Abstract
Coronary artery bypass grafting is among the most commonly performed of all cardiovascular surgical procedures. However, graft failure due to stenosis reduces the long-term benefit of the intervention. This study asks if elevating plasma high density lipoprotein cholesterol (HDL-C) levels by inhibition of cholesteryl ester transfer protein (CETP) activity with des-fluoro-anacetrapib, an analog of the CETP inhibitor anacetrapib, prevents vein bypass-induced neointimal hyperplasia. NZW rabbits were placed on a normal chow diet or chow containing 0.14% (wt/wt) des-fluoro-anacetrapib for 6 weeks. Bypass grafting of the jugular vein to the common carotid artery was performed 2 weeks after starting dietary des-fluoro-anacetrapib supplementation. The animals were euthanised 4 weeks post-bypass grafting. Relative to control, dietary supplementation with des-fluoro-anacetrapib reduced plasma CETP activity by 89 ± 6.9%, increased plasma apolipoprotein A-I levels by 24 ± 5.5%, increased plasma HDL-C levels by 93 ± 26% and reduced intimal hyperplasia in the grafted vein by 38 ± 6.2%. Des-fluoro-anacetrapib treatment was also associated with decreased bypass grafting-induced endothelial expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), endothelial dysfunction, and smooth muscle cell (SMC) proliferation in the grafted vein. In conclusion, increasing HDL-C levels by inhibiting CETP activity is associated with inhibition of intimal hyperplasia in grafted veins, reduced inflammatory responses, improved endothelial function, and decreased SMC proliferation.
Similar content being viewed by others
Introduction
Coronary artery bypass grafting is among the most commonly performed of all cardiovascular surgical procedures. However, graft failure due to stenosis, which impairs blood flow, or can lead to total vessel occlusion, reduces the long-term benefit of this procedure. This is especially true for saphenous vein grafts.
Key factors in the pathogenesis of post-surgical vein graft occlusion are inflammatory responses that result in migration of inflammatory cells into the subendothelium of the vein grafts1, endothelial dysfunction due to decreased bioavailability of nitric oxide, increased endothelin-1 levels and enhanced superoxide production2,3. Smooth muscle cell proliferation and the transformation of smooth muscle cells in the grafted vessel wall from a quiescent, contractile phenotype to a proliferative, synthetic phenotype is also a common occurrence4.
Plasma high density lipoprotein cholesterol (HDL-C) levels are inversely correlated with the risk of having a cardiovascular event5. HDLs have multiple potentially cardioprotective properties. These include an ability to remove cholesterol from macrophages, which initiates the first step of the reverse cholesterol transport pathway6,7. HDLs also attenuate vascular inflammation8, suppress vascular smooth muscle cell (VSMC) proliferation9, promote endothelial repair10, and enhance endothelial function11. These cardioprotective functions of HDLs suggest that increasing endogenous HDL-C levels may reduce vein graft occlusion.
Cholesteryl ester transfer protein (CETP) transfers cholesteryl esters from HDLs to low density lipoproteins (LDLs) and triglyceride-rich lipoproteins in exchange for triglycerides12. Agents that inhibit CETP activity increase the level of HDL-C and apolipoprotein (apo) A-I, the major HDL apolipoprotein, and decrease non-HDL cholesterol levels. We have reported that the CETP inhibitor des-fluoro-anacetrapib, an analog of the CETP inhibitor anacetrapib, increases HDL-C levels13, enhances endothelial repair, improves endothelial function, inhibits vascular smooth muscle cell proliferation and reduces intimal hyperplasia in New Zealand White (NZW) rabbits following endothelial denudation of the abdominal aorta13 and in NZW rabbits with balloon injury and stent deployment in the iliac artery14.
The present study asks whether des-fluoro-anacetrapib treatment is associated with inhibition of neointimal hyperplasia in NZW rabbits with right external jugular vein autologous end-to-side transplantation bypass grafting in the right common carotid artery.
