Abstract
Carbon-14 released from nuclear facilities has been assessed to contribute significantly to the radiation dose that people are exposed to through the food chain. However, the current dose coefficient for members of public, which is the ratio of the 50-year committed effective dose to ingested 1 Bq 14C, recommended by the International Commission on Radiological Protection (ICRP) is not based on experimental human metabolic data for 14C in nutrients and diet. Therefore, to validate the coefficient, we administered 13C-labelled nutrients consisting of four amino acids, three fatty acids, and one monosaccharide to volunteers as substitutes for 14C labelled nutrients and measured the 13C concentration in various excreta samples. Although metabolic models were constructed from the excretion data, a significant fraction of administered 13C was not recovered from some nutrients. The dose coefficients of 14C in uniformly labelled Japanese diet, which were estimated under several assumptions about the unrecoverable fraction, varied from (6.2 ± 0.9) × 10–11 to (8.9 ± 4.4) × 10–10 Sv Bq−1 and were approximately comparable to the current value of 5.8 × 10–10 Sv Bq−1 recommended by the ICRP. Further studies are necessary to elucidate the metabolism of 14C in various nutrients in the unrecoverable fraction.
Similar content being viewed by others
Introduction
Carbon-14 is released from places such as nuclear fuel reprocessing facilities and nuclear power plants1. It is a major radionuclide contributing to the radiation dose to people around the nuclear fuel reprocessing facilities1,2,3. The dose coefficient of 14C, the ratio of 50-year committed effective dose to ingested 1 Bq 14C, is an important parameter for the dose evaluation. The International Commission on Radiological Protection (ICRP), which provides recommendations and guidance on radiological protection, has developed models describing the biokinetics of radionuclides for the calculation of doses from data of intakes or bioassay samples. For estimating the dose coefficient of 14C in diet of members of public, the ICRP has utilized a generic one-compartment model for organic carbon metabolism4, whereas a multi-compartment model was used in a recent publication for unspecified 14C compounds ingested by workers5. The one-compartment model for members of public assumes that 14C is uniformly distributed throughout all body organs and tissues following entry into the systemic circulation. The organic-carbon compartment of the model has a half-life of 40 days, which is derived from the mass balance of carbon in the reference man of 70 kg body weight with body carbon weight of 16 kg and carbon intake and excretion of 300 g carbon per day each6. The dose coefficient of organic 14C in the diet (5.8 × 10−10 Sv Bq−1) was defined using that metabolic model7, which is not based on actual experimental data obtained from 14C in foods or component compounds of major nutrients. Therefore, experimental data of 14C in those compounds are needed to validate the generic one-compartment model of the ICRP or revise it in future publications for members of public following publications for workers8.
In rat tissue and organs, some studies have examined the biokinetics of 14C incorporated into foods or component molecule of major nutrient, e.g., single oral administration of [14C(U)]-glucose, [14C(U)]-glycine, [14C(U)]-leucine, [14C(U)]-lysine, [1–14C]-oleic acid, [1-14C]palmitic acid, and [2-14C]-thymidine9; and continuous ingestion of 14C-wheat10, [14C(U)]-glycine, [14C(U)]-leucine, and 14C-rapeseed11. In sheep, Crout et al. reported the biokinetics of 14C after oral administration of 14C-glucose12. However, most of the human data has been restricted to the 14C in drug molecules in biomedical studies13 and diagnostic use such as 14C-glycocholic acid and 14C-xylose breath tests14, and only few investigations have reported the biokinetics of 14C in component molecule of major nutrients. Malmendier et al.15 examined the metabolism of 14C in intravenously administered [1-14C]-glucose, [1-14C]-glycerol, and [1-14C]-palmitate for 24 h in breath in normal and hyperlipemic patients. Simmons et al.16 reported the 14C excretion in urine and breath until 3 and 7 days, respectively, after intravenous [1-14C]-alanine administration for human volunteers. However, both studies monitored the breath for a short time of up to 7 days after the administration. Berlin and Tolbert17 reported the breath excretion of 14C following intravenous injection of glycine-[2-14C] decreased in four successive phases with half-lives of 0.12, 0.97, 6.1, and 71.5 days. Stenström et al.18 orally administered 14C-triolein and found two phase excretions in the breath with half-lives of 2 and several hundred days. Gunnarsson et al.19 also reported that the excretion of 14C in breath after oral 14C-triolein administration decreased in three phases with half-lives of 0.04, 2, and 150 days. Although the data are limited, several studies have reported fractions of excreta with longer half-lives than that of the ICRP generic one-compartment model as described above.
