollutant exposure (P > 0.68), fast increase in concentration (P > 0.12) or air quality threshold exceedance (P > 0.68). An association between O3 exposures cannot be ruled out, especially when considering the Lag 2 prior to the event and in warm seasons.
Similar content being viewed by others
Introduction
The link between air pollution and respiratory disease has been established, even if the role of each pollutant has not yet been clearly identified. Li et al.1 demonstrated, with a case–crossover design, that air pollutants have a short term effect on a variety of acute respiratory diseases. Particulate matter with diameter ≤ 10 μm and ≤ 2.5 µm (PM10 and PM2.5), nitrogen dioxide (NO2), sulfur dioxide (SO2) and carbon monoxide (CO) had positive associations with outpatient visits for upper respiratory tract infection, acute bronchitis, community acquired pneumonia, acute exacerbation of bronchiectasis and acute exacerbation of chronic obstructive pulmonary disease (COPD). Two meta-analyses confirmed these results for COPD related emergency department visits, hospital admission and mortality2,3. Concerning ozone (O3), a positive association with outpatient visits for acute exacerbation of asthma and COPD was demonstrated1,4,5. For other respiratory diseases, such as pneumothorax, very few studies have documented the relationship with air pollution. Relationships were reported between the occurrence of primary spontaneous pneumothorax (PSP) and interactions with higher values of air pollutants and other meteorological parameters, such as atmospheric pressure, temperature and humidity6,7,8,9. Abul et al.10 showed a link between an increase in O3 and the occurrence of PSP. In a previous study, we demonstrated a lack of connection between PSP and NO2 and PM10 exposure, but could not rule out an association between O3 exposure and PSP11. Secondary spontaneous pneumothorax (SSP) occurs in the context of underlying pulmonary disease. Among several recognized causes of SSP, the most common aetiology is COPD, which accounts for 70–80%12. Therefore, it is important to analyse a potential link between SSP and air pollution. The objectives of this study were: (1) to estimate the relationships between SSP and short term air pollution exposure with PM10, NO2 and O3; and (2) to investigate a time lag effect between exposure and the occurrence of SPP.
Methods
The design of this study is a time-stratified case–crossover13. This design is used to study environmental conditions, particularly air pollution14,15,16,17. This study implicated Emergency Departments from 14 medium-sized cities distributed over all the territory of France (Fig. 1).
Geographical location of the 14 participating centers. Seven university teaching hospitals, namely Angers, Besançon, Clermont-Ferrand, Dijon, Grenoble, Poitiers and Toulouse; and seven general, non-academic hospitals, namely Belfort, Béthune, Boulogne sur Mer, Lomme, Roubaix, Mulhouse and Vesoul. Maximal distance between two hospitals: north–south 798 km; east–west 593 km (maps created with the software Microsoft Word 2013 15.0 and Paint 6.1 from Microsoft Windows 7 Professionnel https://www.microsoft.com/fr).
For each subject, a case period was compared to multiple other control periods dispersed over the month concerned by the occurrence of the event. The date of admission to the Emergency Department determined the date of occurrence of the SSP. Case period was defined as the period leading up to the date of admission, concerning the total time during which the subject was exposed to the risk of occurrence of SPP. A period of 4 days was retained, i.e., Lag 1–Lag 4, before the occurrence of the event. Control periods were defined as the same weekdays as the case period within the same month. The exposure period corresponded as all case and control periods for each subject.
Study population
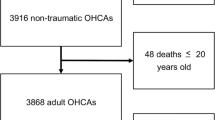
All patients with pneumothorax aged over 18 years and who provided written informed consent from, the period of June 1, 2009, to May 31, 2013, were included. Patients with traumatic and PSP were excluded. Recurrent episodes, defined as a second episode of SSP during the period of inclusion, were excluded. Subjects living more than 30 km (19 miles) from an air pollutant monitoring station, or in a different study area were also excluded.
Data collected from the Emergency Department medical records were the following: age, sex, date of admission, home address, smoking status, and history of COPD, emphysema, pneumothorax and asthma. All research was performed in accordance with relevant regulations and informed consent was obtained from all the participants. The study was approved by the French National authority for the protection of privacy and personal data (CNIL) on December 23, 2014 under the number 913594.
