Abstract
Transgenic maize, Zea mays L., modified to express insecticidal proteins from the bacterium Bacillus thuringiensis Berliner, was introduced in 1996 to control Ostrinia nubilalis Hübner (Lepidoptera: Crambidae), a key maize pest in North America. The high-dose/refuge concept, developed to delay or prevent resistance evolution to this technology, has been exemplified by O. nubilalis as no cases of practical resistance were identified in >20 years. This study documents the first case of practical resistance to Cry1F Bt maize by O. nubilalis in North America. Four collections of O. nubilalis were made from Cry1F maize in Nova Scotia, Canada with unexpected injury (UXI) ranging from 30–70%. Greater survival of UXI collections was observed when larvae were exposed to the highest concentration of 200 ng Cry1F cm−2 in diet-overlay bioassays compared to susceptible laboratory colonies. Larvae also fed and survived on Cry1F leaf tissue in 7 d bioassays. A collection from non-Bt maize, 120 km west of the UXI region, also survived 200 ng Cry1F cm−2, but was susceptible to Cry1F leaf tissue. Detection of Cry1F-resistant O. nubilalis in what might be considered an insignificant maize-growing region indicates that a number of preventable causal factors may have been related to inadequate stewardship of Bt maize technology.
Similar content being viewed by others
Introduction
Transgenic maize, Zea mays L., modified to express insecticidal proteins from the bacterium Bacillus thuringiensis Berliner, commonly referred to as Bt maize, has been widely adopted for control of key maize insect pests in North America, since introduced in 1996. In Canada, approximately 85% of maize acres were planted with Bt maize in 20171. In North America, the primary target pest of Bt maize has historically been the European corn borer Ostrinia nubilalis Hübner (Lepidoptera: Crambidae). The distribution of O. nubilalis overlaps with most of the maize-producing regions in the US and Canada2. Ostrinia nubilalis is a stalk boring pest whose feeding injury in maize results in yield loss, increased infection of secondary pathogens that cause stalk and ear rots, ear drop, stalk breakage, and lodging, which impedes harvest2. The cost of O. nubilalis management and incurred yield losses were estimated to exceed $1 billion USD before the introduction of Bt maize2. Adoption of Bt maize for O. nubilalis control has resulted in widespread suppression of this pest population in North America, providing economic and environmental benefits to both Bt and non-Bt maize producers3,4.
The first commercially available Bt maize events, Bt-11 (Syngenta LLC, Research Triangle Park, NC) and MON810 (Monsanto Company, St. Louis, MO) expressing Cry1Ab protein were approved for use in Canada in 1996 and 1997, respectively5,6. In 2002, event TC1507 (co-developed by Dow AgroSciences LLC, Indianapolis, IN and Pioneer Hi-Bred International, Johnston, IA) which expresses Cry1F protein was approved in Canada7. To delay or prevent resistance to Bt maize, defined as a heritable decrease in susceptibility by the target pest population, insect resistance management (IRM) strategies were developed for implementation by trait providers and growers. The high-dose/refuge (HDR) strategy, which was developed for O. nubilalis, has been the foundation for IRM in North America8 and has been adapted for other target pests of Bt maize. A highly toxic protein will kill nearly all (>99.9%) homozygous susceptible insects and ~95% of heterozygous susceptible insects9. A non-Bt refuge is intended to support an unexposed, susceptible portion of the pest population that will mate with rare, resistant survivors from the Bt crop, thereby maintaining low numbers of individuals carrying resistance alleles within the population. Models for single high-dose Bt toxins predicted 15–30 years of durability with implementation of 5–20% refuge10,11. The IRM strategy for Bt maize and O. nubilalis has been considered exemplary, as practical resistance, defined as field-evolved resistance that reduces the efficacy of the Bt crop and has practical implications for pest control12, has not been documented in Canada (J.L.S., unpublished data) or the U.S.13,14,15,16. Bt-resistant O. nubilalis have only been reported from field collections in North America in two instances to date. A population collected from Minnesota in 2001 survived a diagnostic concentration of Cry1Ab during routine susceptibility monitoring and survived feeding on Cry1Ab leaf tissue; however, larvae were unable to survive on whole plants expressing Cry1Ab13. A collection was identified from Iowa in 2004 that exhibited survival at a diagnostic concentration of Cry1F and survived exposure to Cry1F leaf tissue in assays14. Neither case resulted in classification as practical resistance because subsequent monitoring in these regions did not detect reduced susceptibility14. Practical resistance to Bt maize has evolved in 15 other lepidopteran pests16 in such as Helicoverpa zea L.17, Spodoptera frugipera J.E. Smith18, and Striacosta albicosta Smith19,20, and the coleopteran, Diabrotica spp. LeConte21,22,23, presumably because the high-dose requirement was not met between Bt events and their target pest.