Ingested carbon is excreted via several physiological routes including in the breath, urine, faeces, hair, nails, epithelial cells of the skin and mucous membrane, mucus, and other secretions. The ICRP has classified the routes into four excretion pathways, breath, urine, faeces, and others (other*, hereafter) and estimated the distribution ratio of carbon via the pathways in their reference man6. However, the previous studies on carbon excretion in humans described above examined only the respiration and urinary pathways. Regarding the other* pathway, Stenström et al. reported the specific activity of 14C in hair from personnel at a research laboratory and pointed out the possibility of using hair as a bioassay sample20. To the best of our knowledge, no data has been reported for the human body on the metabolism of 14C incorporated into compounds covering a broad range of nutrients and food, considering every four excretion routes of the ICRP.
We previously reported the biokinetics of carbon in the human body estimated using data of the 13C/12C ratio in breath, urine, faeces, and hair, representing carbon excretion through the breath, urine, faeces, and other* pathways, respectively, after a single oral administration of 13C-labelled glucose to three healthy male volunteers21. The 13C/12C ratio in breath, urine, and hair samples was measured for up to ~112 days after administration. The excretion of 13C in the evaluated pathways decreased in two exponential phases with fast and slow rates, and the 13C/12C ratio of the other* pathway was higher than that of the breath and urine pathways toward the end of the experiment. Although this finding suggests that the carbon pool of the other* pathway had a longer half-life than that of the breath and urine pathways, we could not get the statistically significant 13C half-life of the slow decreasing fraction in the other* pathway.
In this paper, we report the results of experiments administering 13C-labelled four amino acids and three fatty acids to volunteers. First, 13C-labelled leucine and palmitic acid were orally administered once to the volunteers and the excretion through the four routes of interest were measured for up to ~160 day after the administration. Then, the contribution of carbon pools of each route to the cumulative burden of 14C for 50 years was calculated assuming the same metabolism of 13C and 14C. Since the urine and faeces routes did not have significant contributions, we focused on only the breath and other* routes in experiments on the remaining nutrient components. In addition, once daily dosing administered four successive times, instead of the previously used single administration, was adopted for a more accurate measurement of 13C excretion in the final stage of the experiments. An experiment on 13C-labled glucose administered four times was also conducted and, finally, the dose coefficient of 14C in the Japanese diet was estimated using integrated biokinetic models of each nutrient component.
Results and Discussion
Design of experiments
We selected the following amino acids as representative of proteins: leucine from branched chain amino acids, glutamic acid from major amino acids, glycine from collagen components, and phenylalanine from aromatic amino acids. Palmitic, oleic, and linoleic acids were selected as representative saturated, mono-unsaturated, and poly-unsaturated fatty acids, respectively while carbohydrates were represented by glucose. All carbon atoms of the compounds used were labelled with 13C (Cambridge Isotopes, Inc., Tewksbury). The compounds were administered to healthy volunteers and samples of various excreta were collected from them and analysed for 13C. All the 13C/12C ratio data presented in this report are shown as increments from the background values measured for the samples before administration, and normalized to the same dose per body weight (1 g 13C/70 kg body weight) prior to the following analysis. When the 13C/12C ratio under the detection limits, <95% confidence limit of the background, was observed, the data of the detection day and after it were not used for the subsequent numerical analysis. The detection limit mostly depended on the amount of 13C administered per body weight and individual difference.