Environmental data
Air pollution exposure was assessed using measurements provided by the French National AASQA Network, which is accredited by the French Ministry for the Environment and whose quality is guaranteed by ISO/TC146 standard for air quality. Measurements, data control and the maintenance of the instrumentation are standardized while respecting French and European standards. Hourly mean concentration of NO2, O3 and PM10 were collected from each monitoring station of the study area. The number of stations ranged between one to nine per area (mean 5.9). Each patient’s home address was used to estimate the exposure to air pollutants for the case and control periods. The association between the patient’s home address and the appropriate monitoring station was determined by the local AASQA team. To select the most representative station for measuring the exposure of each patient, different parameters were considered: the distance between the geolocated address and the station, the geomorphological situation and the proximity of significant sources of air pollution.
Three exposure assessments were selected. First, the maximum and mean absolute quantitative values of PM10, NO2 and O3 were determined. Second, the fast increase in air pollutant concentration was calculated, which was defined as a positive difference in concentration occurring in an interval of 3 h, noted as ∆3 h. For each hour, ∆3 h was calculated as the difference between the concentrations measured at hour h, minus the concentration measured at hour h-3. A fast increase was retained if a ∆3 h exceeded: 20 µg/m3 for PM10, or 40 µg/m3 for NO2 and O3. This threshold value was chosen as the third quartile of the observed distribution of the ∆3 h.
Finally, the air quality threshold exceedance was defined by the European legislation on air quality18. The legal thresholds for each air pollutant were: PM10 daily average = 50 µg/m3, NO2 hourly average = 200 µg/m3, O3 8-h average = 120 µg/m3.
To highlight a lag effect, these assessments were calculated for each of the 4 days before the occurrence of SSP for all the case and control periods, and for the entire exposure period (Lag 1–Lag 4).
Statistical analysis
A conditional logistic regression was used to analyse the relationship between the exposure to air pollution for each case and control period. To control the false discovery rate with a high number of exposure indices, a correction for multiple testing was realized with Benjamini Yekutieli’s adjustement, which is less conservative than a Bonferroni correction19,20,21. To verify that the effects of concentrations of pollutants were not modified by individual factors, sensitivity analyses were performed with age, sex, smoking status, history of pulmonary disease (COPD, emphysema, pneumothorax, and asthma) and centre. Complementary analyses were also performed with warm seasons (spring and summer) versus cool seasons (autumn and winter) to consider the variation of O3 concentration with the mount of sunlight.
Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). A P value < 0.05 was considered statistically significant.
Results
Participants
From June 1, 2009, to May 31, 2013, were admitted in the 14 Emergency department 1946 patients for pneumothorax. Among them, 358 patients presented a SSP. After excluding subjects living more than 30 km (19 miles) from an air pollutant monitoring station or with a recurrent episodes, 135 SSP were included (Fig. 2). The mean age of the population was 55.56 years (standard derivation 18.54 years). The male/female ratio was 4.4. Approximately 60% of the population presented with COPD or emphysema, and 39% declared a previous onset of pneumothorax. Current smokers accounted for 54%. Half of the SSPs were admitted in spring and summer seasons (Table 1).
Air pollution exposure
The pollutant exposures are presented according to the case and the control status in SI Table A (mean (SD), median, maximum, minimum and quartile) and SI Table B (correlation coefficients between the air pollutants).
The odds ratios associated with PM10, NO2 and O3 values of maximum and mean concentrations, for each Lag and the entire period exposure are presented in Table 2. The odds ratios associated with at least one fast increase (∆3 h) are presented in Table 3. The odds ratios associated with at least one exceedance of air quality threshold are presented in Table 4.
Concerning the PM10 exposure assessment, the odds ratios associated with values of maximum and mean concentrations across Lag 1–Lag 4 ranged between 0.93 (0.77–1.13) and 0.99 (0.91–1.08) (Table 2). When considering each Lag, the odds ratios associated with PM10 values of at least one fast increase (∆3 h) ranged between 0.52 (0.27–1.00) and 1.02 (0.56–1.85) (Table 3) ; the odds ratios associated with at least one exceedance of air quality threshold ranged between 0.41 (0.11–1.49) and 1.00 (0.35–2.88) (Table 4).