Materials and Methods
Animal studies
Two groups of male NZW rabbits (n = 8–9/group) weighing 2.5–3.0 kg (Nanowie Small Animal Production Unit, Bellbrae, Victoria, Australia) were maintained on a regular chow diet, or chow supplemented with 0.14% (wt/wt) des-fluoro-anacetrapib (Merck & Co., Inc. Kenilworth, NJ, USA) for 6 weeks.
After 2 weeks of des-fluoro-anacetrapib treatment, vein bypass grafting was performed under general anaesthesia (inhaled isofluorane (4–5% for induction and 1.5–2% for maintenance). A front midline neck incision was made to expose the right external jugular vein and the right common carotid artery in the anaesthetised animals. The branches of the jugular vein were carefully ligated with 8–0 polypropylene sutures. An approximately 2 cm segment of the jugular vein was removed, flushed and kept moist in heparinized saline (100U heparin/mL) for the autologous, reversed vein graft. The animals were systemically heparinized (200 IU/kg), and the right common carotid artery was clamped at the proximal and distal ends. The side of the artery was flushed with heparinized saline (1U heparin/mL) containing 1% (w/v) lignocaine. An 8/0 prolene uninterrupted suture was used to make a reversed vein attachment end-to-side to the artery (proximal end of the vein to the distal part of the artery and vice versa) under 3.5X magnification (Zeiss, Germany). The artery clamps were removed after graft anastomosis, and blood flow was restored into the grafted vein. The incision was closed with 4/0 silk suture, and the sutured skin was covered with iodine spray and wound gel (antiseptic). Pulsatile flow in the grafted vessel was confirmed by palpation. The animals were euthanised 4 weeks after bypass grafting, and the grafted vein and blood were collected. All the procedures were approved by the University of New South Wales Sydney Animal Care and Ethics Committee (Protocol number 15/144A) and performed in accordance with the relevant guidelines and regulations.
Biochemical analyses
Blood was collected into EDTA tubes (BD Biosciences, Franklin Lakes, NJ) following euthanasia and plasma was isolated (1,000 × g, 4 °C, 10 min). CETP activity was assessed as the transfer of [3H]-labelled cholesteryl esters from ultracentrifugally isolated human HDL3 to isolated human LDLs15. This was achieved by incubation of plasma with [3H]cholesteryl ester-labelled HDL3 and unlabelled LDLs at 37 °C for 3 h. The LDLs were precipitated with heparin (5,000 IU/mL):MnCl2 (2 mol/L) (1:1, v/v) and the radioactivity in the supernatant was determined by liquid scintillation counting. The activity of CETP was determined as the % total radiolabelled cholesteryl esters transferred from HDL3 to LDLs15. ApoA-I concentrations were determined immunoturbidometrically with sheep anti-rabbit apoA-I polyclonal antibodies16. Total cholesterol concentrations were determined enzymatically17. Plasma HDL-C levels were determined after polyethylene glycol 6000 precipitation of apoB-containing lipoproteins18. Analyses were carried out using an AU480 Chemistry Analyzer (Beckman Coulter, Fullerton, CA).
