Abstract
Bullfrog farming and trade practices are well-established, globally distributed, and economically valuable, but pose risks for biodiversity conservation. Besides their negative impacts on native amphibian populations as an invasive species, bullfrogs play a key role in spreading the frog-killing fungus Batrachochytrium dendrobatidis (Bd) in the natural environment. Bullfrogs are tolerant to Bd, meaning that they can carry high infection loads without developing chytridiomycosis. To test the potential of bullfrog farms as reservoirs for diverse and virulent chytrid genotypes, we quantified Bd presence, prevalence and infection loads across approximately 1,500 farmed bullfrogs and in the water that is released from farms into the environment. We also described Bd genotypic diversity within frog farms by isolating Bd from dozens of infected tadpoles. We observed individuals infected with Bd in all sampled farms, with high prevalence (reaching 100%) and high infection loads (average 71,029 zoospore genomic equivalents). Average outflow water volume from farms was high (60,000 L/day), with Bd zoospore concentration reaching approximately 50 million zoospores/L. Because virulent pathogen strains are often selected when growing in tolerant hosts, we experimentally tested whether Bd genotypes isolated from bullfrogs are more virulent in native anuran hosts compared to genotypes isolated from native host species. We genotyped 36 Bd isolates from two genetic lineages and found that Bd genotypes cultured from bullfrogs showed similar virulence in native toads when compared to genotypes isolated from native hosts. Our results indicate that bullfrog farms can harbor high Bd genotypic diversity and virulence and may be contributing to the spread of virulent genotypes in the natural environment. We highlight the urgent need to implement Bd monitoring and mitigation strategies in bullfrog farms to aid in the conservation of native amphibians.
Similar content being viewed by others
Introduction
The international wildlife trade facilitates introductions of invasive species and pathogens that threaten native communities. Bullfrogs are native to eastern North America1,2 and are farmed on a large scale in Asia, Central America, and South America to supply the international frog leg trade2,3. Bullfrog farming emerged as an alternative to overharvesting native amphibian species4. However, this practice continues to contribute to the current global amphibian crisis by facilitating biological invasions5,6,7,8,9,10,11. Escapes of bullfrogs from farms have led to the establishment of invasive bullfrog populations6,12,13,14 that negatively influence the local anurofauna by interfering with acoustic communication and jeopardizing reproduction of native anurans15,16, preying on native amphibian species17,18 and competing for resources, thus reducing the fitness of native amphibian populations19,20.
Bullfrogs also play an important role in the dynamics of amphibian chytridiomycosis, a disease caused by the fungus Batrachochytrium dendrobatidis (Bd) that has been linked to amphibian declines worldwide21. Bullfrogs are highly tolerant hosts22,23, meaning that they are able to withstand high Bd infection loads without developing chytridiomycosis (but see exceptions in24,25). Thus, bullfrogs serve as competent pathogen reservoirs12,22,26 and international pathogen vectors11,27,28. Traded bullfrogs have been found to carry multiple genetic lineages of Bd, including the invasive hypervirulent global pandemic lineage (BdGPL) responsible for the decline of amphibians on several continents11,29,30 as well as genotypes from enzootic lineages that tend to be less virulent due to long-term coevolution with their amphibian hosts, as reported for BdASIA-2/BdBRAZIL31,32, BdCAPE, BdCH29 and BdASIA-111.
The combination of bullfrog-Bd interactions and bullfrog farming practices may create ideal conditions for outbreaks of chytridiomycosis and declines of native fauna. High Bd tolerance in bullfrogs suggests that farms could function as reservoirs of particularly virulent Bd genotypes. Novel Bd genotypes introduced through trade may also have higher virulence in native hosts compared to endemic genotypes with which they have co-evolved. Furthermore, translocation of divergent Bd lineages may bring previously isolated Bd genotypes into contact, allowing for the emergence of sexually hybridized strains and possibly giving rise to more virulent hybrids than the parental lineages28,32. In addition, the water used in bullfrog farming is often released into the natural environment without treatment, potentially carrying viable Bd zoospores that could infect native amphibian species. Bullfrogs are also farmed in high densities, providing ideal conditions for (i) Bd transmission among hosts, (ii) hybridization to occur, and (iii) selection to operate. Thus, bullfrog farms may promote continuous spillover of pathogenic zoospores into native anuran communities and propogation of virulent Bd genotypes, but previous studies have not quantified pathogen outflow from bullfrog farms or compared the virulence of genotypes carried by farmed and native host species under controlled experimental conditions.
