Abstract
The African montane bamboo Yushania alpina provides both habitat and food for many species in the Albertine Rift region. In Volcanoes National Park (VNP), Rwanda, it is especially important as a key food resource for the Endangered mountain gorilla Gorilla beringei beringei and Endangered golden guenon Cercopithecus mitis kandti. We examined temporal and spatial variation in bamboo shoots regeneration and consumption by primates, monitored between 2013 and 2018 in 82 16-m2 plots located along transects in VNP. Our analyses revealed a decline in vegetative regeneration of bamboo in recent years, which is mirrored by a decline in bamboo shoot consumption by primates; but an increase in proportional intake. Local declines in regeneration are potentially due to high intensities of herbivory, decreased amounts of rainfall during growing seasons, and natural processes that form part of the life cycle of bamboo. Moreover, spatial variation in bamboo regeneration can be explained by elevation, as well as by stand-level variation in soil acidity, vegetation density, and the density of dead bamboo culms. We discuss the potential mechanisms underlying observed temporal and spatial variations and outline possible effects of a decline in bamboo regeneration for primates and other aspects of biodiversity in VNP.
Similar content being viewed by others
Introduction
Bamboo forests occur in most tropical regions and serve as habitat for a large variety of species. In addition, bamboo shoots, and leaves and stems of mature bamboo, are sought after by both herbivores and people1. However, there are uncertainties regarding the future of bamboo forests, due to the direct and indirect impacts of anthropogenic activities, such as harvesting2 and climate change3,4, on bamboo regeneration and bamboo stand longevity.
The African montane bamboo Yushania alpina (K. Schum.) W.C. Lin. (1974), previously known as Arundinaria alpina and Sinarundinaria alpina and hereafter also referred to as ‘bamboo’, is widely distributed across the mountains of Central and East Africa. It is commonly found on volcanic soils at high elevations, predominantly between 2,600–3,000 m, where it often dominates the vegetation, resulting in monodominant ‘bamboo zones’5. The vegetative and sexual regeneration of Y. alpina typically involves cyclic processes6,7,8. Shoots tend to emerge from rhizomes every few years, especially in periods coinciding or directly succeeding long rains, and flowering cycles occur at intervals ranging from 15 years to 40 years2,9. The entire growth cycle of Y. alpina might last even longer7,8,9, as rhizomes might persist for decades before shoots start to form9. Y. alpina is monocarpic, and death of entire sections of bamboo forest will usually follow synchronized flowering and seed-forming events. It is unclear how bamboo forests regenerate after such events, or to what extent gap-creating disturbances such as fire play a role in these processes10,11, but bamboo might regenerate from seeds and from surviving rhizomes of old bamboo stands12. Outside of flowering events, the death of individual culms provides conditions that promote sprouting, likely similar to compensatory regrowth after herbivory13. In forests with little or no human disturbance, the death of culms and their replacement by new shoots occurs at roughly equal rates9.
Bamboo zones in the Albertine Rift region of East Africa provide habitat to species such as Archer’s robin-chat Cossypha archeri and Rwenzori apalis Apalis ruwenzorii, two birds particularly associated with the open understory found in bamboo forests14. In addition, bamboo is a food resource for various African vertebrates, especially the Endangered mountain gorilla Gorilla beringei beringei and the Endangered golden guenon Cercopithecus mitis kandti15,16,17, also known as golden monkey. Bamboo can make up a considerable portion of the diet of these primates in the Virunga massif (central Albertine Rift region)—up to 15% and 60% of the annual feeding time of mountain gorillas and golden guenons is spend consuming bamboo, respectively15,17,18,19,20—and other large herbivores such as buffalo, elephant and antelopes feed opportunistically on bamboo shoots when available21.