Assessment of neointimal hyperplasia in the grafted veins
The animals were euthanised 4 weeks after the vein grafting procedure. The grafted veins (~3 mm) were collected, fixed with paraformaldehyde (4%, v/v), embedded in paraffin and then sectioned (5 µm) as described19. Morphology was assessed with Verhoeff’s hematoxylin stain. Adjacent sections of the grafted veins were immunostained with mouse anti-rabbit vascular cell adhesion molecule-1 (VCAM-1) (1:400), mouse anti-rabbit intercellular adhesion molecule-1 (ICAM-1) (1:200) monoclonal antibodies (both gifts from Dr. M.Cybulsky, University of Toronto)19, an anti-mouse proliferating cell nuclear antigen (PCNA) monoclonal antibody (dilution 1:200; Dako, Glostrup, Denmark), a mouse monoclonal antibody against alpha smooth muscle actin (α-SMC actin) (dilution 1:50; Abcam, Cambridge, UK), a mouse monoclonal antibody against macrophage clone RAM11 (RAM11) (dilution 1:200; Dako), and a mouse monoclonal antibody against CD18 (1:200) (AbD Serotec, Raleigh, NC). A polyclonal goat anti-mouse IgG-HRP (dilution 1:200, Dako) was used as a secondary antibody. The Horseradish Peroxidase (HRP)-3,3′ Diaminobenzidine (DAB) system (Envision Mouse Kit, Dako), and counterstaining with haematoxylin was used for visualization of the stained samples. Sections were imaged with a light microscope (Zeiss, Germany). Planimetry (Adobe Photoshop V6.0) was performed by tracing the area of the intima and media. Results are reported as total pixel numbers. Intimal hyperplasia was evaluated as the intima/media ratio. DAB staining was quantified with ImageJ software (http://rsb.info.nih.gov/ij/) using the polygon tool to quantify the total intima/media cross-sectional area and lumen circumference. The threshold for positive staining was defined by an independent observer that was blinded to the treatment. Positively stained areas were quantified by de-convolution. To account for variations in grafted vein size, the number of pixels staining positive for VCAM-1- and ICAM-1 was divided by the circumference of the lumen19. Total cell profiles and PCNA+ cells were quantified manually (40x magnification). All the samples were coded and analysed by a single operator that was blinded to the treatment.
Assessment of endothelial function
Isometric tension experiments for grafted vein rings (~3 mm in length) were performed 4 h after the animals were euthanised. The rings were positioned in cold Krebs buffer solution (Sigma-Aldrich, St Louis, MO, Catalogue Number: K3753), aerated with 95% O2/5% CO2 and mounted in a Myobath (World Precision Instruments, Sarasota, FL, USA) containing 20 mL of Krebs buffer solution aerated at 37 °C with 95% O2/5% CO219. The viability of the rings was confirmed by incremental constriction (2.5 g load) in the presence of phenylephrine (Sigma-Aldrich, Catalogue Number: P6126)19. After pre-constricting the rings to 80% maximal response, endothelium-dependent vasodilation was quantified in the presence of incremental doses (0.001–10 µmol/L) of acetylcholine (Sigma-Aldrich, Catalogue Number: A6625), and sodium nitroprusside (Sigma-Aldrich, Catalogue Number: 71778).
Statistical analysis
Data are expressed as mean ± SEM. Differences between groups were evaluated using an unpaired Student’s t test. Between group differences in acetylcholine and sodium nitroprusside dose response curves were evaluated by one-way ANOVA for repeated-measures with Bonferroni corrections. All statistical tests were performed using GraphPad Prism software version 7.03 (GraphPad Software, Inc. San Diego, CA). Result are expressed as the mean ± SEM. A 2-tailed p < 0.05 was considered significant.
Results
Des-fluoro-anacetrapib treatment inhibits CETP activity and increases intimal hyperplasia in grafted veins in NZW rabbits
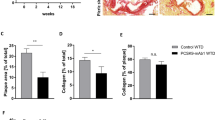
Two groups of NZW rabbits (n = 8–9/group) were studied. Dietary supplementation with 0.14% (wt/wt) des-fluoro-anacetrapib reduced CETP activity by 89 ± 6.9% relative to the animals that were maintained on regular chow (Fig. 1A, p < 0.05). Plasma apoA-I levels increased from 0.46 ± 0.04 mg/mL for the control animals to 0.57 ± 0.03 mg/mL in the des-fluoro-anacetrapib-treated animals (Fig. 1B, p < 0.05). HDL-C levels increased from 0.42 ± 0.05 mmol/L in the control animals to 0.81 ± 0.14 mmol/L in the des-fluoro-anacetrapib-treated animals (Fig. 1C, p < 0.05).