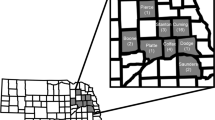
In the fall of 2018, unexpected injury (UXI) by O. nubilalis to a maize hybrid expressing event TC1507 at a number of fields in Nova Scotia, Canada was reported to the authors, only 12 years after Cry1F hybrids were first marketed in the region. The Maritime Province of Nova Scotia is a predominantly bedrock peninsula surrounded by the Atlantic Ocean (Fig. 1). Nova Scotia is a relatively small maize growing region24; approximately 14,000 ha were grown in 2018 (G. Murray, personal communication). Collections of O. nubilalis were made from four fields with unexpected injury near Truro, NS and one from a non-Bt field approximately 120 km west near Kentville, NS. Second generation offspring of the NS field collections were tested for their susceptibility to Cry1F proteins in diet-overlay concentration response bioassays and to Cry1F and Cry1Ab in leaf tissue bioassays and compared to known susceptible populations of O. nubilalis collected from the Province of Ontario, Canada. The results of this investigation confirm that O. nubilalis collected in Nova Scotia are resistant to Cry1F. This is the first case of practical resistance to Cry1F or any Bt protein among O. nubilalis in North America. Although there are many questions surrounding the development of Cry1F resistance in O. nubilalis in what might be considered an insignificant maize growing region, a number of causal factors related to inadequate stewardship of Bt maize technology may have been overlooked by stakeholders in that region.
Location of Ostrinia nubilalis collections made from four fields with unexpected injury to Cry1F maize hybrids (UXI-1 to UXI-4) and one field of non-Bt maize (Non-UXI) in 2018 in Nova Scotia (NS), Canada. Susceptible laboratory colonies were collected from Winger and Delaware, Ontario (ON) in 2010 and 2016, respectively.
Results
Diet bioassays
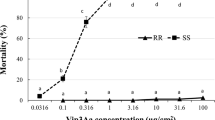
Greater than 80% survival was observed among all of the NS UXI collections exposed to the highest concentration of 200 ng Cry1F cm−2 7 days after introduction (DAI) (Fig. 2). Similarly, more than 95% of the larvae from the non-UXI site in NS survived the highest concentration (Fig. 2). Due to the lack of a mortality response within the concentration range tested, lethal concentration values for the UXI collections could not be estimated, but exceeded 200 ng cm−2 (Table 1). In contrast, 100% mortality was observed in the Delaware, ON collection at the highest concentration tested (30 ng cm−2), and for Winger, ON, 97% mortality was observed at 25 ng cm−2 and no survival was observed at any higher concentration (50–200 ng cm−2). The mortality responses of the Delaware and Winger, ON collections were alike based on overlapping confidence intervals; LC50 values were 6.7 and 6.5 ng cm−2, respectively (Table 1).
Mortality of neonate (<24 hr old) Ostrinia nubilalis exposed to Cry1F protein in diet-overlay bioassays. Larvae were second generation offspring of O. nubilalis collected from four fields with unexpected injury to Cry1F maize hybrids (UXI-1 to UXI-4) and one field of non-Bt maize (Non-UXI) in 2018 in Nova Scotia (NS), Canada. Susceptible laboratory colonies were collected from Winger and Delaware, Ontario (ON) in 2010 and 2016, respectively.
Leaf tissue bioassays
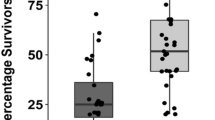
Proportional survival of O. nubilalis larvae on Cry1F or Cry1Ab leaf tissue depended on the interaction among treatment, collection, and DAI (F12, 2330 = 11.31, p < 0.0001). At 2–3 DAI, survival of UXI collections on Cry1F tissue ranged from 66–84%; survival of the non-UXI collection, and susceptible Ontario colonies was 23, 7 and 22%, respectively (Fig. 3). Survival on Cry1Ab tissue at 2–3 DAI ranged from 37–72% among UXI collections, 35% for non-UXI, and 2 and 8% of Ontario colonies (Fig. 3). By 4–5 DAI, survival of UXI collections declined to 30–63% on Cry1F tissue (Fig. 2). Neither of the Ontario collections nor the non-UXI collection survived on Cry1F or Cry1Ab tissue beyond 4–5 DAI (Fig. 3). On Cry1Ab tissue, survival among UXI collections ranged from 1–3% at 4–5 DAI. No survival of any collection was observed on Cry1Ab tissue by 7 DAI (Fig. 3). At 7 DAI, 36–66% of larvae from the UXI collections were still alive, except UXD-3 which had <10% survival (Fig. 3).
Mean proportional survival ± SE of neonate (<24 hr old) Ostrinia nubilalis larvae in Cry1F or Cry1Ab maize leaf tissue bioassays at (A) 2–3, (B) 4–5, and (C) 7 days after introduction (DAI). Larvae were second generation offspring of O. nubilalis collected from four fields with unexpected injury to Cry1F maize hybrids (UXI-1 to UXI-4) and one field of non-Bt maize (NON-UXI) in 2018 in Nova Scotia (NS), Canada. Susceptible laboratory colonies were collected from Winger and Delaware, Ontario (ON) in 2010 and 2016, respectively. Means with the same letter are not significantly different (Tukey’s HSD test, p > 0.05).
Percent defoliation of Cry1F and Cry1Ab leaf tissue also depended on the interaction among treatment, collection, and DAI (F12, 1947 = 20.44, p < 0.0001). On all sampling dates, larvae from UXI collections consumed more leaf tissue than the non-UXI collection and susceptible Ontario colonies, and UXI collections consumed more Cry1F than Cry1Ab leaf tissue (Fig. 4). Larvae from susceptible collections and the non-UXI collection never consumed more than 2% of the Cry1F or Cry1Ab leaf tissue provided. By 7 DAI, larvae from UXI collections had consumed 8–21% of Cry1F leaf tissue (Fig. 4).