Concerning the NO2 exposure assessment, the odds ratios associated with values of maximum and mean concentrations across Lag 1–Lag 4 ranged between 0.87 (0.70–1.09) and 0.99 (0.82–1.21) (Table 2). When considering each Lag, the odds ratios associated with NO2 values of at least one fast increase (∆3 h) ranged between 0.37 (0.18–0.77) and 1.24 (0.65–2.38) (Table 3). No exceedance of air quality threshold of NO2 was observed.
Concerning the O3 exposure assessment, the odds ratios associated with values of maximum and mean concentrations across Lag 1–Lag 4 ranged between 0.95 (0.84–1.07) and 1.02 (0.93–1.13) (Table 2). When considering each Lag, the odds ratios associated with O3 values of at least one fast increase (∆3 h) ranged between 1.03 (0.61–1.74) and 1.40 (0.84–2.32) (Table 3) ; the odds ratios associated with at least one exceedance of air quality threshold ranged between 0.91 (0.29–2.81) and 1.77 (0.63–4.95) (Table 4).
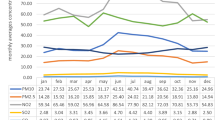
The complementary analyses O3 values separately performed on warn and cold seasons are presented in SI Figure A and SI Table C.
No significant association between the occurrence of SSP and the air pollutant exposure assessment were observed. All PCorr values were superior to 0.12.
Discussion
In this study, no significant association between the occurrence of SSP and short term exposure to PM10, NO2, and O3 were identified for quantitative absolute values, fast increase in air pollutants and peak of pollution. Nevertheless, a link between O3 and the occurrence of the event cannot be ruled out.
Our study had some strengths. We were careful to avoid selection bias. During the study period, all patients with a diagnosis of pneumothorax [International Classification of Diseases (ICD) 10th revision code = J93] were retrospectively identified by medical informatics queries of the emergency unit databases in the participating centres. The diagnosis of SSP (ICD code J93.12) was checked by research assistants in the medical files of the Emergency Department’s admissions database.
Concerning the air pollution exposure for each patient, a partnership was realized with the French National AASQA Network. The aim was to identify the appropriate monitoring station in relation to each patient’s address. Considering the geomorphological situation and the sources of primary pollutant, allows us to determine the most reliable level of air pollutant exposure.
The time-stratified case–crossover design, which is an advanced matching technique, makes it possible to take into account the characteristics of individual patients. This design is recognized to consider temporal trends and to reduce differential errors due to the measure of exposure. This methodology is specifically adapted to the analysis of associations between rapid onset disease with a short induction period, and acute exposure14,22.
To the best of our knowledge, our study is the first in the literature to evaluate the link between SSP and air pollution. Patients with SSP have mainly underlying chronic lung diseases, such as COPD. In their study on the epidemiology of pneumothorax, Gupta et al.12 demonstrated that COPD causes 70–80% of SSP cases. In our study, approximately 60% of patients with SSP presented an underlying COPD or an emphysema. A significant impact between acute exacerbation of COPD and air pollution was demonstrated1,2,3,5. Long-term exposure to air pollutants is significantly associated with increasing emphysema23,24. With these chronic inflammatory diseases, these population are most vulnerable and present a greater risk of pulmonary complications. A diffuse histopathological change in the lung parenchyma under the visceral pleural could lead to the occurrence of a pneumothorax. Diffuse increased porosity at the periphery of both lungs has been identified, which could be associated with localized rupture of a bleb, or a bulla25,26. Short-term O3 exposure could induce airway hyperresponsiveness, increase oxidant stress and stimulate inflammatory responses in the respiratory system associated with a chronic inflammatory disease, which could lend credence to the hypothesis of a stronger link between SSP and air pollutants27.
Nevertheless, in our study, a link between O3 exposure and SSP cannot be ruled out. In the literature, Park et al.7 showed that increased concentrations of O3 on two previous days before the occurrence of PSP are significantly associated. Huang et al.5 demonstrated that the strongest association between O3 exposure and COPD mortality was found in the moving average concentration from the day of the occurrence of COPD to Lag 2. Yang et al.28 concluded at an association among respiratory mortality and increased average O3 2 days before the occurrence of the event. Although non significant, our study results associated with the fast increase of 40 µg/m3 and the peak of 8-h average of 120 µg/m3 are compatible with the occurrence of SSP and a fast increase O3 concentration to Lag 2 prior to the event.