Dietary supplementation with des-fluoro-anacetrapib inhibits plasma CETP activity, and intimal hyperplasia in grafted veins in NZW rabbits. NZW rabbits received chow (control) or chow supplemented with 0.14% (wt/wt) des-fluoro-anacetrapib (dfAna) for 6 weeks. A right external jugular vein autologous end-to-side transplantation bypass graft was carried out after 2 weeks of des-fluoro-anacetrapib treatment. The animals were sacrificed 4 weeks after bypass grafting. Panel (A): plasma CETP activity. Panel (B): plasma apoA-I levels. Panel (C): plasma HDL-C levels. Panel (D): A representative Verhoeff’s hematoxylin-stained cross-section of the centre of a grafted vein (bar = 500 µm). Panel (E): Quantification of intima-to-media ratio of cross-sections of grafted veins. Data are expressed as individual points with the cross symbol indicating the mean ± SEM, n = 8 for the control group, n = 9 for the dfAna group, #p < 0.05 vs Control.
We have reported elsewhere that des-fluoro-anacetrapib treatment inhibits intimal hyperplasia in NZW rabbits with balloon injury of the abdominal aorta13 and balloon injury and stent deployment in the iliac artery14. In the present study, right external jugular vein autologous end-to-side transplantation bypass grafting of the common carotid artery also led to neointimal formation in the grafted veins, as determined by the increased intima/media ratio, the control animals. (Fig. 1D, red arrows)). Grafted vein neointimal hyperplasia in the des-fluoro-anacetrapib-treated rabbits was, by contrast, decreased by 38 ± 6.2% compared to what was observed for the control animals (Fig. 1D,E, p < 0.05).
Des-fluoro-anacetrapib treatment inhibits endothelial expression of VCAM-1 and ICAM-1 in grafted veins in NZW rabbits
The grafted veins in the control animals that did not receive des-fluoro-anacetrapib had high endothelial expression levels of VCAM-1 (Fig. 2A) and ICAM-1 (Fig. 2B). By contrast, endothelial expression of VCAM-1 (Fig. 2A) and ICAM-1 (Fig. 2B) in the des-fluoro-anacetrapib-treated rabbits was decreased by 65 ± 9.9% (Fig. 2C) and 51 ± 14% (Fig. 2D), respectively, compared to what was observed for the control animals (p < 0.05 for both).
Des-fluoro-anacetrapib treatment decreases endothelial VCAM-1 and ICAM-1 expression in grafted veins in NZW rabbits. Right external jugular vein autologous end-to-side transplantation bypass grafting of the right common carotid artery was carried out in NZW rabbits maintained on regular chow (control) or chow supplemented with 0.14% (wt/wt) des-fluoro-anacetrapib (dfAna) as described in the legend to Fig. 1. VCAM-1 (Panel A) and ICAM-1 immunostaining (Panel B) of representative grafted vein cross-sections is shown (bar = 100 µm). Quantification of endothelial expression of VCAM-1 and ICAM-1 is shown in Panels (C,D), respectively. Data are expressed as individual points with the cross symbol indicating the mean ± SEM, n = 8 for the control group, n = 9 for the dfAna group, #p < 0.05 vs Control.
Des-fluoro-anacetrapib treatment reduces endothelial dysfunction in grafted veins in NZW rabbits
Endothelial dysfunction was evident in the grafted veins in the control animals (Fig. 3A, open circles). Des-fluoro-anacetrapib treatment was associated with a maximal increase in endothelium-dependent relaxation in pre-constricted rings from the grafted veins in response to acetylcholine of 1.7 ± 0.2-fold relative to control (Fig. 3A, closed circles) (p < 0.05). Endothelium-independent relaxation with sodium nitroprusside was indistinguishable for the control and des-fluoro-anacetrapib-treated animals (Fig. 3B).