Mean percent defoliation ± SE of Cry1F or Cry1Ab maize leaf tissue by Ostrinia nubilalis larvae at (A) 2–3, (B) 4–5, and (C) 7 days after introduction (DAI). Larvae were second generation offspring of O. nubilalis collected from four fields with unexpected injury to Cry1F maize hybrids (UXI-1 to UXI-4) and one field of non-Bt maize (NON-UXI) in 2018 in Nova Scotia (NS), Canada. Susceptible laboratory colonies were collected from Winger and Delaware, Ontario (ON) in 2010 and 2016, respectively. Means with the same letter are not significantly different (Tukey’s HSD test, p > 0.05).
Discussion
This study documents the first case of practical resistance to Bt maize among O. nubilalis in North America. Observations of Cry1F hybrids with 30–70% injury by O. nubilalis in Nova Scotia in 2018 and survival of offspring on Cry1F leaf tissue in laboratory bioassays provide evidence that practical resistance to Cry1F has developed among O. nubilalis in Nova Scotia. Furthermore, offspring of these field collections survived exposure to a concentration of 200 ng Cry1F cm−2 in diet-overlay bioassays which is much higher than the diagnostic concentration of 60 ng Cry1F cm−2 for Cry1F resistance, derived from extensive testing of U.S. O. nubilalis populations25,26. There are no previously reported data on the susceptibility of O. nubilalis in Nova Scotia to any Bt proteins. The lack of baseline susceptibility data from this region highlights the limitations of historical susceptibility monitoring efforts and the ability to identify field-evolved resistance; however, unexpected injury to Bt maize has not been previously reported in this region. Nova Scotia populations of O. nubilalis collected in 2018 were compared with those collected in Ontario in 2010 and 2016 that have been maintained as laboratory colonies shown to be susceptible to Cry1F protein in this study. Since LC50 values for Cry1F could not be estimated for Nova Scotia O. nubilalis, resistance ratios (RR) of LC50 values of Nova Scotia and susceptible laboratory colonies could not be calculated. A pest population is generally considered resistant when its LC50 value is 10 times greater than the LC50 value of a comparable susceptible population27. In this study, the LC50 values of Nova Scotia populations would have needed to exceed 51–65 ng Cry1F cm−2 for a RR >10 which is plausible given the greatest mean mortality observed at 200 ng cm−2 was 18.5%.
In addition to requiring a high-dose and non-Bt refuge, the HDR strategy relies on a number of assumptions, including that the frequency of resistance alleles within a population are low (<10−3), that resistance is recessive, and that random mating occurs between susceptible and resistant insects28. F1 and F2 screening of O. nubilalis from the Midwestern U.S. from 2003 to 2009 determined that Cry1F resistance allele frequencies were higher than 10−3 26. As allele frequencies did not change significantly during the years of that study, it was suggested that this may have reflected the frequency of alleles within O. nubilalis populations before the introduction of Cry1F maize26. In contrast, allele frequencies for Cry1Ab resistance were found to be very rare among O. nubilalis collected in France and the U.S.29. In laboratory selection experiments, a Cry1F-resistant colony of O. nubilalis was developed with 3000-fold resistance after 35 generations of selection25. Experiments with the laboratory-selected colony and the Iowa field collection showed that a high level of Cry1F resistance was controlled by an autosomal, single locus, recessive factor with weak fitness costs30,31.
The results of this study indicate that the four UXI collections from the Truro, NS region have developed greater tolerance to Cry1F than the non-UXI collection taken from non-Bt maize near Kentville, NS. Although all NS collections exhibited tolerance to Cry1F at 200 ng cm−2 in diet-overlay bioassays, the UXI collections had greater survival and caused greater defoliation than the non-UXI collection in leaf tissue bioassays. Similarly, the laboratory-selected Cry1F-resistant strain of O. nubilalis described by Pereira et al.31 was able to survive concentrations of >12,000 ng Cry1F cm−2 in diet-overlay bioassays25; however, survival on vegetative Cry1F leaf tissue was approximately 20% lower than on non-Bt isoline tissue. Our leaf tissue bioassay results demonstrated variability in susceptibility among collections from UXI fields which suggests that three of the UXI collections may be homozygous resistant to Cry1F and that the UXI-3 and non-UXI collections may remain heterozygous susceptible, as the dose of Cry1F expressed in leaf tissue has been shown to be high enough to kill or significantly inhibit the development of heterozygotes31. Variability of Cry1F expression among maize tissues32 may also impact the development and detection of resistance as the dose expressed in vegetative stage plants may be higher than in reproductive stage plants, as Pereira et al.31 observed greater survival of Cry1F-resistant larvae on R1 stage maize than vegetative stage maize. A short-coming of our study is that a non-Bt near-isoline was unavailable for use as a negative control in the leaf tissue bioassay. Without collaboration with Bt crop developers, public researchers are restricted to using commercially available corn hybrids of which Bt and non-Bt isolines are very rare. Moreover, testing of O. nubilalis collected in this study against the Bt crop developers’ Cry1F protein was not permitted, however, we were able to obtain Cry1F protein from a public source.