The concentrations of O3 are generally higher in warm seasons than in cool seasons. In fact, the concentration and distribution of O3 are linked with regard to the main determinants: emissions and sunlight. Primary pollutant emissions from urban traffic include nitrogen oxides, carbon monoxide, and volatile organic compound produce O3 through photochemical transformations. A strong correlation between O3 concentrations and temperature is established29; increasing temperature also increases the emission of volatile organic compound by processes such as evaporation30. Ozone concentration increases almost linearly with the ambient temperatures and with greater intensity of sunlight, which is why O3 concentrations are higher in warm seasons and in urban areas29,31,32. In the literature, Abul et al.10 showed an association between the occurrence of PSP and higher levels of O3 in the spring. Gryparis et al.33 evaluated the relationship between acute effects of O3 and respiratory mortality in 23 Europeans cities. O3 concentrations are higher during the summer and are associated with an increase respiratory mortality during warmer seasons. Zanobetti et al. and Peng et al.34,35 demonstrated the same results for American and Canadian cities. Although our study results are non significant, the ORs concerning the associations between maximum and mean O3 concentrations and the occurrence of SSP are higher in warm seasons than in cool seasons for each Lag and for the entire period of exposure before the event. A link between short term O3 exposure and these diseases cannot be ruled out in warm seasons.
In this regard, two points merit discussion, namely the seasonality and the associations of different meteorological factors.
In the literature, the occurrence of pneumothorax and respiratory disease seems to have a seasonal effect. However, the results are contradictory. In fact, Park et al. and Aissa et al.7,36 demonstrated a greater occurence of pneumothorax in warm versus cold seasons. Schieman et al. and Motono et al.37,38 concluded that there was an absence of a significant link between pneumothorax and seasonality. Concerning respiratory diseases, such as COPD, a relationship is established with a higher concentration of O3 during the summer33,34,35. However, Chen et al., Yin et al. and Wong et al.39,40,41 showed in East Asia and southern China that O3, despite a higher concentration in warm versus cool seasons, had stronger effects on respiratory mortality in cool seasons The main explanation for this finding is the people’s behavior in relation to the climate. American, Canadian and European cities are exposed to a continental climate with extremely cold winters and hot summers with a great range of temperatures. People also spend longer amounts of time outdoors during warm seasons than in cool seasons. They are exposed in warm seasons to higher outdoors O3 pollution and pollen concentration, which can increase respiratory disease exacerbation33. Southeast Asia has a subtropical climate with mild winter and hot and damp summers. During cool seasons, people are more likely to go outdoors and are also more exposed to O3. During warm stay at home with the air conditioner and are also less exposed to O35,41. Therefore, the exposition of air pollutants depends on lifestyle and people’s behavior. Climate, with the associations of different meteorological factors, is therefore an interesting parameter to evaluate the occurrence of pneumothorax.
Concerning the different meteorological factors, the associations of air pollutants and different parameters, such as atmospheric pressure, temperature and humidity, were not included in our study. In fact, Bertolaccini et al.8 concluded that the occurrence of PSP was significantly triggered by higher daily mean NO2, lower daily mean O3 and daily decrement of standard variation of temperature and wind speed. In the second study of Bertolaccini et al.9, PSP was significantly more likely to occur on warm windy days with high atmospheric pressure and high mean NO2 concentrations. Park et al.7 demonstrated that increased concentrations of air pollutants associated with an abrupt change in atmospheric pressure were significantly associated with an increased incidence rate of PSP. Temperature and wind speed might affect the transport of air pollutants and allergens, leading to bronchiolar spasm, mucous retention and coughing7,37. The association with different factors could have a synergistic effect, and a resulting impact on air pollution. All environmental factors should be taken into account to evaluate their relationship with pneumothorax.
Conclusions
Our study failed to identify any significant relationship between SSP and short-term PM10 and NO2 exposure. A link with O3 exposures cannot be ruled out, especially when considering 2 days prior to the event and in warm seasons. Therefore, a potentiating effect of different meteorological factors remains to be demonstrated.
References
Li, R. et al. Impact of air pollutants on outpatient visits for acute respiratory outcomes. Int. J. Environ. Res. Public Health 14, 1 (2017).