Selection of major excretion routes of 13C in amino acid and fatty acid
We orally administered 13C-labelled leucine or palmitic acid to three male volunteers who were sedentary workers and then collected their breath, urine, faeces, and hair samples at different times depending on the compounds and excreta (Fig. 1). As shown in Figure 1, the 13C/12C ratio of the breath and hair samples increased after administration then decreased in two exponential phases. The peak time of breath samples from the leucine-administered group (0.11 ± 0.02 days, mean ± standard deviation) was earlier than that of the palmitic acid-administered group (0.30 ± 0.03 days, P < 0.01), suggesting that the compounds had different absorption rates from the alimentary tract (Fig. S1 in the supplementary information). The peak time of the 13C/12C ratio in faeces samples after administration of labelled leucine and palmitic acid varied from 0.75 to 1.9 days and from 0.75 to 3.75 days among subjects, respectively. The excretion rate of administered 13C into the faeces is considered to depend on subject-specific values such as residence time of faeces in the colon, which was independent of the administered compounds.
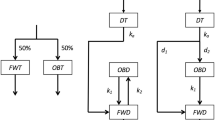
For each compound, we developed a metabolic model comprising four carbon excretion pathways: breath, urine, faeces, and other* (Fig. 2). Since the 13C/12C ratios of each excreta sample showed a dual exponential decrease after peaking, two compartments representing fast and slow carbon metabolism were set for each of the four excretion pathways. However, every compartment in those models does not represent any tissue and organ. In addition, one upstream compartment was used for each pathway to fit the prediction curve of the model to the increase phase observed in the breath and faeces samples immediately after administration (Fig. 1 and Fig. S1).
Structure of model developed to analyse 13C excreted through each excretion pathway after ingestion of 13C labelled compounds. Rr is ratio of total recoverable carbon through four excretion pathways to administered 13C. dm, dmn, distribution factors of 13C to each compartment. Cm, Cmn, 13C mass in the carbon pool (g). kmn, excretion rate constant (day−1).
The rate of 13C excretion for each pathway (Em, g d−1) is given by the following equations:
where, m is the excretion pathway symbol of B, U, F, and O corresponding to the excretion pathways of breath, urine, faeces, and other*, respectively; n is the symbol of f and s corresponding to the compartments with fast and slow metabolism, respectively; km and kmn are the excretion rate constants from compartment Cm and Cmn, respectively; dm is the distribution ratio of 13C into compartment Cm to the administered 13C; dmn is the distribution ratio of 13C into compartment Cmn to 13C from compartment Cm; Rr is the recovery ratio of administered 13C. The sum of distribution ratios of compartments with fast and slow metabolism was fixed to unity (i.e. dBf + dBs = 1). Since the sum of the distribution ratios of each pathway (dB + dU + dF + dO) was not forced to unity, to prevent underestimation of these parameters, Rr was over unity for some cases.
The parameter values were estimated using the data of each volunteer using a least square method (Table 1). Although some parameters were not statistically significant (p > 0.05, most parameters for the urine, faeces, and other* pathway in the palmitic acid model, and in the faeces pathway in the leucine model), we included those parameters in the following analysis.
We calculated the 50-year cumulative body burdens of 13C from orally ingested 1 g 13C in leucine and palmitic acid using the above models. Then, the variation of the burden in response to a ± 10% change of each parameter was calculated using the mixed data from all subjects for each compound group. The results are shown in Fig. 3 for the top 10 parameters causing larger variation and most were in the breath and other* pathways, whereas only two parameters causing smaller variations in the faeces pathway were included. All the statistically insignificant parameters in the regression analysis were not sensitive to the burden. The contribution of urine and faeces pathways to the burden from orally ingested 1 g of 13C in leucine were (0.37 ± 0.25)% and (0.7 ± 1.5)%, respectively, and those for palmitic acid were (0.038 ± 0.030)% and (0.034 ± 0.070)%, respectively. Therefore, we concluded that the urine and faeces pathway could be omitted from the following experiment on carbon dynamics in our study. For glucose, we previously reported the negligible contribution of the urine and faeces pathways to the cumulative burden21.