Volcanoes National Park (VNP) in Rwanda constitutes part of the Virunga massif, a mountain range in the Albertine Rift region. Extensive bamboo zones are located near the boundary of the park, at elevations of approximately 2,500–3,000 m a.s.l.21. No previous studies of bamboo ecology exist for VNP, but we can gain some insights from studies conducted in the neighbouring Mgahinga Gorilla National Park (MGNP) in Uganda. Here, flowering events were observed in patches of a few hectares in the 1980s22, though current bamboo shoot regeneration in the region seems to result mainly from vegetative reproduction from rhizomes. Most bamboo stands in MGNP seemed to be in a building phase (see Agnew8 for an explanation of classifications of stages in the life cycle of bamboo) around eight years ago (~in 2010), as the diameter of young culms surpassed that of old culms2. Canopies tend to become increasingly dense and closed during this building phase, which usually promotes a shift in understory vegetation from pioneer species to shade-tolerant plants, and eventually leads to changes in abiotic factors such as a decline in soil pH8.
Interestingly, Sheil et al.2 mention that a suspected decline in bamboo regeneration was the main incentive to conduct their study of bamboo ecology in MGNP. In recent years, park staff of VNP as well as researchers affiliated with The Dian Fossey Gorilla Fund’s Karisoke Research Center hinted at similar trends in VNP. Such declines, if confirmed, could lead to limitations in food availability for species, such as gorillas and golden guenons. In addition, it could affect various aspects of the ecology of these primates, including reproductive behaviour, and could have consequences for a range of other biota (e.g., birds) associated with bamboo habitat.
A lack of bamboo shoot regeneration may be driven by many factors. It could indicate that bamboo stands in the region underwent natural succession towards a mature phase, during which there is no increase in culm height or diameter8. It has been suggested that there is a decrease in shoot development near the end of this mature phase, before the onset of flowering (ref.23 cited in ref.2). In addition, lack of bamboo sprouting could result from high levels of herbivory24. Current knowledge of gorilla25 and golden guenon17 feeding ecology suggests that both species are unlikely to cause systematic mortality of their main food plants, but localized resource depression has been observed15 and patterns of resource use could change under increased population densities of both primates. Alternatively, herbivory, as well as trampling by other large herbivores, such as elephant and buffalo, could have an opposite effect and result in increases in shoot development through compensatory regrowth25,26,27—thus indicating that bamboo regeneration could actually be limited by a lack of large herbivores. Additionally, there are indications that bamboo competes for resources with other plants species, especially lianas28. These plants, in turn, are also consumed by large herbivores, thus indirectly affecting bamboo growth. Finally, bamboo regeneration could be affected by human-induced disturbances and stress, especially harvesting and climate change, or potentially even by indirect alterations such as changes in gap-creating wildfire regimes11. We know that human use can have both positive and negative effects on bamboo regeneration2,29, but the effects of climate change on Y. alpina have not been studied at all. However, we know that VNP experiences changes in both temperature and rainfall30, important determinants of the distribution of Y. alpina27,31,32, and thus we do predict effects of climate change on Y. alpina regeneration, similar to the declines in regeneration following climate change seen in other bamboo species3,4.
With this study, we aim to improve our understanding of bamboo ecology in VNP in order to inform conservation and management of bamboo-associated species. In VNP, bamboo shoots emerge during two distinct seasons (hereafter the early and late growing seasons), following the onset of rainy seasons. Although long-term changes in availability of key gorilla food plants was the focus of previous studies in VNP, no analyses of temporal trends in bamboo shoot availability are currently available20. Here, we address whether there were any trends in bamboo shoot regeneration in selected plots in VNP (Fig. 1) over a 6-year period, from 2013 to 2018. In addition, we aimed to determine whether trends in bamboo shoot regeneration were linked to bamboo consumption by primates and explored which bioclimatic and environmental factors influence both temporal and spatial variation in bamboo shoot regeneration.
Results
Temporal, spatial, and climatic effects on bamboo regeneration and consumption
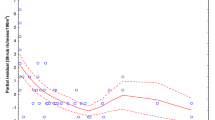
Between 2013 and 2018, we found a significant (p < 0.001), 83-percent decline in the number of newly regenerated shoots, a trend that was especially pronounced for the late growing season (GAMM; Fig. 2; Table 1). The largest portion of this decline occurred before 2015, with only a minor decline in bamboo shoot regeneration in recent years (2016–2018). This decrease in bamboo shoot regeneration was reflected by a significant decline in the absolute number shoots consumed by primates over the years (p < 0.001; from 9.26 on average in a plot in 2013 to 2.33 in 2018), and a significant increase in proportional consumption (p < 0.001; from an average of 17% of shoots consumed in 2013 to 38% consumed in 2018).