Des-fluoro-anacetrapib treatment protects against grafted vein endothelial dysfunction in NZW rabbits. Right external jugular vein autologous end-to-side transplantation bypass grafting of the right common carotid artery was carried out in NZW rabbits maintained on regular chow (control) or chow supplemented with 0.14% (wt/wt) des-fluoro-anacetrapib (dfAna) as described in the legend to Fig. 1. Panel (A): Endothelial-dependent relaxation of pre-contracted grafted vein rings in response to acetylcholine (ACh). Panel (B): Endothelial-independent relaxation of pre-contracted grafted vein rings in response to sodium nitroprusside (SNP). Data are expressed as mean ± SEM, n = 8 for the control group, n = 9 for the dfAna group, *p < 0.05.
Des-fluoro-anacetrapib treatment reduces smooth muscle cell proliferation in grafted veins in NZW rabbits
Smooth muscle cell (SMC) proliferation was apparent in the grafted veins of the control animals as judged by the numerous PCNA+ cells (Fig. 4A). Treatment with des-fluoro-anacetrapib was associated with a reduction in the number of PCNA+ cells in grafted veins of 43 ± 5.5% relative to what was observed for the control animals (Fig. 4B) (p < 0.05).
Des-fluoro-anacetrapib treatment reduces grafted vein smooth muscle cell proliferation in NZW rabbits. Right external jugular vein autologous end-to-side transplantation bypass grafting of the right common carotid artery was carried out in NZW rabbits maintained on regular chow (control) or chow supplemented with 0.14% (wt/wt) des-fluoro-anacetrapib (dfAna) as described in the legend to Fig. 1. Panel (A): immunostaining of PCNA+ cells in grafted vein cross-sections (bar = 50 µm). Panel (B): Quantification of cell proliferation expressed as the % PCNA+ cells relative to the total number of cells in the intima and media. Data are expressed as individual points with the cross symbol indicating the mean ± SEM, n = 8 for the control group, n = 9 for the dfAna group, #p < 0.05 vs Control.
Cellular characterization of the vein grafts
As indicated by α-actin staining, the number of smooth muscle cells in the grafted veins of the control animals was increased relative to what was observed for the des-fluoro-anacetrapib-treated animals (Fig. 5A). A small number of neutrophils (CD18 + cells) were also evident in the neointimal region of the grafted veins (Fig. 5B). Macrophages (RAM11 + cells) were not detected in the grafted veins of the des-fluoro-anacetrapib-treated or control animals (Fig. 5C).
Cellular characterization of grafted veins in NZW rabbits treated with des-fluoro-anacetrapib. Right external jugular vein autologous end-to-side transplantation bypass grafting of the right common carotid artery was carried out in NZW rabbits maintained on regular chow (control, n = 8) or chow supplemented with 0.14% (wt/wt) des-fluoro-anacetrapib (dfAna, n = 9) as described in the legend to Fig. 1. Representative photomicrographs of grafted vein cross-sections (bar = 50 µm) from control and dfANa-treated animals immunostained for α-SMC actin (Panel A), CD18 (Panel B, red arrows) and RAM11 (Panel C) are shown.
Des-fluoro-anacetrapib treatment does not have a direct effect on vascular cell proliferation and inflammation
To ascertain whether the reduction in intimal hyperplasia in the des-fluoro-anacetrapib-treated animals could be attributed to a direct interaction of the inhibitor with intimal smooth muscle cells, human micro-vascular endothelial cells (HMECs) (Supplemental Fig. 1A) and human aortic smooth muscle cells (HASMCs) (Supplemental Fig. 1B) were incubated with des-fluoro-anacetrapib. Cell proliferation did not increase in either of these incubations (Supplemental Fig. 1). Moreover, incubation with des-fluoro-anacetrapib did not inhibit VCAM-1 (Supplemental Fig. 1C) or ICAM-1 (Supplemental Fig. 1D) mRNA levels in tumour necrosis factor (TNF)-α−activated HMECS.