Metabolisms of 13C administered as various 13C -labelled compounds
We also administered the remaining target nutrient compounds,13C-labelled glutamic acid, glycine, phenylalanine, oleic acid, linoleic acid, and glucose to the volunteers who were healthy university students, three males and females each, and then we collected only breath and hair samples. All volunteers were administered each compound once daily for 4 successive days.
Figure 4 shows the 13C/12C ratio of the breath and hair samples after the first administration of each 13C-labelled compound. The 13C/12C ratios of hair samples decreased faster in the groups of volunteers administered oleic acid, linoleic acid, and glucose than in the other groups and reached the detection limit before the end of experiments. Consequently, we did not analyse parameters relating the slow carbon compartment in the other* pathway for some subjects. In contrast, the 13C/12C ratios of the groups of volunteers administered glycine and phenylalanine were kept relatively high throughout the experimental period, and were higher than those in the breath samples of the same volunteers toward the end of the experiment. These results reflected the higher utilization of carbon in amino acids for protein synthesis such as in hair growth than carbon in fatty acids and monosaccharides. The results of leucine and palmitic acid-administered group also showed a similar tendency (Fig. 1). However, amino acids in samples from volunteers in the glutamic acid-administered group decreased faster than other amino acids did. This observation is consistent with the reported high use of dietary glutamic acid in the small intestine22.
The metabolic model was also developed for each compound. The values of parameters of the breath and other* pathways were estimated and are summarized in Table 2. The parameters for subjects with only fast C compartment in the other* pathway were included in the summarization. Since the difference of results between sexes was not clear, the average values for individual data of both sexes were also shown in Table 2 and discussed below. The estimated distribution ratio of 13C for the breath pathway (dB) for leucine, glutamic acid, glycine, and phenylalanine were (68 ± 5)%, (84 ± 8)%, (80 ± 9)%, and (64 ± 7)%, respectively. Berlin and Tolbert17 reported an 86% recovery through the breath pathway after intravenous administration of glycine-[2-14C], which was consistent with the value of glycine in our experiment. Compared to glutamic acid and glycine, smaller proportions of leucine and phenylalanine were excreted through the breath pathway while a considerable portion was excreted through the other* pathway (dO, (30 ± 12)% and (35 ± 8)%, respectively). Leucine stimulates protein synthesis via the mammalian target of rapamycin complex 1 (mTORC1) mediating signalling pathway23 and, therefore, it might activate this pathway and consequently reduced its oxidation. However, such an effect has not been reported for phenylalanine. The distribution ratio of 13C of glucose in the breath pathway (dB, 90 ± 15%) was similar to the value reported in our previous paper (86 ± 10%)21.
The mean slow excretion rate constants (kBs) of the breath pathway varied from 0.061 to 0.21 day−1 among the examined amino acids. These values were 5–20 times larger than the slow transfer coefficient of 0.0099 day−1 from the systemic tissue compartment to the carbon dioxide compartment in the generic model for workers recommended by the ICRP-1345. However, the slow excretion constants (kOs) of the other* pathway for the examined amino acids were from 0.0059 to 0.012 day−1 and comparable to the value of the slow compartment in the generic model for workers. Our result provides evidence to supports the existence of a carbon pool with a slow metabolic rate.
Estimation of dose coefficient for members of public from ingested dietary 14C
As shown in Table 2B,C, the recovery ratios (Rr) of the administered 13C in oleic and linoleic acids were markedly small at 0.62 and 0.68, respectively, while most of the administered 13C in the other examined compounds including palmitic acid were recovered in our experiment (Tables 1 and 2). Takeda et al.9 reported that the 14C concentration in adipose tissue in rats 100 days after administration of 14C-labelled oleic acid was three times higher than that of palmitic acid. Although palmitic acid is a main constituent of adipose tissue fatty acids in humans24, in this study, we assumed that the unrecoverable fraction of 13C in both unsaturated fatty acids and all nutrient compounds examined was stored in adipose tissue.