We found a significant difference between regeneration in the two growing seasons (GAMM; p < 0.001; Fig. 2B, Tables 1 and 2), with overall levels of regeneration being lower in the early as compared to the late growing season. There was also a significant increase in shoot regeneration with elevation (p < 0.001), though this was predominantly caused by relatively high shoot regeneration at high elevations in the early growing season and was not observed for the late growing season (evidenced by a significant interaction effect between season and elevation; p < 0.001). Overall consumption was significantly higher in the late as compared to the early growing season (p < 0.001), and again higher at high elevations (p < 0.001), at least in the early growing season (interaction effect season x elevation; p < 0.001). Despite differences in overall consumption, we found no significant difference between the seasons in the proportional consumption of shoots (p = 0.760). Specifically, we found that both bamboo shoot regeneration and consumption in the early growing season was higher at high elevations than at low elevations, whereas the reverse was true for the late growing season. Finally, we found a significant increase in the growth rate of bamboo shoots over the years (p < 0.001) and a negative effect of elevation on growth rate (p < 0.001), but no differences in growth rates between the growing seasons (p = 0.877).
The decline we found in bamboo shoot regeneration mirrored the significant (GAMM; p < 0.001; Table 3) decline in the amount of rainfall during bamboo growing seasons over the 2013–2018 period, from an average of ~182 mm (late 2013 / early 2014) to ~121 mm (late 2017/early 2018), with no significant effect of season (p = 0.115). In contrast, we found that minimum/maximum temperatures increased from an average of 11.8/23.4 °C (calculated over late 2013 and early 2014) to 13.1/23.7 °C (late 2017 and early 2018), though these increases in temperatures were not significant (p > 0.05 for both minimum and maximum temperatures).
Relationships between bamboo shoot regeneration and consumption
There was a positive relationship between the number of bamboo shoots that emerged in a given growing season and the number of bamboo shoots consumed that same season (GLM; b = 0.022, SE = 0.001, t-value = 23.968, p < 0.001; Fig. 3A). However, primates did not seem to increase their intake linearly in proportion to availability. If anything, there seemed to be proportionally less consumption in plots with particularly high (100 shoots or more) numbers of young bamboo shoots (GLM; b = −0.005, SE = 0.002, t-value = −2.150, p = 0.032; Fig. 3B), and hardly ever were more than 50 shoots consumed in any one season in any one plot. Finally, we found a negative relationship between bamboo shoot consumption by primates in one given growing season and bamboo shoot regeneration in the next growing season (GAMM; b = −2.266, SE = 0.198, t-value = −11.426, p < 0.001; Fig. 4).
Relationships between number of bamboo shoots available in a plot and consumption by primates. Panel (A) shows the number of shoots consumed by primates versus bamboo shoot availability, with the blue line and grey band representing a quadratic relationship and ±95% CI. Panel (B) shows primates never consumed more than half of shoots consumed in plots with particularly high numbers of shoots (>100).
The relationship between primate herbivory and bamboo shoot regeneration. Depicted is the relationship between the number of bamboo shoots consumed in a survey plot in a given season (i) and the difference in the number of bamboo shoots (as compared to the current season) that emerge a season later (N(i + 1)-N(i)). Blue line and grey band represent a linear relationship and ±95% CI.
Environmental correlates of bamboo shoot regeneration
We found that spatial patterns in bamboo shoot regeneration between 2013 and 2018 were potentially (co-)determined by at least three environmental variables (GLM; p < 0.05; Table 4). Specifically, bamboo shoot regeneration was negatively related to soil pH (p = 0.016) but increased with higher vertical understory densities (p < 0.001) and numbers of dead bamboo culms (p = 0.006) found in a plot.