In Brazil, ranaculture began in the 1930s and escalated throughout the country in the 1970s33, coinciding with sharp historical amphibian declines throughout the Brazilian Atlantic Forest, which have been recently attributed to the emergence of Bd34. Recently, a Brazilian ordinance proposed that introduced aquatic species, including bullfrogs, should be considered native to foster aquaculture development35, which is expected to exacerbate introductions of bullfrogs into native amphibian communities. To test bullfrog farms in Brazil as a source of virulent Bd genotypes for native amphibians, we sampled Bd from farmed bullfrogs across life stages (tadpoles, juveniles and adults), as well as from the outflow water from frog farms. We also isolated and genotyped Bd strains found in farmed bullfrogs and performed infection experiments testing for differences in Bd virulence among isolates from native hosts and farmed bullfrogs. We hypothesized that because of the high Bd tolerance in bullfrogs23,26, isolates from bullfrogs would be more pathogenic to a native Brazilian host species than those isolated from native frogs. Combined, our results provide important information about the dynamics of Bd in the amphibian trade that should be used to guide actions directed at conserving native anurans in Brazil and elsewhere.
Results
Bullfrog farm assessment
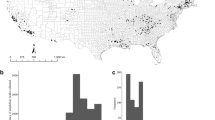
The observed Bd prevalence in farmed tadpoles ranged from 0 (2 farms) to 48%. We detected Bd+ juveniles at all farms, with prevalence reaching 100% and infection loads up to 71,029 zoospore genome equivalents (g.e.; Fig. 1a, Table 1). Bd prevalence varied among amphibian developmental stages (F = 4.226; P = 0.027), with juveniles exhibiting higher Bd prevalence than tadpoles (Tukey P = 0.02), but not adults (Fig. 2a). We detected the same pattern after accounting for a conservative estimate of false positive error (Supplementary Table S1, Table S2). Prevalence of Bd was similar across life stage when accounting for a conservative estimate of false negative error (Supplementary Table S1, Table S2). Juveniles presented higher infection loads than adults (t = 6.55, df = 1, P < 0.001; Fig. 2b).
We consistently recorded high Bd zoospore concentrations in outflow water, with an average concentration of 114 Bd zoospore g.e. per liter. Outflow of water averaged 60,000 L per day, which is estimated to release approximately 50 million zoospore g.e. per day (Fig. 3).
We cultured 36 Bd isolates [7 from farm #4, 7 from farm #8, 8 from farm #9, and 14 from farm #10 (Fig. 1b)] from tadpoles showing clinical signs of chytridiomycosis (dekeratinized jaw sheath and tooth rows). We detected isolates from BdGPL-2 at all four farms and isolates from BdASIA-2/BdBRAZIL at two farms. One tadpole was infected with both lineages.
Laboratory infection experiment
We found a significant effect of Bd isolate on survival of the native toadlet Brachycephalus ephippium (χ2 = 37.269; df = 5; P < 0.0001; Fig. 4a). Isolates associated with the highest and lowest host mortality rates were both cultured from bullfrogs (Fig. 4a). However, Bd isolates cultured from bullfrogs and native frogs led to similar rates of host mortality (χ2 = 0.082; df = 1; P = 0.774).
Patterns in Bd infection loads were consistent with our survival analysis. Average pathogen loads differed among B. ephippium exposed to different Bd isolates (F(6,49) = 68.918; P < 0.0001; Table 2, Fig. 4b, Supplementary Table S3). However, loads were similar between toadlets exposed to isolates cultured from bullfrogs and native frogs (F(1,46) = 0.002; P = 0.965). Individuals that died during the experiment showed similarly high Bd infection loads (>105 g.e.), independent of isolate (F(5,35) = 1.674; P = 0.168; Table 3).
Discussion
Past studies have shown that invasive bullfrog populations harbor high prevalences of Bd and may contribute to the global spread of Bd through the international food trade8,11,12. However, we lack information on how frog farming practices may facilitate the spread of Bd to nearby native host communities and whether bullfrog farms function as a reservoir for highly virulent Bd strains. Our study demonstrates that bullfrog farms constantly release substantial quantities of Bd zoospores into the surrounding natural environment. The high concentration of Bd zoospores in frog pens probably results from movement of water among infected frog pens36,37. Releases of Bd zoospores from bullfrog farms may not only maintain the presence of Bd in the natural environment, but also may introduce the pathogen to new sites.
The high prevalence and infection loads observed in farmed bullfrogs can be explained by the high densities of frogs in these farms, which promotes pathogen transmission by direct contact among individuals38 or by circulating through frog pens36,37. Ideally, the density of animals should not surpass 20 tadpoles per liter of water, or 100 juveniles/50 adults per m2 33,39,40. We observed high bullfrog densities of all frog life stages at sampled farms (Supplementary Fig. S1). Thus, stress caused by high host densities in captivity may lower host immune capacity and increase pathogen loads39.