With violation of the assumption that Cry1F resistance alleles in O. nubilalis populations are rare, the functionally recessive nature of Cry1F resistance indicates that implementation of refuge is critical to maintaining O. nubilalis susceptibility to Cry1F by production of susceptible insects to mate with potentially resistant ones14,31. Since 2005, industry has moved towards Bt maize products that express a pyramid of two or more high-dose toxins targeting O. nubilalis to prolong Bt event durability with smaller refuge requirements that are more likely to be implemented by growers10. The likelihood of resistance development to a pyramid of high-dose toxins is lower than to a single toxin; however the risk increases when single toxin hybrids are not removed from the market and selection pressure is sustained33,34. In the Nova Scotia case, information obtained from maize producers and seed providers in the region where resistant populations have been found, confirm that maize hybrids expressing only Cry1F, rather than a pyramid of toxins targeting O. nubilalis, were used extensively since 2006 up to and including 2018. Although a 20% structured refuge is typically required for single toxin Bt maize products in Canada35, data on compliance with this requirement are not available from the region in question. The Canadian Food Inspection Agency, the federal body responsible for regulation of plants with novel traits (PNT) requires that IRM plans are developed for each approved PNT; however, Bt crop developers are responsible for monitoring grower compliance to IRM requirements36. Assuming that the frequency of Cry1F resistance alleles within the Nova Scotia O. nubilalis populations was similar to that found in U.S. populations25, a lack of non-Bt refuge may have facilitated the development of this resistant population.
In addition to the selection pressure placed on O. nubilalis populations in this region due to the use of a single Bt toxin, the geographic characteristics of this region should be investigated for their contribution to resistance development. The phenology of O. nubilalis in the Maritimes region of Canada is not well understood. Determination of the pheromone races, voltinism, distribution, and host range of O. nubilalis populations in this region would provide greater understanding of the historical isolation or mixing of O. nubilalis under Cry1F selection pressure and the probability of successful mitigation of Cry1F resistance in this region. Using a similar recent case for mitigation of a Cry1F-resistant population of Diatraea grandiosella Dyar detected in Arizona37, a number of strategies should be implemented immediately in the Maritimes region and in neighbouring provinces and states such as New Brunswick, Prince Edward Island, Quebec, Maine, New York, New Hampshire, and Vermont to reduce the size and spread of Cry1F-resistant O. nubilalis populations.
The sale and planting of Bt maize hybrids expressing only Cry1F should cease immediately to discourage survival of resistant individuals. Maize producers should only plant hybrids expressing a pyramid of at least two Bt toxins that O. nubilalis remain susceptible to. Although cross-resistance between Cry1F and Cry1Ab has not been documented previously25, Bt hybrids expressing only Cry1Ab, pyramids expressing Cry1Ab and Cry1F, or Cry1Ab and Vip3A, should not be used, to decrease selection pressure against Cry1Ab. Elevated survival of NS UXI collections on Cry1Ab leaf tissue compared to susceptible laboratory colonies and the non-UXI collection at 2–3 DAI was observed in this study; therefore further determination of the susceptibility of the Cry1F-resistant collections to Cry1Ab is needed.
In fields where unexpected injury to Cry1F maize has occurred, maize stalks should be chopped and buried using tillage in the fall to reduce the frequency of resistance alleles in O. nubilalis populations38. Cry1F resistance has not been shown to be associated with strong fitness costs in O. nubilalis, therefore, survival rates of homozygous susceptible, heterozygous resistant, and homozygous resistant O. nubilalis from non-Bt plants may not differ significantly30. As shown by Pereira et al., very few heterozygous resistant O. nubilalis are able to survive on reproductive stage Cry1F plants31; therefore, destruction of a significant source of resistant alleles in the overwintering phase will aid in mitigating the resistant population. Lastly, collections of O. nubilalis from neighbouring provinces and states should be tested to determine the spread of Cry1F-resistant populations. This case highlights the importance of monitoring Bt susceptibility of O. nubilalis populations in potentially geographically isolated regions where this technology is deployed, especially where single-toxin maize products are grown. We encourage diligence on the part of regulatory and industry stakeholders to increase monitoring efforts for susceptibility of O. nubilalis populations and unexpected injury to Bt maize for similar regions, and discontinue the use of non-pyramided Bt-maize products.