Bloemsma, L. D., Hoek, G. & Smit, L. A. M. Panel studies of air pollution in patients with COPD: Systematic review and meta-analysis. Environ. Res. 151, 458–468 (2016).
DeVries, R., Kriebel, D. & Sama, S. Outdoor air pollution and COPD-related emergency department visits, hospital admissions, and mortality: A meta-analysis. COPD 14(1), 113–121 (2017).
Zu, K. et al. Concentration-response of short-term ozone exposure and hospital admissions for asthma in Texas. Environ. Int. 1(104), 139–145 (2017).
Huang, J. et al. The burden of ozone pollution on years of life lost from chronic obstructive pulmonary disease in a city of Yangtze River Delta, China. Environ. Pollut. 1(242), 1266–1273 (2018).
Han, C., Lim, Y.-H., Jung, K. & Hong, Y.-C. Association between ambient air pollution exposure and spontaneous pneumothorax occurrence. Epidemiol. Camb. Mass. 30(Suppl 1), S48-56 (2019).
Park, J. H. et al. Air pollutants and atmospheric pressure increased risk of ED visit for spontaneous pneumothorax. Am. J. Emerg. Med. 36(12), 2249–2253 (2018).
Bertolaccini, L., Alemanno, L., Rocco, G. & Cassardo, C. Air pollution, weather variations and primary spontaneous pneumothorax. J. Thorac. Dis. 2(1), 9–15 (2011).
Bertolaccini, L. et al. Analysis of spontaneous pneumothorax in the city of Cuneo: Environmental correlations with meteorological and air pollutant variables. Surg Today. 45(5), 625–629 (2015).
Abul, Y. et al. Spontaneous pneumothorax and ozone levels: Is there a relation?. Multidiscip. Respir. Med. 6, 16 (2011).
Marx, T. et al. Does air pollution really impact the onset of spontaneous pneumothorax? A French case–crossover study. Environ. Int. 127, 317–323 (2019).
Gupta, D. et al. Epidemiology of pneumothorax in England. Thorax 55(8), 666–671 (2000).
Maclure, M. The case–crossover design: A method for studying transient effects on the risk of acute events. Am. J. Epidemiol. 133(2), 144–153 (1991).
Bateson, T. F. & Schwartz, J. Control for seasonal variation and time trend in case–crossover studies of acute effects of environmental exposures. Epidemiology. 10(5), 539–544 (1999).
Lumley, T. & Levy, D. Bias in the case–crossover design: Implications for studies of air pollution. Environmetrics 11(6), 689–704 (2000).
Levy, D., Lumley, T., Sheppard, L., Kaufman, J. & Checkoway, H. Referent selection in case–crossover analyses of acute health effects of air pollution. Epidemiology 12(2), 186–192 (2001).
Janes, H., Sheppard, L. & Lumley, T. Overlap bias in the case–crossover design, with application to air pollution exposures. Stat. Med. 24(2), 285–300 (2005).
Ministère de l’écologie, de l’énergie, du développement durable et de la mer, en charge des technologies vertes et des négociations sur le climat. Code de l’environnement. Décret n°2010–1250 relatif à la qualité de l’air. Section 1 : Surveillance de la qualité de l’air ambiant Article R221–1. 2010.
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57(1), 289–300 (1995).
Benjamini, Y. & Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29(4), 1165–1188 (2001).
Reiner, A., Yekutieli, D. & Benjamini, Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19(3), 368–375 (2003).
Janes, H., Sheppard, L. & Lumley, T. Case–crossover analyses of air pollution exposure data: Referent selection strategies and their implications for bias. Epidemiology 16(6), 717–726 (2005).
Adar, S. D. et al. Air pollution and percent emphysema identified by computed tomography in the Multi-Ethnic study of Atherosclerosis. Environ. Health Perspect. 123(2), 144–151 (2015).
Wang, M. et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA 322(6), 546–556 (2019).
Noppen, M. et al. Fluorescein-enhanced autofluorescence thoracoscopy in patients with primary spontaneous pneumothorax and normal subjects. Am. J. Respir. Crit. Care Med. 174(1), 26–30 (2006).
Tschopp, J.-M. & Marquette, C.-H. Spontaneous pneumothorax: Stop chest tube as first-line therapy. Eur. Respir. J. 49, 4 (2017).