We found lower Bd prevalence in bullfrog tadpoles than in juveniles and adults. Prevalence of Bd in farmed bullfrog tadpoles approximated or exceeded prevalences found in wild tadpoles in the Brazilian Atlantic Forest and savannah (Cerrado)34, the biomes where our sampled farms were located. Although visual inspection can be used as a proxy for Bd diagnosis34,36,38,41,42, it is possible that mouthpart dekeratinization may not be observed at early stages of infection36; thus, the prevalence we detected in bullfrog farms may be an underestimate. When we applied the error estimation to our raw data, Bd prevalence increased significantly, making our findings even worse. The above method uses different species and environmental conditions, and considers individuals Bd+ with zoospore genomic equivalents (g.e.) ≥0.1, while the present study ≥1 g.e. This may be generating an overestimated false negative value, however, considering the legitimacy of these data, the prevalence in bullfrog tadpoles may be higher than observed, and therefore, therefore we are most concerned about the high prevalence found in the bullfrog farms. Hence, we argue that tadpoles in bullfrog farms may play a key role as reservoirs of the pathogen43, maintaining high concentrations of zoospores in the water and promoting infection or reinfection of individuals.
We showed that juveniles had higher Bd prevalence compared to tadpoles, and higher infection loads compared to adults. Our findings corroborate other studies showing low infection loads in adults43 and juveniles with higher prevalence, infection loads, and mortality rates compared to adults44. During metamorphosis, tadpoles undergo several physiological processes that reshape the immune system, leading to stress that can increase susceptibility to Bd infection45,46. In addition, newly metamorphosed individuals may be more affected by Bd infection, since this is the stage at which keratinization of the skin occurs, providing new substrate and making them an excellent host for keratinophilic pathogens such as Bd47. In the wild, high prevalence and infection loads of juveniles may result in greater spread of Bd, since this stage of development is associated with high dispersion rates48,49.
The presence of Bd at all farms and host life stages suggests that this pathogen has the potential to interfere economically in ranaculture by negatively influencing the commercial production of bullfrogs. Although this species tolerates Bd infection23,26, previous study shows that bullfrog tadpoles displayed multiple cardiac alterations in response to infection50, possibly incurring a high energy cost to developing animals, affecting metamorphosis and potentially reducing growth and post-metamorphic survival. Furthermore, other sublethal effects of Bd have been reported in tadpoles, such as behavioral changes and reduced feeding51. Chytrid infection may also reduce frog immune responses, increasing susceptibility to other infections52. Therefore, controlling chytrid infections in frog farms would potentially increase profits, due to enhanced growth and quality of frogs produced.
In addition to serving as a reservoir of the pathogen, bullfrogs may host high genetic diversity of Bd53. The presence of two Bd lineages within our focal farms, BdGPL and BdASIA-2/BdBRAZIL, even in the same tadpole, creates a high potential for hybridization. Hybrid lineages, such as the one between BdGPL × BdASIA-2/BdBRAZIL in Brazil’s Atlantic forest28,54, may be more virulent than the parental lineages32, in accordance with the hybrid vigor theory55. Hybridization of Bd lineages is apparently rare in the wild11,54, but frog farms may exacerbate the risk of new hybrids32,56.
The evolution of virulence is expected in bullfrog farms due to short generation times of Bd57, high host tolerance23,26, and high host population densities38. However, we found no support for higher virulence of isolates originating from bullfrog farms compared to those originating from wild frogs. Instead, individuals of B. ephippium died when Bd infection load surpassed 100,000 zoospores g.e., independent of isolate or origin (bullfrog or native host)58. In contrast, an infection load threshold of 10,000 zoospores was considered to be fatal for aquatic-breeding species59,60, indicating that these thresholds vary among species with contrasting life histories. The high variation in Bd load that we observed between host individuals could be attributed to multiple factors that can act alone or synergistically. Variation in innate and adaptive host immune responses61,62, gene expression63,64, previous contact with the pathogen65 and host stress levels66 may modulate the intensity of infection. Also, infections from different strains may result in different Bd loads32.
Our study emphasizes the prominent role that bullfrog farms may play in the spread of Bd into native host communities. In addition, it suggests that Bd strains that evolve on bullfrog farms are likely to impact susceptible native species through the release of zoospores into the environment, a form of pathogen spread. Our results indicate that bullfrog farms, which are distributed across the world, provide a potential environment for survival, reproduction and dissemination of Bd inside and outside farms. A possible way to reduce the spread of chytrid fungus through farming would be to treat the water that is released to the environment. Bullfrogs should also be treated for Bd infection using previously reported treatment methods67. Additional studies aimed at treatment and control of Bd in amphibian farming systems would be beneficial to native amphibian conservation efforts.