Methods
Insect collections
Four fields with unexpected injury (UXI) by O. nubilalis to maize hybrids expressing Cry1F protein were visited by the author in early September 2018 after identification by the grower (Fig. 1, Table 2). Seed sales representatives visited the fields in question during the previous week and confirmed Cry1F expression using QuickStix for Cry1F protein (EnviroLogix, Inc., Portland, ME). Additionally, a non-Bt maize field near Kentville, NS, approximately 120 km west and upwind of the UXI fields, infested with O. nubilalis, was visited within the same week to make a collection of O. nubilalis from a site lacking Cry1F exposure (Fig. 1, Table 2). The level of infestation was estimated at each site by counting the number of plants exhibiting symptoms of O. nubilalis injury within 100 examined; across all sites, plants ranged from the R3 (milk) to R5 (dent) stage39 (Table 2). Symptomatic plants were cut into sections using pruning shears and placed into mesh bags and shipped to the laboratory at the University of Guelph in Ridgetown, ON, which were received within 4 days of collection. The expression of Cry1F protein was re-confirmed in leaf tissue from a random subsample of five stalks per field using QuickStix for Cry1F protein; all plants from UXI fields tested positive for Cry1F. Stalks were carefully split, larvae were extracted, and placed into tubs containing meridic diet (Table 2)40. Rearing conditions were 26:18 °C, 60% RH, and photoperiod of 16:8 (L: D) h for all life stages. Larvae pupated in a corrugated cardboard ring, and the ring was transferred to a cage (30 cm × 30 cm × 60 cm, mesh roof) at the first sign of adult eclosion. Adults were provided with a water source via paper towel wicking ad libitum, and the cage sides were misted with water daily. Wax paper sheets were placed over the cage roof as an oviposition substrate, replaced daily, and placed into a plastic sweater box with moistened paper towel. Colonies of O. nubilalis originally collected from Winger and Delaware, ON in 2010 and 2016, respectively, and maintained for 95 and 17 generations, respectively, were used as susceptible laboratory controls for all experiments (Table 2).
Diet bioassays
Diet used for bioassays was formulated in the same manner as that used for rearing. One mL of diet per well was dispensed into 128-well bioassay trays (Bio-16, CD International, Pitman, NJ) using a repeater pipette. The diet surface area was 2.0 cm2. Wells were covered with transparent, adhesive, ventilated covers (Frontier Agricultural Sciences, Newark, DE) after diet had solidified. Trays were stored at 4 °C until required for bioassays. Lyophilized protein standard containing 100% purity truncated Cry1F was obtained from M. Pusztai-Carey (Case-Western University, Cleveland, OH). The protein standard was stored at −80 °C inside a desiccator (Desi-Vac Container™, Thermo Fisher Scientific, Hampton, NH) containing dessicant (Drierite, W.A. Hammond Drietite Co. Ltd., Xenia, OH). Cry1F protein solutions were diluted in series from the stock solution using 10 mM CAPS (3-cyclohexylamino)-1-propane sulfonic acid) buffer solution with pH adjusted to 10.5 using 10 N NaOH. Five concentrations plus a negative control were used for each bioassay. The concentrations tested for all collections included: 0, 12.5, 25, 50, 100, and 200 ng cm−2 except for Delaware-Sus ON where the concentrations tested were: 0, 1.9, 3.8, 7.6, 15.0, and 30.0 ng cm−2; the negative control was treated with 10 mM CAPS buffer solution. The appropriate concentration of Bt protein or control solution was applied to the diet surface of each well in 30 µl aliquots using a repeater pipette.
Trays were repeatedly tilted in all directions to cover the entire diet surface with solution. Trays were left in a fume hood until the solvent component of the solution had evaporated. One unfed, neonate (<24 h old) larva was transferred from the plastic sweater box to each well of the bioassay tray using a fine-point paint brush. Tray covers were replaced following introduction, placed into rearing conditions and covered with cardboard to prevent positive phototaxis and condensation build-up. For each bioassay, 24 or 36 larvae were treated per concentration. Larval mortality and individual larval weight were recorded at 7 DAI. Severely stunted larvae that were alive but weighed less than 0.1 mg were considered dead. Each bioassay was repeated 3 times for each collection.
Leaf tissue bioassays
The maize hybrid P72-11HR which expressed Cry1F was purchased from Pioneer Hi-Bred (Chatham, ON) and the hybrid HZ3011A which expressed Cry1Ab was purchased from Horizon Seeds (Courtland, ON). A non-Bt near-isoline was not available for either hybrid to use as a negative control. Before germination, Bt expression was verified from each seed lot by testing every 16th seed selected for planting using QuickStix for Cry1F protein. Seeds were commercially pre-treated with insecticide and fungicide treatments; therefore, all seeds were washed following the methods of Gassmann et al.21 before germination. Seeds were germinated on moistened filter paper in a growth room at 26 °C, 60–70% RH, photoperiod of 16:8 (L:D) h. Once germinated, individual seeds were planted 2 cm deep in nursery plug pots (Stuewe and Sons, Inc., Corvallis, OR) with moistened soil for 7–10 d. At the VE stage, plants were transferred to the greenhouse and transplanted into black 8-L plastic pots containing a moistened soil-less mixture prepared from a 1:1 blend of Juniper Pro-Line all-purpose mix (JVK, St. Catherines, ON) and Gro-Coir #3 (Gro-Bark Limited, Waterloo, ON). Each pot was planted with three seedlings of either treatment and pot placement was randomized under the light bank. Plants were grown in a greenhouse (mean daily T ± SD = 25 ± 3 °C; mean daily RH ± SD 52 ± 10%) under natural light supplemented by high-pressure sodium lamps (400 W S51 Lumalux®, Osram Sylvania, Mississauga, Ontario) placed 120 cm above the greenhouse bench, with each lamp covering 2 m2, at a photoperiod of 16:8 h L:D. Two weeks after planting, pots were fertilized weekly with 100 mL of Peters Excel 15-5-15 Cal-Mag Special (JVK, St. Catherines, ON) at a concentration of 4 mg mL−1. Individual pots were watered as required.