Discussion
Vein graft surgery has a high failure rate. Patency can decrease from 98% immediately after surgery to < 88% within the first month after surgery, and to 60% after 10 years as a result of intimal hyperplasia, atherosclerosis, and rupture of plaques in the vein grafts20,21. Anti-platelet agents and statins are the only medications currently used for prevention of vein graft failure20. As vein grafts will continue to be used in the foreseeable future, it is apparent that additional approaches for improving the patency of the grafted vessels are needed. The present study, which indicates that increasing HDL-C levels by inhibiting CETP activity is associated with reduced intimal hyperplasia in grafted veins, may be a further strategy for improving vein graft patency.
We have previously shown that increasing HDL-C levels with the CETP inhibitor des-fluoro-anacetrapib accelerates endothelial repair, enhances endothelial function, inhibits vascular SMC proliferation and reduces intimal hyperplasia in NZW rabbits with endothelial denudation of the abdominal aorta13 or balloon injury and stent deployment in the iliac artery14. In the previous studies, dietary supplementation with 0.14% (wt/wt) des-fluoro-anacetrapib increased plasma HDL-C levels and reduced CETP activity to a similar extent as in the present study. We have also reported that treating NZW rabbits with des-fluoro-anacetrapib increases HDL particle size, but does not affect plasma non-HDL-C or triglyceride levels13,14. It therefore follows that the protective effect of des-fluoro-anacetrapib against grafted vein neointimal formation is likely due to the increase in HDL-C levels.
Inflammatory responses, endothelial dysfunction and cellular proliferation are all associated with grafted vein neointimal formation1,22,23,24. In the present study smooth muscle cells were the dominant cell type in the grafted veins. A small number of neutrophils were also detected in the neointima. Although macrophages play an important role in vascular inflammation, they were not detected in the grafted veins, possibly because the animals were maintained on a normal chow diet for the duration of the study.
Our results indicate that the beneficial effects of dietary supplementation with des-fluoro-anacetrapib in terms of inhibiting endothelial cell inflammation decreasing expression of VCAM-1 and ICAM-1, reducing endothelial dysfunction and inhibiting smooth muscle cell proliferation in grafted veins are directly attributable to the increase in HDL-C levels in the des-fluoro-anacetrapib-treated animals and cannot be explained by an interaction of the inhibitor with smooth muscle cells or endothelial cells. These results are also in line with an exploratory analysis from the CASCADE trial in which lower HDL-C levels were associated with increased graft occlusion and intimal hyperplasia in the grafted veins25. Patients with HDL-C levels > 60 mg/dL also had significantly lower intimal hyperplasia at 12 months after coronary bypass surgery in that study25.
This may explain why there was reduced neointimal formation in the grafted veins of the des-fluoro-anacetrapib treated NZW rabbits. It is also possible that the reduced SMC proliferation and neointimal formation in the grafted veins of the des-fluoro-anacetrapib treated NZW rabbits may have occurred in an SR-B1/PDZK1- and PI3K/Akt-dependent manner as we have previously reported for des-fluoro-anacetrapib treated NZW rabbits with endothelial denudation of the abdominal aorta13,14.
HDLs and apoA-I are well-established independent inverse predictors of cardiovascular events. However, three large-scale randomized cardiovascular outcome clinical trials of CETP inhibitors have failed to meet their endpoints26. In the recent REVEAL trial, by contrast, anacetrapib significantly reduced major coronary events27. Although anacetrapib has a long terminal half-life because of accumulation in adipose tissue, and its development has stopped28,29, the results of the present study have identified a potential application of CETP inhibition as a therapy for preventing graft failure due to stenosis. Further studies in patients undergoing coronary artery bypass grafting are warranted.