Materials and Methods
Bullfrog farm assessment
We sampled 10 bullfrog farms in the state of São Paulo, located in Brazilian Atlantic Forest and savannah (Cerrado), southeastern Brazil. Our sampling quantified Bd prevalence and infection loads in farmed bullfrogs. We sampled 35 juveniles from each of eight farms and 35 adults from each of nine farms. For each individual, we swabbed the inguinal region and each limb 5 times, specifically between the digits68. We used disposable gloves to handle each individual frog to avoid cross-contamination. Additionally, we sampled 100 tadpoles from each of nine farms for the presence of Bd (Supplementary Fig. S2a). We selected tadpoles at Gosner’s stage 25 and visually inspected jaws sheaths and tooth rows. We did not use molecular methods of detection for tadpoles because dekeratinization of the oral region is a reliable proxy for Bd infection in tadpoles36,41, including amphibians sampled in Brazil34,69. We considered individuals with jaw sheath dekeratinization to be infected with Bd (Bd+), regardless of the level of dekeratinization of the tooth rows (Supplementary Fig. S2). Although visual inspection for mouthpart dekeratinization is used for Bd diagnosis in tadpoles34,41, some studies show that tadpoles with early stages of infection may not present mouthpart dekeratinization36, and that tadpoles with fully dekeratinized mouthparts no longer have substrate for Bd growth34, therefore leading to false positives and negatives42. Thus, in order to validate our method, we ran qPCR analyses with a subsample of 51 bullfrog tadpoles with dekeratinized mouthparts from one of the focal bullfrog farms. We found a very low proportion of false positives (two individuals = 4%), indicating that visual inspection for mouthpart dekeratinization is a realiable method for Bd detection in bullfrogs from the state of São Paulo (Supplementary Table S4). In addition, and to be even more conservative, we included an estimation of false negatives and false positives in our analyses (see below) based on previous studies. We applied as a possible error the largest estimates observed in a recent study42: 12.3% for false positives and 18.7% for false negatives (Supplementary Table S1).
In addition to detecting and quantifying Bd from individual frogs, we collected water samples and standardized a filtering protocol to detect the pathogen in the aquatic environment. We measured the volume of water released by farms into the surrounding natural environment (L/s). We measured outflow rate as the amount of water released per second. Then we collected 1 liter and filtered 500 ml of this water from each farm (Supplementary Fig. S1d). Using a vacuum pump, we filtered the sampled water with a permeable membrane (0.45 μm pore size) (Supplementary Fig. S3). After filtering, we extracted DNA from membranes and quantified Bd zoospores and zoosporangia using qPCR as described below.
qPCR, Bd isolation and sequencing
We extracted Bd DNA from swab samples of juveniles and adult amphibians using PrepMan ULTRA (Life Technologies) and performed quantitative PCR analyses for Bd detection and quantification68. We considered samples with zoospore genomic equivalents (g.e.) ≥1 to be Bd+ 70. We estimated Bd infection prevalence (for tadpoles, juveniles and adults) as the number of infected individuals divided by the total number of sampled individuals for each farm.
We cultured Bd from bullfrog tadpoles showing mouthpart dekeratinization, following protocols by Vieira & Toledo71 and Fisher et al.72. After isolation, we transferred Bd cultures into Petri dishes containing 1% Tryptone agar and incubated cultures for one week. We then extracted DNA from the culture, following protocols by James et al.73. We genotyped each Bd isolate using a sequence of 6 SNP markers (Supplementary Table S5) as described by Schloegel et al.28 and sequenced them in the Sequencing Core Lab at the University of Michigan.
Laboratory infection experiment
We conducted experimental inoculations in the laboratory to test for effects of different Bd isolates on amphibian hosts. We used Brachycephalus ephippium (Anura: Brachycephalidae) as an experimental host. Brachycephalus ephippium is a direct-developing species endemic to Brazil’s Atlantic Forest74. Direct developers carry low Bd prevalence in the wild and often show low resistance to chytridiomycosis58. Thus, they are an ideal model organism for infection trials. We collected B. ephippium (about 2 cm in snout-vent length) in the municipality of Mogi das Cruzes, state of São Paulo, Brazil. We individually housed each wild-caught individual in plastic bags with leaf-litter to avoid potential cross-contamination among frogs while in the field. We swabbed all individual frogs and only used those that tested negative for Bd in the field. During the experiment, we individually housed each frog in plastic boxes (22 × 15 × 8 cm) containing autoclaved moist Sphagnum moss. We monitored frogs daily and fed them calcium-fortified pinhead crickets. We carried out the experiment in a temperature-controlled room, with temperatures set at 20 °C and a 12 h day-night cycle.