Maize tissues were collected from the greenhouse on the day of bioassay tray preparation. Wells within a 32-well bioassay tray (CD-32, CD International, Pitman, NJ) were filled with 2.5 mL of 2.5% agar. A 9 cm2 piece of filter paper (P5 grade, Fisherbrand™) was placed on the agar, and once fully moistened by the agar solution, a 4 cm2 piece of V6 stage maize leaf tissue was placed into each well. Expression of Bt events was confirmed in each leaf used in the assay before tissue was placed in the bioassay trays using QuickStix for Cry1F protein. Sixteen wells were treated with Cry1F or Cry1Ab leaves per replicate. Five, unfed, neonate (<24 hr old) larvae were placed onto the leaf tissue in each well, transferred from the plastic sweater box using a fine-point paint brush. Transparent, adhesive, ventilated lids were placed over each well (Frontier Agricultural Sciences, Newark, DE). Infested trays were placed into rearing conditions for the duration of the experiment and covered with cardboard. New tissue was added to each well every 2–3 d to ensure fresh, unlimited food. For each bioassay, 80 larvae were exposed to each treatment and each bioassay was replicated at least 4 times for each collection. At 2–3, 4–5, and 7 DAI, survival and defoliation were assessed. Survival was assessed by gently prodding larvae with a fine-tipped paint brush; larvae that did not respond with movement were considered dead. The number of larvae surviving at each time point was divided by the initial number of larvae infested to calculate proportional survival. Percent defoliation was assessed visually as the area of leaf tissue consumed by the larvae.
Statistical analysis
Diet bioassays
Only diet bioassays with ≥80% survival in the control were used in the analysis. Mortality data were analyzed to determine LC50, LC95, and LC99 values (concentration of Bt protein that resulted in mortality of 50, 95, and 99% of the individuals in a collection, respectively) with 95% confidence intervals and slope of the concentration-response curve using PROC PROBIT in SAS 9.4 (SAS Institute, Cary, NC). The OPTC option was used which estimated the natural response rate of larvae in the control41. Pearson’s chi-square test was used to test the fit of observed values compared to the predicted probit model; results were rejected if p < 0.1042. Weights of surviving larvae from each concentration were pooled to determine the mean weight per larva and transformed to percent growth inhibition relative to the mean weight of larvae in the control. To determine the EC50 (concentration of Bt protein that inhibits growth in 50 of the individuals in a collection) for each collection, growth inhibition data were analyzed by nonlinear regression fitted to a probit model using a SAS program written by D. Travnicek (Department of Biometry, University of Nebraska-Lincoln)43. Pairwise comparisons among collections were made between LC50 values and significant differences were declared for non-overlapping 95% confidence intervals.
Leaf tissue bioassays
The effects of treatment, collection, DAI, and their interactions on proportional survival and percent defoliation were analyzed as a generalized linear mixed model using PROC GLIMMIX in SAS 9.4. Replication, replication by treatment, and well within replication by treatment were considered random effects. Dependent variables were treated as repeated measures as observations were taken from wells within replication by treatment at 2–3, 4–5, and 7 DAI. PROC UNIVARIATE and the Shapiro-Wilk statistic were used to test residuals for normal distribution and studentized residuals were calculated to test for outliers using Lund’s test42. Proportional survival and percent defoliation followed a normal distribution and no outliers were detected. Least squares means (LSMEANS) were estimated and pairwise comparisons were made using Tukey-Kramer tests (α = 0.05).
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Dunlop, G. 2017 Bt Corn IRM Compliance in Canada. (CropLife Canada, 2018).
Mason, C. E. et al. European corn borer ecology, management, and association with other corn pests. (North Central Regional Extension Publication No. NCR 0327, 2018).
Hutchison, W. D. et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 330, 222–225 (2010).
Dively, G. P. et al. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. USA 115, 3320–3325, https://doi.org/10.1073/pnas.1720692115 (2018).
Canadian Food Inspection Agency. DD1996-12: Determination of Environmental Safety of Northrup King Seeds’ European Corn Borer (ECB) Resistant Corn (Zea mays L.), http://www.inspection.gc.ca/plants/plants-with-novel-traits/approved-under-review/decision-documents/dd1996-12/eng/1303862914603/1303863058362 (1996).
Canadian Food Inspection Agency. DD1997-19: Determination of the Safety of Monsanto Canada Inc.‘s Yieldgard™ Insect Resistant Corn (Zea mays L.) Line MON810, http://www.inspection.gc.ca/plants/plants-with-novel-traits/approved-under-review/decision-documents/dd1997-19/eng/1303947308350/1303947444023 (1997).
Canadian Food Inspection Agency. DD2002-41: Determination of the Safety of Dow AgroSciences Canada Inc. and Pioneer Hi-Bred International’s Insect Resistant and Glufosinate - Ammonium Tolerant Corn (Zea mays L.) Line 1507, http://www.inspection.gc.ca/plants/plants-with-novel-traits/approved-under-review/decision-documents/dd2002-41/eng/1311872961735/1311873078751 (2002).