We further presumed that the carbon in adipose tissue had a mean residence time of 1.6 years, as estimated by Arner et al.25 for adipocyte lipids by analysing 14C originating from nuclear weapon tests. Since the ICRP does not provide the tissue weighting factor for adipose tissue, we also set two assumptions for this, 0 and 0.12 used for the remaining organs in the ICRP-103 guidelines26. The dose coefficients obtained with each assumption are called non conservative estimation (ER) and conservative estimation (EC) hereafter. The recovered fraction was assumed to be distributed uniformly in the whole-body carbon with a tissue weighting factor of unity. In considering the possible existence of additional carbon compartment, which was not detected in our study but have a longer half-life than that in the present slow carbon compartments, we adopted the EC as a moderate estimation. In addition to these estimations, we calculated the most conservative estimation (EX) adopting the tissue weighting factor of unity for unrecovered carbon. We solely adopted the value for the maximal estimation.
Figure 5A shows the 50-year committed effective dose from 1 Bq of the 14C in labelled compounds after single ingestion calculated using each model developed. The ER values of the amino acids were generally higher than those of the fatty acids, whereas the EC and EX values were the opposite. Glucose showed similar ER values to those of the unsaturated fatty acids, whereas the EC and EX values are similar to those of amino acids. The assumption about the unrecoverable fraction played an extremely important role in our estimation of the dose because of the assumed long half-life of unrecovered carbon. The relative contribution of each pathway including the unrecoverable fraction to the EC is shown in Fig. 5B. It is noteworthy that parameters of the urine and faeces pathway in the first experiment were used for the other compounds (details are shown below), and the contribution of these pathways was very minor (<0.8%). As shown in Fig. 5B, the unrecoverable fraction contributed significantly to the committed dose when we adopted a tissue weighting factor of 0.12 to adipose tissue.
Dose estimation for ingested 14C. (A) Committed effective dose from 1 Bq of 14C in each compound. Error bars show standard deviation among subjects (n = 3–6). (B) contribution of 13C via each excretion pathway and unrecoverable carbon to the conservative committed dose (EC). (C) Dose coefficient for members of public from dietary 14C according to nutrition survey in Japan. Error bars show standard deviation.
We estimated the dose coefficient for members of public based on ingestion of a Japanese diet27, which was uniformly labelled by 14C and had the nutritional composition shown in Table 3. The nutrition in the diet was classified into groups which was represented by each single compound examined in our study. The proportion of carbon ingested as the nutrition groups to total carbon ingestion rate was obtained by using literature data28,29 (details are shown below). The total daily ingestion of 300 g carbon per day of ICRP reference man was divided by the proportion of carbon ingestion rate each and inputted to each single compound model. The dose coefficient was obtained by summing up the 50-year committed effective dose from each representative compound comprising the diet with a total ingestion of 1 Bq 14C.
The ER of the Japanese diet was estimated to be (6.2 ± 0.9) × 10−11 Sv Bq−1 (Fig. 5C), which was nine and two times smaller than the current dose coefficient of 5.8 × 10−10 Sv Bq−1 for members of public as recommended by the ICRP-727 and 1.6 ×10−10 Sv Bq−1 for workers adopting the generic multi-compartment model by ICRP-1345, respectively. The EC and EX were (5.6 ± 2.6) × 10−10 and (8.9 ± 4.4) × 10−10 Sv Bq−1, respectively (Fig. 5C). These conservative values were not significantly different from the current dose coefficient for members of public7.
As described before, there is no data about metabolism of carbon in a broad range of nutrients administered orally, this work firstly provides the experimental base of dose coefficient for members of public. Since the unrecoverable fraction was very important for the estimation of the dose coefficient in our research, further studies are necessary to clarify the metabolism of 14C in various nutrients.