We exposed frogs to three Bd isolates from bullfrogs sampled at farms for this study and three Bd isolates previously isolated and genotyped from native amphibian hosts. Our experimental design consisted of seven treatments (six Bd isolates and a negative control) with eight frogs per treatment (Supplementary Table S6). All isolates were within the BdGPL-2 clade28, which is the dominant form in the Brazilian Atlantic Forest54. We cultured Bd isolates in Petri dishes with tryptone agar at 17 °C for five days. We then harvested Bd zoospores by flooding Petri dishes with distilled water and waiting for approximately one hour for zoospore release from zoosporangia32,75. We then quantified zoospores in a Neubauer hemocytometer and standardized the inoculum concentration (4.6 × 106 zoospores/ml) among isolates.
For inoculations, we placed frogs in individual Petri dishes containing 1 ml of Bd inoculum (treatment) or 1 ml of autoclaved distilled water (control) for 45 minutes. This procedure occurred only once for each animal. We swabbed each individual 16 and 31 days following inoculation, which is sufficient time for multiple Bd generations76. We monitored amphibians daily and swabbed dead or dying individuals. Experimental protocols were approved by the local animal care committee (CEUA UNICAMP #4688-1/2017). We used the same DNA extraction and qPCR protocols described above to detect and quantify Bd68.
Statistical analyses
Bullfrog farm assessment
We used a General Linear Model (GLM) with a binomial distribution (logit link) to test for differences in Bd prevalence among host developmental stages (tadpole, juvenile and adult); we performed a Tukey HSD a posteriori test for multiple comparisons. In addition, we repeated this analysis taking into account the estimated rates of false negatives and false positives associated with the visual inspection method. We also ran a GLM with normal distribution (identity link) to test for differences in Bd infection loads among host developmental stage (juvenile and adult). We log-transformed (log10) infection load data to obtain GLMs with normally distributed residuals.
Laboratory infection experiment
We built survival curves (Parametric Survival analyses) to test for effects of isolation source (native frogs vs. bullfrogs) on the survival of individual hosts. We performed a similar analysis to test for effects of isolate on host survival. In addition, we used a Generalized Linear Mixed Model (GLMM) to test whether Bd infection loads in B. epphipium exposed to bullfrog Bd isolates were higher than those exposed to isolates from native frogs, including the six Bd isolates as a random effect. For these analyses, we included samples collected halfway through the experiment (day 16) and included swabs of individuals that died before day 16; we excluded the control group from this analysis. We also performed a Tukey HSD a posteriori test for pairwise multiple comparisons among means. We also used a simple Analysis of Variance (ANOVA) to test for differences in average infection loads at the point of mortality among individuals exposed to different isolates. Finally, we used Proportional Hazards analysis, including the interaction between infection load and isolates, to test whether survival depended on these factors. We also excluded the control group from this analysis.
Ethics statement
All experiments were performed in accordance with university guidelines and regulations for animal care and husbandry. Our collecting permit was provided by ICMBio (SISBio #54656-3; 27745-13; 17242-3). Experimental protocols were approved by Universidade Estadual de Campinas (UNICAMP) and the local animal care committee [Comissão de Ética no Uso de Animal – CEUA (#4688-1/2017)]. This research was accessioned at SISGen platform (SISGEN #A1E9E10).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Barrasso, D. A., Cajade, R., Nenda, S. J., Baloriani, G. & Herrera, R. Introduction of the American bullfrog Lithobates catesbeianus (Anura: Ranidae) in natural and modified environments: an increasing conservation problem in Argentina. S. Am. J. Herpetol. 4, 69–75 (2009).
Frost, D. R. Amphibian Species of the World: an Online Reference. Version 6.0. American Museum of Natural History, New York, USA. Available from http://research.amnh.org/herpetology/amphibia/index.html (accessed March 2018) (2018).
FAO (Food and Agriculture Organization) 2005–2018. Cultured Aquatic Species Information Programme - Rana catesbeiana. Text by Flores Nava, A. In FAO Fisheries and Aquaculture Department, Rome, http://www.fao.org/fishery/culturedspecies/Rana_catesbeiana/en (2018).
Carpenter, A. I. et al. Over-harvesting. Pages 26–31 in Gascon, C. et al. editors. Amphibian conservation action plan. IUCN/SSC Amphibian Specialist Group, Gland, Switzerland and Cambridge, UK (2007).
Kats, L. B. & Ferrer, R. P. Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers. Distrib. 9, 99–110 (2003).
Fisher, M. C. & Garner, T. W. The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biol. Rev. 21, 2–9 (2007).
Laufer, G., Canavero, A., Núñez, D. & Maneyro, R. Bullfrog (Lithobates catesbeianus) invasion in Uruguay. Biol. Invasions 10, 1183–1189 (2008).
Schloegel, L. M. et al. Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biol. Conserv. 142, 1420–1426 (2009).
Carpenter, A. I., Andreone, F., Moore, R. D. & Griffiths, R. A. A review of the international trade in amphibians: the types, levels and dynamics of trade in CITES-listed species. Oryx 48, 565–574 (2014).