Glaser, J. A. & Matten, S. R. Sustainability of insect resistance management strategies for transgenic Bt corn. Biotechnol. Adv. 22, 45–69, https://doi.org/10.1016/j.biotechadv.2003.08.016 (2003).
United States Environmental Protection Agency. Subpanel on Bacillus thuringiensis (Bt) plant-pesticides and resistance management, https://archive.epa.gov/scipoly/sap/meetings/web/pdf/finalfeb.pdf (1998).
Storer, N. P., Thompson, G. D. & Head, G. P. Application of pyramided traits against Lepidoptera in insect resistance management for Bt crops. GM Crops & Food 3, 154–162, https://doi.org/10.4161/gmcr.20945 (2012).
Kang, J. et al. Modeling the Impact of Cross-pollination and Low Toxin Expression in Corn Kernels on Adaptation of European Corn Borer (Lepidoptera: Crambidae) to Transgenic Insecticidal Corn. Environ. Entomol. 41, 200–211, https://doi.org/10.1603/en11133 (2012).
Tabashnik, B. E., Mota-Sanchez, D., Whalon, M. E., Hollingworth, R. M. & Carrière, Y. Defining Terms for Proactive Management of Resistance to Bt Crops and Pesticides. J. Econ. Entomol. 107, 496–507, https://doi.org/10.1603/EC13458 (2014).
Siegfried, B. D. et al. Ten years of Bt resistance monitoring in the European Corn Borer: What we know, what we don’t know, and what we can do better. Am. Entomol. 53, 208–214 (2007).
Siegfried, B. D. & Hellmich, R. L. Understanding successful resistance management. GM Crops & Food 3, 184–193, https://doi.org/10.4161/gmcr.20715 (2012).
Tabashnik, B. E. et al. Insect resistance to transgenic Bt crops: lessons from the laboratory and field. J. Econ. Entomol. 96, 1031–1038 (2003).
Tabashnik, B. E. & Carrière, Y. Global Patterns of Resistance to Bt Crops Highlighting Pink Bollworm in the United States, China, and India. J. Econ. Entomol. https://doi.org/10.1093/jee/toz173 (2019).
Dively, G. P., Venugopal, P. D. & Finkenbinder, C. Field-evolved resistance in corn earworm to Cry proteins expressed by transgenic sweet corn. Plos One 11, e0169115, https://doi.org/10.1371/journal.pone.0169115 (2016).
Huang, F. et al. Cry1F resistance in fall armyworm Spodoptera frugiperda: single gene versus pyramided Bt maize. Plos One 9, e112958, https://doi.org/10.1371/journal.pone.0112958 (2014).
Smith, J. L., Rule, D. M., Lepping, M. D., Farhan, Y. & Schaafsma, A. W. Evidence for field-evolved resistance of Striacosta albicosta (Lepidoptera: Noctuidae) to Cry1F Bacillus thuringiensis protein and transgenic corn hybrids in Ontario, Canada. J. Econ. Entomol. 110, 2217–2228, https://doi.org/10.1093/jee/tox228. (2017).
Ostrem, J. S. et al. Monitoring susceptibility of western bean cutworm (Lepidoptera: Noctuidae) field populations to Bacillus thuringiensis Cry1F protein. J. Econ. Entomol. 109, 847–853, https://doi.org/10.1093/jee/tov383 (2016).
Gassmann, A. J., Petzold-Maxwell, J. L., Keweshan, R. S. & Dunbar, M. W. Field-evolved resistance to Bt maize by western corn rootworm. Plos One 6, e22629 (2011).
Gassmann, A. J. et al. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc. Natl. Acad. Sci. USA 111, 5141–5146, https://doi.org/10.1073/pnas.1317179111 (2014).
Ludwick, D. C. et al. Minnesota field population of western corn rootworm (Coleoptera: Chrysomelidae) shows incomplete resistance to Cry34Ab1/Cry35Ab1 and Cry3Bb1. J. Appl. Entomol. 141, 28–40, https://doi.org/10.1111/jen.12377 (2017).
Statistics Canada. Nova Scotia leads Atlantic Canada in corn and apple area, https://www150.statcan.gc.ca/n1/pub/95-640-x/2016001/article/14802-eng.htm (2017).
Pereira, E. J. G., Lang, B. A., Storer, N. P. & Siegfried, B. D. Selection for Cry1F resistance in the European corn borer and cross-resistance to other Cry toxins. Entomol. Exp. Appl. 126, 115–121, https://doi.org/10.1111/j.1570-7458.2007.00642.x (2008).
Siegfried, B. D. et al. Estimating the frequency of Cry1F resistance in field populations of the European corn borer (Lepidoptera: Crambidae). Pest Manage. Sci. 70, 725–733, https://doi.org/10.1002/ps.3662 (2014).
Tabashnik, B. E., Rensburg, J. B. J. V. & Carriere, Y. Field-evolved insect resistance to Bt crops: Definition, Theory, and Data. J. Econ. Entomol. 102, 2011–2025 (2009).
Alstad, D. N. & Andow, D. A. Managing the Evolution of Insect Resistance to Transgenic Plants. Science 268, 1894–1896, https://doi.org/10.1126/science.268.5219.1894 (1995).