Mudway, I. S. & Kelly, F. J. Ozone and the lung: A sensitive issue. Mol. Aspects Med. 21(1), 1–48 (2000).
Yang, C. et al. Alternative ozone metrics and daily mortality in Suzhou: The China Air Pollution and Health Effects Study (CAPES). Sci. Total Environ. 1(426), 83–89 (2012).
Bloomer, B. J., Stehr, J. W., Piety, C. A., Salawitch, R. J. & Dickerson, R. R. Observed relationships of ozone air pollution with temperature and emissions. Geophys. Res. Lett. 36, 9 (2009).
Stockwell, W. R., Lawson, C. V., Saunders, E. & Goliff, W. S. A review of tropospheric atmospheric chemistry and gas-phase chemical mechanisms for air quality modeling. Atmosphere 3(1), 1–32 (2012).
Baklanov, A., Molina, L. T. & Gauss, M. Megacities, air quality and climate. Atmos. Environ. 1(126), 235–249 (2016).
Bernard, N. L., Gerber, M. J., Astre, C. M. & Saintot, M. J. Ozone measurement with passive samplers: Validation and use for ozone pollution assessment in Montpellier, France. Environ. Sci. Technol. 33(2), 217–222 (1999).
Gryparis, A. et al. Acute effects of ozone on mortality from the “air pollution and health. Am. J. Respir. Crit. Care Med. 170(10), 1080–1087 (2004).
Zanobetti, A. & Schwartz, J. Mortality displacement in the association of ozone with mortality. Am. J. Respir. Crit. Care Med. 177(2), 184–189 (2008).
Peng, R. D. et al. Acute effects of ambient ozone on mortality in Europe and North America: Results from the APHENA study. Air Qual. Atmos. Health. 6(2), 445–453 (2013).
Aissa, S. et al. Influence of weather conditions on the onset of spontaneous pneumothorax in the region of Sousse (Tunisia): Analysis of time series. BioMed Res. Int. 2019, 1793973 (2019).
Schieman, C. et al. Weather and chinook winds in relation to spontaneous pneumothoraces. Can. J. Surg. J. Can Chir. 52(5), E151-155 (2009).
Motono, N. et al. Atmospheric temperature and pressure influence the onset of spontaneous pneumothorax. Clin. Respir. J. 12(2), 557–562 (2018).
Chen, R. et al. Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. Am. J. Respir. Crit. Care Med. 196(1), 73–81 (2017).
Yin, P. et al. Ambient ozone pollution and daily mortality: A nationwide study in 272 Chinese cities. Environ. Health Perspect. 125(11), 117006 (2017).
Wong, C. M., Ma, S., Hedley, A. J. & Lam, T. H. Effect of air pollution on daily mortality in Hong Kong. Environ. Health Perspect. 109(4), 335–340 (2001).
Acknowledgements
We thank the Official Air Quality Monitoring Associations (AASQA) and their experts ATMO Franche-Comté (Anaïs Détournay), ASPA Alsace (Pierre Robelet), Atmosf’air Bourgogne (Shirley Salomon), Air Rhône-Alpes (Sabrina Pontet), ATMO Auvergne (Sabrina Pontet), Atmo Nouvelle-Aquitaine (Agnes Hulin), Atmo Hauts-de-France (Aurélie Dapvril), ORAMIP ATMO (Pierre-Yves Robic), Air Pays de la Loire (Gilles Levigoureux), for providing data and for the association between home addresses and air quality monitoring stations. This study was financed by a Grant from the University Hospital of Besançon (APICHU 2012).
Author information
Authors and Affiliations
Contributions
Study conception and design: N.B., A.L.P., M.G., J.C.D., F.M., T.D. Acquisition, analysis and interpretation of data: T.M., N.B., A.L.P., B.M., J.B.P., F.M., T.D. Statistical analysis: T.M., M.P., F.M. All the authors participated in drafting of the manuscript, approved the version to be published, and agree to be held accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marx, T., Bernard, N., Parmentier, AL. et al. Short term association between air pollution (PM10, NO2 and O3) and secondary spontaneous pneumothorax. Sci Rep 10, 11823 (2020). https://doi.org/10.1038/s41598-020-68831-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68831-4
This article is cited by
-
Air pollution and hospital admissions for critical illness in emergency department: a tertiary-center research in Changsha, China, 2016–2020
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.