GISD (Global Invasive Species Database). Available from, http://193.206.192.138/gisd/search.php (accessed March 2018) (2018).
O’Hanlon, S. J. et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360, 621–627 (2018).
Garner, T. W. et al. The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana. Biol. Letters 2, 455–459 (2006).
Lau, M., van Dijk, P. P. & Syed, G. P. Managing problems of overexploitation and trade in amphibians. In Stuart, S. et al. editors. Threatened Amphibians of the World, Lynx Edicions, Barcelona (2008).
Both, C. et al. Widespread occurrence of the American bullfrog, Lithobates catesbeianus (Shaw, 1802) (Anura: Ranidae), in Brazil. S. Am. J. Herpetol. 6, 127–134 (2011).
Medeiros, C. I., Both, C., Grant, T. & Hartz, S. M. Invasion of the acoustic niche: variable responses by native species to invasive American bullfrog calls. Biol. Invasions 19, 675–690 (2017).
Forti, L. R. et al. Perspectives on invasive amphibians in Brazil. PLoS One 12, e0184703 (2017).
Toledo, L. F., Silva, R. R. & Haddad, C. F. B. Anurans as prey: an exploratory analysis and size relationships between predators and their prey. J. Zool. 271, 170–177 (2007).
Leivas, P. T., Savaris, M., Lampert, S. & Lucas, E. M. Predation of Odontophrynus americanus (Anura: Odontophrynidae) by the invasive species Lithobates catesbeianus (Anura: Ranidae) in an Araucaria Forest remnant in Southern Brazil. Herpetol. Notes 6, 603–606 (2013).
Kiesecker, J. M., Blaustein, A. R. & Miller, C. L. Potential mechanisms underlying the displacement of native red‐legged frogs by introduced bullfrogs. Ecology 82, 1964–1970 (2001).
Boone, M. D., Little, E. E. & Semlitsch, R. D. Overwintered bullfrog tadpoles negatively affect salamanders and anurans in native amphibian communities. Copeia 2004, 683–690 (2004).
Scheele, B. C. et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463 (2019).
Hanselmann, R. et al. Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biol. Conserv. 120, 115–119 (2004).
Eskew, E. A., Worth, S. J., Foley, J. E. & Todd, B. D. American bullfrogs (Lithobates catesbeianus) resist infection by multiple isolates of Batrachochytrium dendrobatidis, including one implicated in wild mass mortality. EcoHealth 12, 513–518 (2015).
Mazzoni, R. et al. Emerging pathogen in wild amphibians and frogs (Rana catesbeiana) farmed for international trade. Emerg. Infect. Dis. 9, 995–998 (2003).
Gervasi, S. S. et al. Experimental evidence for American Bullfrog (Lithobates catesbeianus) susceptibility to chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth 10, 166–171 (2013).
Daszak, P. et al. Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetol. J. 14, 201–207 (2004).
Kriger, K. M. & Hero, J. Chytridiomycosis, Amphibian Extinctions, and Lessons for the Prevention of Future Panzootics. EcoHealth 6, 6–10 (2009).
Schloegel, L. M. et al. Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol. Ecol. 21, 5162–5177 (2012).
Farrer, R. A. et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. P. Natl. Acad. Sci. USA 108, 18732–18736 (2011).
Rosenblum, E. B. et al. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. P. Natl. Acad. Sci. USA 110, 9385–9390 (2013).
Becker, C. G. et al. Variation in phenotype and virulence among enzootic and panzootic amphibian chytrid lineages. Fungal Ecol. 26, 45–50 (2017).
Greenspan, S. E. et al. Hybrids of amphibian chytrid show high virulence in native hosts. Sci. Rep. 8, 9600, https://doi.org/10.1038/s41598-018-27828-w (2018).
Ferreira, C. M., Pimenta, A. G. C. & Paiva-Neto, J. S. Introdução à ranicultura. Bol. Inst. Pesca 33, 1–15 (2002).
Carvalho, T., Becker, C. G. & Toledo, L. F. Historical amphibian declines and extinctions in Brazil linked to chytridiomycosis. P. R. Soc. B. 284, 20162254, https://doi.org/10.1098/rspb.2016.2254 (2017).
Brito, M. F. G. et al. Brazil naturalizes non-native species. Science 361, 139–139 (2018).
Rachowicz, L. J. & Vredenburg, V. T. Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Dis. Aquat. Organ. 61, 75–83 (2004).
Berger, L., Hyatt, A. D., Speare, R. & Longcore, J. E. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 68, 51–63 (2005).
Rachowicz, L. J. & Briggs, C. J. Quantifying the disease transmission function: effects of density on Batrachochytrium dendrobatidis transmission in the mountain yellow‐legged frog Rana muscosa. J. Anim. Ecol. 76, 711–721 (2007).