Discussion
Our aim was to test for temporal trends in bamboo shoot availability in Volcanoes National Park (VNP), Rwanda over recent years. We show that, between 2013 and 2018, there was a decline in the number of bamboo shoots that emerged through vegetative regeneration. Although most of this decline occurred before 2015, current rates of bamboo shoot regeneration are at such low levels that even small future declines could eventually lead to a complete lack of bamboo shoot regeneration in our study plots. Moreover, we provide evidence that this decline in bamboo shoot availability is mirrored by a decline in the consumption of shoots by primates, and by an increase in proportional consumption. If proportional consumption increases further, then competition for bamboo shoots as a food resource might rise accordingly.
Trends in climatic conditions (e.g., VNP has seen a decline in rainfall and increase in temperatures, this paper), increases in primate population sizes which may have led to an increased intensity of herbivory, and natural succession of bamboo stands are all factors that possibly contribute to observed declines in bamboo shoot regeneration. Though it is difficult, for now, to separate causative from correlative relationships, we briefly discuss these potential causes of declines in shoot regeneration. Regarding climatic conditions, we note that Y. alpina generally grows in regions where annual rainfall is between 800 and 2,000 mm and mean annual temperature ranges from 10 to 20 °C27,31,33. Although the current climate in VNP approaches such climatic conditions (minimum and maximum annual temperature 12–24 °C at lower elevations of the park and ca. 1,000 mm rain annually), we did find that rainfall during the growing seasons has declined in recent years, whereas temperatures have increased. Following these trends in climatic conditions, we could tentatively argue that abiotic conditions become increasingly less favourable at lower elevations, and more favourable at higher elevation, which could in turn explain the observed higher rates of regeneration at higher elevations. We propose that locally obtained microclimatic data (i.e., as measured within plots and across elevations) is incorporated in future explorations of the relationships between trends in climatic conditions and bamboo shoot regeneration. Climatic data with such ‘high spatial resolution’ will also allow us to explore possible effects of changes in rainfall seasonality and possible elevational range shifts for bamboo and associated biota34.
Effects of increased herbivory may also have contributed to observed temporal patterns of declines in bamboo shoot regeneration. For one, we found evidence of an increase in proportional consumption of bamboo shoots over recent years, as well as a relationship between the availability of bamboo shoots in a given growing season and consumption by herbivores, though possibly with an upper limit to the amount of bamboo shoots consumed at one particular location at a given time. This relationship confirms the importance of bamboo shoots as a food resource among primates in VNP but also leads to questions regarding possible effects of increases in competition for bamboo shoots, for example as a result of growing gorilla populations35. In this context, we also point to signals that high levels of herbivory may add to local declines in bamboo shoot regeneration in a subsequent season—which could reflect a form of resource depression also observed by Watts15—a topic for additional experimental studies.
Finally, we consider the role of natural processes in the bamboo life cycle and succession of bamboo stands. Interestingly, the growth rate of bamboo shoots increased over recent years. This is possibly due to altered rates of photosynthesis linked to climate change36, but may also indicate that bamboo stands have reached a ‘building phase’ in which we see an increase in the diameters and heights of subsequent generations of regenerated bamboo culms8. Nevertheless, given the fact that the complete reproductive cycle of Y. alpina (from seed germination and seedling recruitment after a mass flowering event to death of a bamboo stand) takes anywhere from 15 to 40 years to complete, we deem it likely that at least some of the bamboo stands in VNP have reached the mature phase8, when there is no further increase in bamboo size, with a lack of regeneration at some locations as a result.
With regards to spatial variation in bamboo shoot regeneration, we found relatively high rates of shoot regeneration in dense bamboo stands with large numbers of dead bamboo culms. High rates of shoot regeneration may result from altered understory conditions (e.g., sunlight reaching the forest floor10) or from compensatory regrowth after the death of bamboo culms and the availability of high densities of rhizomes from which new shoots might emerge. In addition, our finding that shoots regenerate more frequently at locations with a relatively low soil pH may, with caution, be linked to increased overall bamboo productivity on such soils37. Alternatively, the pH of soils in close vicinity to bamboo stands is known to decline as bamboo stands mature8, and relatively high rates of bamboo regeneration on more acidic soils can thus also potentially be linked differences in the age of bamboo in different stands—which may itself influence stand-level characteristics such as rhizome densities. More experimental approaches (e.g., changing soil characteristics within certain plots) may clarify the links between environmental correlates and spatial patterns in bamboo regeneration, as well as explore the possible effects of disturbances, such as soil trampling, bamboo harvesting, and fire11,25,26,27,29,38.