Materials and Methods
Administration of 13C-labelled compounds
In the first experiment to select the major excretion routes for carbon in amino and fatty acids, an aliquot of uniformly 13C-labelled leucine and palmitic acid (Cambridge Isotopes, Inc., Tewksbury) was administered to healthy adult Japanese male sedentary workers aged 41 ± 9 and 40 ± 5 years old (n =3 each), respectively. The dose was 1.0 g 13C per person. We followed the administration procedure described in a previous paper21. Briefly, the 13C-labelled compounds were orally ingested by each volunteer at 12:15 immediately before eating lunch on experimental day 0. An additional 0.1 g of 13C in the compounds was similarly administered on day 112 after the initial administration to mark that day in the hair samples (see below). To avoid background fluctuations in the 13C/12C ratio by over-eating of 13C-rich foods, nutritionally balanced foods were provided to the volunteers throughout the experimental period from day −7 to 112, although they were able to eat other foods under certain circumstances.
In the second experiment using various 13C-labelled compounds, uniformly 13C-labelled glutamic acid, glycine, phenylalanine, oleic acid, linoleic acid, and glucose (Cambridge Isotopes, Inc., Tewksbury) were administered to healthy adult Japanese male and female volunteers (n = 3, each) aged 25 ± 7 years once a day at 12:05 on 4 successive days. The doses of those compounds were 8.9, 7.1, 11.9, 11.0, 16.4, and 35.6 mg 13C kg−1 day−1, respectively. Additional doses of 13C-labelled compounds were administered on day 160 for marking the 13C/12C ratio of hair samples and the volunteers freely ate meals without calorific restrictions.
Sample collection and determination of 13C/12C ratio
In the first experiment, breath, urine, faeces, and hair samples were periodically collected and the 13C/12C ratio was analysed following the procedure described in a previous paper21. Briefly, breath and urine samples were collected daily when the volunteers rose in the morning from day −7 to 112. Faecal samples were collected once before day 0 and at every defecation from day 0 to 14. On day 0, additional breath and urine samples were collected frequently after administration. Five hair samples from each subject were collected on day 119 and used as representative samples of the other* pathway. Then, 10 cm of each hair starting from the root was cut into 1-mm sections and the 13C/12C ratio determination of each showed two peaks in the 13C/12C ratio corresponding to the first and second administration of 13C-compounds. According to the section number of the two peaks, the experimental date for each section between the two peaks was determined under an assumption of uniform hair growth. In the second experiment, breath samples were collected until day 160 and hair samples were obtained by brushing on day 167.
The 13C/12C ratio was measured using a mass spectrometer (Delta V Advantage; Thermo Electron, Waltham, MA, USA) coupled with an elemental analyser (FlashEA 1112NC; Thermo Electron) and a gas chromatograph (MAT GC; Thermo Electron) for combusting organic samples and purifying CO2. Breath samples were collected by the volunteers themselves, and some samples showed clearly inconceivable results such as similar 13C/12C ratios over considerable periods of breath sample collection, which may have been caused by collecting the samples on a single day instead of once daily sampling on sequential days. The whole data of such individuals were discarded, which decreased the number of samples from three in Table 2. Some volunteers also dropped out of the sampling procedure for various reasons.
Constructing multi-compartment metabolic model by regression analysis
The 13C distribution ratios and excretion rate constants of all compartments in the four excretion pathways for leucine and palmitic acid were determined using the least squares fitting method with the statistical analysis software R version 2.15.2., while only parameters for the breath and other* pathway were determined for the other six examined compounds. The detailed procedures used were described in a previous published paper21. When urine and hair samples had no increase phase, the values of kU and kO were fixed to those of kB obtained from the same volunteers in the regression analysis.
Estimation of dose coefficient for members of public from dietary 14C
The 50-year cumulative body burden of 14C after a single ingestion of 1 Bq of 14C in each compound (BC Bq d) was calculated using the metabolic model and its parameters described above, under the assumption of the same metabolic behaviour of 13C and 14C. The parameters of the urine and faeces pathway for leucine and palmitic acid were used for the other amino acids and fatty acids. For glucose, the parameters of the urine and faeces pathways reported in our previous paper21 were adopted. The total cumulative burden was derived as a sum of burdens from the recoverable and unrecoverable fractions with designated proportions Rr and 1−Rr, respectively. For the recoverable fraction, the burden was calculated using following equation:
where, every dm was divided by Rr when Rr was larger than unity.