Cribb, A. Y., Afonso, A. M. & Mostério, C. M. F. Manual Técnico de Ranicultura. Embrapa Agroindústria de Alimentos, Brasília, 13–71 (2013).
Seixas Filho, J. T., Pereira, M. M. & Mello, S. C. R. P. Manual de Ranicultura para o Ranicultor. H. P. Comunicação, 155 (2017).
Knapp, R. A. & Morgan, J. A. Tadpole mouthpart depigmentation as an accurate indicator of chytridiomycosis, an emerging disease of amphibians. Copeia 2006, 188–197 (2006).
Navarro-Lozano, A., Sánchez-Domene, D., Rossa-Feres, D. C., Bosch, J. & Sawaya, R. J. Are oral deformities in tadpoles accurate indicators of anuran chytridiomycosis? PloS One 13, e0190955 (2018).
Briggs, C. J., Knapp, R. A. & Vredenburg, V. T. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. P. Natl Acad Sci USA B 107, 9695–9700 (2010).
Langhammer, P. F., Burrowes, P. A., Lips, K. R., Bryant, A. B. & Collins, J. P. Susceptibility to the amphibian chytrid fungus varies with ontogeny in the direct-developing frog, Eleutherodactylus coqui. J. Wildlife Dis. 50, 438–446 (2014).
Rollins‐Smith, L. A. Metamorphosis and the amphibian immune system. Immunol. Rev. 166, 221–230 (1998).
Fernández-Loras, A., Fernández-Beaskoetxea, S., Arriero, E., Fisher, M. C. & Bosch, J. Early exposure to Batrachochytrium dendrobatidis causes profound immunosuppression in amphibians. Eur. J. Wildlife Res. 63, 99, https://doi.org/10.1007/s10344-017-1161-y (2017).
Berger, L. et al. Cytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. P. Natl. Acad. Sci USA 95, 9031–9036 (1998).
Hamilton, W. D. & May, R. M. Dispersal in stable habitats. Nature 269, 578 (1977).
Semlitsch, R. D. Differentiating migration and dispersal processes for pond‐breeding amphibians. J. Wildlife Manage. 72, 260–267 (2008).
Salla, R. F. et al. Cardiac adaptations of bullfrog tadpoles in response to chytrid infection. J. Exp. Zool. 323A, 487–496 (2015).
DeMarchi, J. A., Gaston, J. R., Spadaro, A. N., Porterfield, C. A. & Venesky, M. D. Tadpole food consumption decreases with increasing Batrachochytrium dendrobatidis infection intensity. J. Herpetol. 49, 395–398 (2015).
Miller, D. L. et al. Concurrent infection with ranavirus, Batrachochytrium dendrobatidis, and Aeromonas in a captive anuran colony. J. Zoo Wildlife Med. 39, 445–449 (2008).
Goka, K. et al. Amphibian chytridiomycosis in Japan: distribution, haplotypes and possible route of entry into Japan. Mol. Ecol. 18, 4757–4774 (2009).
Jenkinson, T. S. et al. Amphibian-killing chytrid in Brazil comprises both locally endemic and globally expanding populations. Mol. Ecol. 25, 2978–2996 (2016).
Whaley, W. G. Heterosis. Bot. Rev. 10, 461–498 (1944).
Ghosh, P. & Fisher, M. C. Dr Jekyll and Mrs Hyde: Risky hybrid sex by amphibian-parasitizing chytrids in the Brazilian Atlantic Forests. Mol. Ecol. 25, 2961–2963 (2016).
Hamilton, W. D. Sex versus non-sex versus parasite. Oikos 35, 282–290 (1980).
Mesquita, A. F. et al. Low resistance to chytridiomycosis in direct-developing amphibians. Sci. Rep. 7, 16605 (2017).
Vredenburg, V. T., Knapp, R. A., Tunstall, T. S. & Briggs, C. J. Dynamics of an emerging disease drive large-scale amphibian population extinctions. P. Natl. Acad. Sci. USA 107, 9689–9694 (2010).
Kinney, V. C., Heemeyer, J. L., Pessier, A. P. & Lannoo, M. J. Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: Affirmation of Vredenburg’s “10,000 Zoospore Rule”. Plos One 6, e16708, https://doi.org/10.1371/journal.pone.0016708 (2011).
Ramsey, J. P., Reinert, L. K., Harper, L. K., Woodhams, D. C. & Rollins-Smith, L. A. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 78, 3981–3992 (2010).
Voyles, J., Rosenblum, E. B. & Berger, L. Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: a review of pathogenesis and immunity. Microbes Infect. 13, 25–32 (2011).
Savage, A. E. & Zamudio, K. R. MHC genotypes associate with resistance to a frog-killing fungus. P. Natl. Acad. Sci. USA 108, 16705–16710 (2011).