There are some limitations in the present study. Only one dose of 0.14% (wt/wt) des-fluoro-anacetrapib was used, and we therefore cannot determine if there is a dose-dependent association of des-fluoro-anacetrapib with inhibition of neointimal formation in grafted veins. However, in our previous studies the effects of 0.14% (wt/wt) des-fluoro-anacetrapib on plasma HDL-C levels, CETP activity, angiogenesis in hindlimb ischemia, and endothelial function and repair were larger than those of 0.07% (wt/wt) des-fluoro-anacetrapib. It is therefore likely that des-fluoro-anacetrapib will also protect against grafted vein neointimal formation in a dose-dependent manner. Moreover, as the present study was performed in NZW rabbits, further studies are needed to determine whether CETP inhibition also decreases neointimal formation in grafted veins in humans. A further limitation of this study is that neointimal hyperplasia and endothelial function were evaluated only at the single time point of 4 weeks after the vein graft procedure. This precluded investigation of acute inflammatory cell recruitment and pro-inflammatory cytokine and chemokine production in the first few days after the procedure that could contribute to early vein graft failure30,31.
In conclusion, inhibition of CETP activity inhibits intimal hyperplasia in grafted veins in NZW rabbits by inhibiting endothelial inflammation, improving endothelial function, and reducing SMC proliferation. Further clinical studies are needed to determine whether CETP inhibition, or other HDL raising agents, reduce the incidence of graft failure due to stenosis in humans.
References
Hanyu, M. et al. VCAM-1 expression precedes macrophage infiltration into subendothelium of vein grafts interposed into carotid arteries in hypercholesterolemic rabbits–a potential role in vein graft atherosclerosis. Atherosclerosis. 158, 313–319 (2001).
Cerrato, R. et al. Endothelin-1 increases superoxide production in human coronary artery bypass grafts. Life Sci. 91, 723–728 (2012).
Buttery, L. D. et al. Explanted vein grafts with an intact endothelium demonstrate reduced focal expression of endothelial nitric oxide synthase specific to atherosclerotic sites. J Pathol. 179, 197–203 (1996).
Li, F. D. et al. Intimal thickness associated with endothelial dysfunction in human vein grafts. J Surg Res. 180, e55–e62 (2013).
Gordon, D. J. et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79, 8–15 (1989).
Khera, A. V. & Rader, D. J. Future therapeutic directions in reverse cholesterol transport. Curr Atheroscler Rep. 12, 73–81 (2010).
Rohatgi, A. et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 371, 2382–2393 (2014).
Nicholls, S. J. et al. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 111, 1543–1550 (2005).
van der Vorst, E. P. et al. High-density lipoproteins suppress chemokine expression and proliferation in human vascular smooth muscle cells. FASEB J. 27, 1413–1425 (2013).
Tso, C. et al. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler Thromb Vasc Biol. 26, 1144–1149 (2006).
Spieker, L. E. et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 105, 1399–1402 (2002).
Barter, P. J. et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 23, 160–167 (2003).
Wu, B. J. et al. Cholesteryl ester transfer protein inhibition enhances endothelial repair and improves endothelial function in the rabbit. Arterioscler Thromb Vasc Biol. 35, 628–636 (2015).
Wu, B. J. et al. Reduction of in-stent restenosis by cholesteryl ester transfer protein inhibition. Arterioscler Thromb Vasc Biol. 37, 2333–2341 (2017).
Tollefson, J. H., Liu, A. & Albers, J. J. Regulation of plasma lipid transfer by the high-density lipoproteins. Am J Physiol. 255, E894–E902 (1988).
Clay, M. A., Pyle, D. H., Rye, K. A. & Barter, P. J. Formation of spherical, reconstituted high density lipoproteins containing both apolipoproteins A-I and A-II is mediated by lecithin:cholesterol acyltransferase. J. Biol. Chem. 275, 9019–9025 (2000).
Stähler, F., Gruber, W., Stinshoff, K. & Röschlau, P. A practical enzymatic cholesterol determination. Med. Lab. (Stuttg). 30, 29–37 (1977).