The 50-year committed effective dose (Sv) from ingestion of 1 Bq 14C was calculated from the 50-year cumulative burden by using the ratio of absorbed dose to 14C radioactivity, radiation weighting factor of beta-ray from 14C (1.0) and tissue weighting factors. As previously described, for the unrecoverable fraction, we used a mean residence time of 1.6 years (excretion rate constant of 0.0017 d−1)25 and tissue weighting factors of 0, 0.12, and unity for the estimation of ER, EC, and EX, respectively.
The Japanese typical nutrition intake rates were cited from the annual nationwide National Health and Nutrition Survey conducted by the Ministry of Health, Labour and Welfare, Japan27. In addition, we used the proportion of each amino acid as reported by Iwaya28 to obtain the ingestion rate of each amino acid group including branched chain amino acid (BCAA), major amino acid, glycine, and aromatic amino acid (AAA) groups (Table 3). We assumed that the metabolism of each amino acid group was the same as that of the represented compound examined in our study as shown in Table 3. The lipids and carbohydrates were also represented by each single compound. Then, the ingestion rate of carbon in each nutrition group was calculated according to the weight percentage of carbon in the representative compound (Table 3)29, followed by obtaining the proportion of carbon ingestion rate of each group to the total carbon ingestion rate. Then, the input to each compound model was calculated from the total carbon ingestion rate of 300 g carbon per day of ICRP reference man6 and the proportion of carbon ingestion rate. Finally, the dose coefficient for members of public from dietary 14C was calculated by summing up the committed effective doses of 14C and weighting proportion of 14C in each compound up to 1 Bq 14C in the diet.
Ethical considerations
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the prior approval of the Institute for Environmental Sciences Review Board for Human Subject Experiments. Written informed consent was obtained from each volunteer prior to participation.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on a reasonable request.
References
Koarashi, J., Akiyama, K., Asano, T. & Kobayashi, H. Chemical composition of 14C in airbone release from the Tokai reprocessing plant, Japan. Radiat. Prot. Dosimetry. 114, 551–555 (2005).
MaCartney, M., Baxtre, M. S. & Scott, E. M. Carbon-14 discharged from the nuclear fuel cycle: 2 Local effects. J. Environ. Radioact. 8, 157–171 (1988).
Veluri, V. R., Boone, F. W. & Palms, J. M. The environmental impact of 14C released by a nuclear funnel-reprocessing plant. Nucl. Safety. 17, 580–590 (1976).
International Commission on Radiological Protection (ICRP), Limits for intakes of radionuclides by workers. ICRP Publication 30. Ann. ICRP 2 (Elsevier (1979).
International Commission on Radiological Protection (ICRP), Occupational intakes of radionuclides: Part 1. ICRP Publication 134. Ann. ICRP 45 (Elsevier (2016).
International Commission on Radiological Protection (ICRP), Report on the task group on reference man. ICRP Publication 23. Ann. ICRP (Elsevier (1975).
International Commission on Radiological Protection (ICRP), Age-dependent doses to the members of the public from intake of radionuclides: Part 5 Compilation of ingestion and inhalation coefficients. ICRP Publication 72. Ann. ICRP 26 (Elsevier 1995).
Paquet, F., Bailey, M. R. & Leggett, R. Assessment and interpretation of internal doses: uncertainty and variability. Ann. ICRP. 45, 202–214 (2016).
Takeda, H., Fuma, S., Miyamoto, K., Nishimura, Y. & Inaba, J. Biokinetics and dose estimation of radiocarbon in rats. Radiat. Prot. Dosimetry. 79, 169–174 (1998).
Takeda, H. et al. Biokinetics of radiocarbon ingested as a food in rats. Health Phys. 96, 587–593 (2009).
Takeda, H. et al. Comparative biokinetics of radiocarbon ingested as compounds or foods in rats. Health Phys. 99, 668–673 (2010).
Crout, N. M., Mayes, R. W., Beresford, N. A., Lamb, C. S. & Howard, B. J. A metabolic approach to simulating the dynamics of C-14, H-3 and S-35 in sheep tissues. Radiat. Environ. Biophys. 36, 243–250 (1998).