Bataille, A. et al. Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proc. Biol. Sci. 282, 20143127 (2015).
McMahon, T. A. et al. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511, 224 (2014).
Gabor, C. R., Fisher, M. C. & Bosch, J. A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels. PloS One 8, e56054 (2013).
Moreno, L. F., Morão, P. & Toledo, L. F. Tratamento de anfíbios infectados pelo fungo quitrídio do gênero Batrachochytrium. Herpetol. Bras. 4, 30–34 (2015).
Lambertini, C., Rodriguez, D., Brito, F. B., Leite, D. S. & Toledo, L. F. Diagnóstico do fungo Quitrídio: Batrachochytrium dendrobatidis. Herpetol. Bras. 2, 12–17 (2013).
Vieira, C. A., Toledo, L. F., Longcore, J. E. & Longcore, J. R. Body length of Hylodes cf. ornatus and Lithobates catesbeianus tadpoles, depigmentation of mouthparts, and presence of Batrachochytrium dendrobatidis are related. Braz. J. Biol. 73, 195–199 (2013).
Kriger, K. M., Hiner, H. B., Hyatt, A. D., Boyle, D. G. & Hero, J. M. Techniques for detecting chytridiomycosis in wild frogs: comparing histology with real-time Taqman PCR. Dis. Aquat. Organ. 71, 141–148 (2006).
Vieira, C. A. & Toledo, L. F. Isolamento, cultivo e armazenamento do fungo quitrídio: Batrachochytrium dendrobatidis. Herpetol. Bras. 1, 18–19 (2012).
Fisher, M. C. et al. Development and worldwide use of non-lethal, and minimal population-level impact, protocols for the isolation of amphibian chytrid fungi. Sci. Rep. 8, 7772 (2018).
James, T. Y., Stenlid, J., Olson, A. & Johannesson, H. Evolutionary significance of imbalanced nuclear ratios within heterokaryons of the basidiomycete fungus Heterobasidion parviporum. Evolution 62, 2279–2296 (2008).
Haddad, C. F. et al. Guia dos anfíbios da Mata Atlântica: diversidade e biologia. Anolis Books, 542 (2013).
Jenkinson, T. S. et al. Globally invasive genotypes of the amphibian chytrid outcompete an enzootic lineage in coinfections. P. R. Soc. B 285, 20181894, https://doi.org/10.1098/rspb.2018.1894 (2018).
Longcore, J. E., Pessier, A. P. & Nichols, D. K. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227 (1999).
Acknowledgements
We thank all bullfrog farm owners for allowing access to specimens and water used in experiments. Thanks to JRG for field assistance and helping me with the statistical analyses, CL for field assistance and figure editing, DMC for contributions in for English grammar and spelling, and CMFM for enabling and assisting the sampling in one of the bullfrog farms. Financial support was provided by grants from the São Paulo State Research Foundation (FAPESP #2016/03344-0; #2016/25358-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) and National Council for Scientific and Technological Development (CNPq #300896/2016-6) and US National Science Foundation (DEB-1601259; DBI-1711032). This study was completed in partial fulfilment of the degree of Master of Science by Luisa de Pontes Ribeiro at Universidade Estadual de Campinas (UNICAMP) whose thesis is entitled “Chytrid fungus in bullfrog farms of the state of São Paulo and its implications for native anurans conservation”.
Author information
Authors and Affiliations
Contributions
L.F.T., C.G.B. and L.P.R. conceived the idea of the study. L.P.R. and T.C. collected field data, cultured Bd, prepared inocula and conducted the experiment. L.P.R. conducted DNA extractions and qPCRs. D.S.L. contributed with molecular analysis and infrastructure. T.Y.J. and T.S.J. genotyped Bd isolates. C.G.B., L.F.T. and L.P.R. performed statistical analyses. L.P.R. wrote the manuscript with important contributions from the other authors. S.E.G. made great contributions in the writing of the manuscript and reviewed carefully for English grammar and spelling. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ribeiro, L.P., Carvalho, T., Becker, C.G. et al. Bullfrog farms release virulent zoospores of the frog-killing fungus into the natural environment. Sci Rep 9, 13422 (2019). https://doi.org/10.1038/s41598-019-49674-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49674-0
This article is cited by
-
Contribution of host species and pathogen clade to snake fungal disease hotspots in Europe
Communications Biology (2024)
-
Coinfection with chytrid genotypes drives divergent infection dynamics reflecting regional distribution patterns
Communications Biology (2023)
-
Chytrid in the clouds: an alternative passive transport of a lethal pathogen for amphibians
Hydrobiologia (2023)
-
Genetic structure of American bullfrog populations in Brazil
Scientific Reports (2022)
-
Habitat Disturbance Linked with Host Microbiome Dispersion and Bd Dynamics in Temperate Amphibians
Microbial Ecology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.