Allen, J. K., Hensley, W. J., Nicholls, A. V. & Whitfield, J. B. An enzymic and centrifugal method for estimating high-density lipoprotein cholesterol. Clin. Chem. 25, 325–327 (1979).
Wu, B. J. et al. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler Thromb Vasc Biol. 30, 968–975 (2010).
de Vries, M. R., Simons, K. H., Jukema, J. W., Braun, J. & Quax, P. H. Vein graft failure: from pathophysiology to clinical outcomes. Nat Rev Cardiol. 13, 451–470 (2016).
Yahagi, K. et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 13, 79–98 (2016).
Zhang, J. Y. et al. Local application of paeonol prevents early restenosis: a study with a rabbit vein graft model. J Surg Res. 212, 278–287 (2017).
Hattori, K. et al. Celiprolol reduces the intimal thickening of autogenous vein grafts via an enhancement of nitric oxide function through an inhibition of superoxide production. J Vasc Surg. 46, 116–123 (2007).
Miyake, T. et al. Inhibitory effects of NFkappaB decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit vein graft model. J Mol Cell Cardiol. 41, 431–440 (2006).
Jerzewski, K., Ruel, M., Voisine, P., Le May, M. R. & Kulik, A. Does high-density lipoprotein influence the development of saphenous vein graft disease after coronary bypass surgery?: exploratory analysis from the CASCADE trial. J Cardiothorac Surg. 8, 172 (2013).
Barter, P. J. & Rye, K. A. Cholesteryl ester transfer protein inhibition is not yet dead–Pro. Arterioscler Thromb Vasc Biol. 36, 439–441 (2016).
Bowman, L. et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 377, 1217–1227 (2017).
Krishna, R. et al. Pharmacokinetics and pharmacodynamics of anacetrapib following single doses in healthy, young Japanese and White male subjects. J Clin Pharmacol. 58, 254–262 (2018).
Gotto, A. M. et al. Lipids, safety parameters, and drug concentrations after an additional 2 years of treatment with anacetrapib in the DEFINE study. J Cardiovasc Pharmacol Ther. 19, 543–549 (2014).
Karper, J. C. et al. Toll-like receptor 4 is involved in human and mouse vein graft remodelling, and local gene silencing reduces vein graft disease in hypercholesterolemic APOE*3Leiden mice. Arterioscler Thromb Vasc Biol. 31, 1033–1040 (2011).
Meng, X. B. et al. Small interfering RNA targeting nuclear factor kappa B to prevent vein graft stenosis in rat models. Transplant Proc. 45, 2553–2558 (2013).
Author information
Authors and Affiliations
Contributions
B.J.W. and Y.L. were responsible for experimental design, carried out the experiments, performed data analysis, and wrote the manuscript. B.J.W. and Y.S. carried out the experiments. K.L.O., D.J., P.J.B. and K.A.R. contributed to the experimental design and revision of the manuscript. All authors approve submission of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.J. is an employee of Merck & Co, Inc. The other authors have no conflicts of interest to disclose. This work was supported by Merck & Co., Inc, the National Health and Medical Research Council (NHMRC) of Australia (Grants 482800 and 1037903) to KAR and PJB and an NHMRC Career Development Fellowship (1122854) to KLO. YS was supported by the Medical University of Graz, Doctoral College of Metabolic and Cardiovascular Disease and the Austrian Science Fund (Grant DK-MCD W1226-B18).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, B.J., Li, Y., Ong, KL. et al. The Cholesteryl Ester Transfer Protein Inhibitor, des-Fluoro-Anacetrapib, Prevents Vein Bypass-induced Neointimal Hyperplasia in New Zealand White Rabbits. Sci Rep 9, 16183 (2019). https://doi.org/10.1038/s41598-019-52510-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52510-0
This article is cited by
-
The effect of lncRNA MIR155HG-modified MSCs and exosome delivery to synergistically attenuate vein graft intimal hyperplasia
Stem Cell Research & Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.