Lappin, G. & Garner, R. C. Current perspective of 14C-isotope measurement in biomedical accelerator mass spectrometry. Anal. Bioanal. Chem. 378, 356–364 (2004).
Gunnarsson, M. et al. Long term biokinetics and radiation exposure of patients undergoing 14C-glycocholic acid and 14C-xylose breath tests. Cancer Biother. Radiopharm. 22, 762–771 (2007).
Malmendier, C. L., Delcroix, C. & Berman, M. Interrelation in the oxidative metabolism of free fatty acids, glucose, and glycerol in normal and hyperlipemic patients. J. Clin. Invest. 54, 461–476 (1974).
Simmons, P. S., Nissen, S. & Haymond, M. W. Total body absorbed radiation dose from 1-14C-alanine for human studies. Health Phys. 43, 561–565 (1982).
Berlin, N. I. & Tolbert, B. M. Metabolism of glycine-2-14C in man: V. further consideration of pulmonary excretion of 14CO2. Proc. Soc. Exp. Biol. Med. 88, 386–389 (1953).
Stenström, K. et al. Application of accelerator mass spectrometry (AMS) for high-sensitivity measurements of 14CO2 in long term studies of fat metabolism. Appl. Radiat. Isot. 47, 417–422 (1996).
Gunnarsson, M. et al. Biokinetics and radiation dosimetry for patients undergoing a glycerol tri[1-14C]oleate fat malabsorption breath test. Appl. Radiat. Isot. 58, 517–526 (2003).
Stenström, K., Unkel, I., Nilsson, C. M., Raaf, C. & Mattsson, S. The use of hair as an indicator of occupational 14C contamination. Radiat. Environ. Biophys. 49, 97–107 (2010).
Masuda, T. et al. Biokinetics of 13C in the human body after oral administration of 13C-labeled glucose as an index for the biokinetics of 14C. J. Radiol. Prot. 36, 532–546 (2016).
Reeds, P. J., Burrin, D. G., Davis, T. A. & Stroll, B. Amino acid metabolism and the energetics of growth. Arch. Tieremahr. 51, 187–97 (1998).
Dickinson, J. M. et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J. Nutr 141, 856–862 (2011).
Insull, W., Lang, P. D., His, B. P. & Yoshimura, S. Studies of arteriosclerosis in Japanese and American men. J. Clin. Invest. 48, 1313–1327 (1969).
Arner, P. et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 478, 110–113 (2011).
International Commission on Radiological Protection (ICRP). The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37 (Elsevier (1997).
Ministry of Health, Labour and Welfare, Japan. The National Health and Nutrition Survey, Japan [(accessed on 12th March 2020)]; Available online: https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html. (In Japanese).
Iwaya, M. Amino acid composition of dietary protein ingested by the Japanese and its nutritional evaluation. Jpn. J. Nutr. Diet. 42, 297–304 (1984).
Nakamura, M. & Yuyama, Y. Development of a composition database for various types of biomass. Tech. Report NIRE 203, 57–80 (2005).
Acknowledgements
We wish to express our sincere gratitude to the volunteers for their willing and extended cooperation. This study was performed under a contract with the government of Aomori Prefecture, Japan.
Author information
Authors and Affiliations
Contributions
T.M. conceived the idea and designed the study; H.T. participated in the study design; T.M., K.M. and T.Y. acquired the data; T.M. and T.T. analysed the data; H.H., Ya. T. and Yu. T. participated in the study coordination; T.M. and S.H. interpreted the data and wrote the text; H.T., T.T., H.H., Ya. T. and Yu. T., commented on the manuscript writing; All authors critically reviewed the data and the manuscript; SH supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Masuda, T., Yoshioka, T., Takahashi, T. et al. Estimation of dietary 14C dose coefficient using 13C-labelled compound administration analysis. Sci Rep 10, 8156 (2020). https://doi.org/10.1038/s41598-020-64954-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64954-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.