Bourguet, D. et al. Frequency of alleles conferring resistance to Bt maize in French and US corn belt populations of the European corn borer, Ostrinia nubilalis. Theor. Appl. Genet. 106, 1225–1233, https://doi.org/10.1007/s00122-002-1172-1 (2003).
Pereira, E. J. G., Storer, N. P. & Siegfried, B. D. Fitness costs of Cry1F resistance in laboratory-selected European corn borer (Lepidoptera: Crambidae). J. Appl. Entomol. 135, 17–24, https://doi.org/10.1111/j.1439-0418.2009.01488.x (2011).
Pereira, E. J. G., Storer, N. P. & Seigfreid, S. D. Inheritance of Cry1F resistance in laboratory-selected European corn borer and its survival on transgenic corn expressing the Cry1F toxin. Bull. Entomol. Res. 98, 621–629 (2008).
United States Environmental Protection Agency. Biopesticide registration action document Bacillus thuringiensis Cry1F corn, http://bch.cbd.int/database/attachment/?id=10711 (2004).
Zhao, J.-Z. et al. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proceedings of the National Academy of Sciences of the United States of America 102, 8426–8430, https://doi.org/10.1073/pnas.0409324102 (2005).
Roush, R. T. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 353, 1777–1786, https://doi.org/10.1098/rstb.1998.0330 (1998).
Canadian Corn Pest Coalition. Bt Corn Products/Traits Available in Canada as of May 2019, https://www.cornpest.ca/bt-corn/bt-corn-products-traits-available-in-canada-as-of-may-2019/ (2019).
Macdonald, P. & Yarrow, S. Regulation of Bt crops in Canada. J. Invertebr. Pathol. 83, 93–99, https://doi.org/10.1016/S0022-2011(03)00059-4 (2003).
United States Environmental Protection Agency. White paper on resistance in lepidopteran pests of Bacillus thuringiensis (Bt) plant-incorporated protectants in the United States, https://www.epa.gov/sites/production/files/2018-07/documents/position_paper_07132018.pdf (2018).
Schaafsma, A. W., Meloche, F. & Pitblado, R. E. Effect of mowing corn stalks and tillage on overwintering mortality of European corn borer (Lepidoptera: Pyralidae) in field corn. J. Econ. Entomol. 89, 1587–1592, https://doi.org/10.1093/jee/89.6.1587 (1996).
Abendroth, L. J., Elmore, R. W., Boyer, M. J. & Marlay, S. K. Corn growth and development. Vol. Bulletin PMR 1009 (Iowa State University Extension, 2011).
Guthrie, W. D. In Handbook of Insect Rearing Vol. 2 (eds P. Singh & P. D. Moore) (Elsevier, 1985).
SAS Institute. In SAS/STAT 9.2 User’s Guide 112 (SAS Institute Inc., Cary, NC, https://support.sas.com/documentation/cdl/en/statugprobit/61823/PDF/default/statugprobit.pdf, 2008).
Bowley, S. R. A hitchhiker’s guide to statistics in biology. Generalized linear mixed model edition., (Plants et al., Inc., 2015).
Marcon, P. C. R. G., Young, L. J., Steffey, K. L. & Siegfried, B. D. Baseline susceptibility of European corn borer (Lepidoptera: Crambidae) to Bacillus thuringiensis toxins. J. Econ. Entomol. 92, 279–285 (1999).
Acknowledgements
The authors are thankful to the grower cooperators who allowed us to collect O. nubilalis on their farms and were willing to assist in this research. We are extremely appreciative to Marianne Pushtai-Carey for supplying Cry1F protein. We thank Gordon (Sonny) Murray for help in finding collection locations and assistance with collecting. Thanks to Samuel Capannelli from the Ontario Ministry of Agriculture, Food and Rural Affairs for creating Figure 1. This research was not supported by any external funding.
Author information
Authors and Affiliations
Contributions
J.S. and A.S. designed the study. J.S. and Y.F. performed the experiments. J.S. analyzed the data. J.S. wrote the manuscript and prepared the figures. All authors reviewed and edited the paper and critically discussed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smith, J.L., Farhan, Y. & Schaafsma, A.W. Practical Resistance of Ostrinia nubilalis (Lepidoptera: Crambidae) to Cry1F Bacillus thuringiensis maize discovered in Nova Scotia, Canada. Sci Rep 9, 18247 (2019). https://doi.org/10.1038/s41598-019-54263-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54263-2
This article is cited by
-
Genetic mutations linked to field‐evolved Cry1Fa-resistance in the European corn borer, Ostrinia nubilalis
Scientific Reports (2023)
-
Recent trends in management strategies for two major maize borers: Ostrinia nubilalis and Sesamia nonagrioides
Journal of Pest Science (2023)
-
CRISPR-mediated mutations in the ABC transporter gene ABCA2 confer pink bollworm resistance to Bt toxin Cry2Ab
Scientific Reports (2021)
-
Shared and Independent Genetic Basis of Resistance to Bt Toxin Cry2Ab in Two Strains of Pink Bollworm
Scientific Reports (2020)
-
Cadherin repeat 5 mutation associated with Bt resistance in a field-derived strain of pink bollworm
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.