Regardless of the cause of the observed decline of bamboo regeneration, we may briefly address possible consequences of a potential lack of shoot availability for some of the animals that use it as a food or habitat resource. With regards to the mountain gorillas of VNP, we deem it probable that mountain gorillas can show some dietary flexibility20 if bamboo shoots become scarce, especially as it is clear that bamboo does not form a substantial part of the diet of all gorillas in VNP15. On the other hand, although there is no current direct evidence of food competition39, a decrease in bamboo shoot regeneration might become a problem if the population of mountain gorillas in VNP continues to increase. Besides, even if gorillas are not physically limited by a lack of bamboo shoot availability, there might be indirect consequences that are mediated through changes in behaviour. For example, the activity budget and energy expenditure of individual gorillas can change considerably as a result of the seasonal increase in bamboo shoot consumption40, and a lack of bamboo shoot availability can have unknown consequences for various gorilla behaviours, such as play and processes of weaning.
A lack of bamboo shoot availability might also not directly affect the nutrient uptake by individual golden guenons, as a large portion of their diet is made up of parts of mature bamboo (especially leaves) in addition to the occasional intake of other food items (fruits, flowers, leaves of other plants, insects)17,41. However, a lack of bamboo shoot regeneration is likely to impact golden guenon behaviour and population dynamics. For example, it may induce shifts in home-ranges or changes in reproductive patterns, especially as current birthing seasons seem linked to bamboo growing seasons17. Finally, we note that additional research will be needed to determine whether, and to what extent, a lack of bamboo availability leads to an increase in crop-raiding by primates.
Diminished vegetative regeneration, or even death of certain bamboo stands, does not have to be a worrisome development with only negative consequences, as it may be part of a natural succession cycle. For example, as bamboo mass-flowering and die-off events may increase the survivorship and growth of saplings of overstory tree species42, we could hypothesize that mass die-off of bamboo in VNP may have positive effects on the regeneration of the keystone tree Hagenia abyssinica43. Moreover, it is unlikely that bamboo will die-off instantaneously across VNP and both of the main consumers of bamboo (mountain gorillas and golden guenons) are likely to show some dietary flexibility. Moreover, although we found significant declines in bamboo regeneration in our study plots, it should be noted that 82 survey plots represent only a small portion of all the bamboo forest found in the Virunga Massif. Bamboo stands across the Virunga Massif, including VNP, show spatial variation in age, culm density and a host of other variables, and we consider it highly unlikely that bamboo regeneration will come to a simultaneous halt across the entire region. Nevertheless, we deem it important that conservation and research efforts focus on continuous monitoring of bamboo regeneration, in order to detect further changes. Specifically, there is a need for continuous monitoring of the responses of consumers of bamboo shoots to local decline/absences of this resource. Such research is urgent, as both mountain gorillas and golden guenons are endangered and occur in small populations in a relatively small and isolated geographic region, which could limit these primates’ opportunities to adapt to changes in food availability.
Methods
Study area and data collection
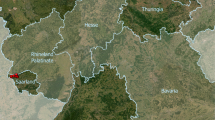
We studied vegetative regeneration of Yushania alpina in Volcanoes National Park (VNP) in north-western Rwanda, located between 1°21′–1°35′S and 29°22′–29°44′E (Fig. 1). Between September 2013 and June 2018, we collected data in 82 plots placed along 21 transects that were set up to cover an elevational range from 2,610 to 3,040 m a.s.l. Transects were placed approximately 200 m apart, to reduce spatial autocorrelation among our data, and varied in length (400–1,600 m) according to the width (as measured along the slope of the mountains) of the bamboo zone. Along each transect, we placed 4 m × 4 m plots at 200 m intervals. During the study, we visited each plot biweekly during the course of two distinct bamboo shooting seasons (the short (March–May) and long rainy season (September–December), hereafter called the early and late growing seasons, respectively). In each plot, we counted all bamboo shoots, marked them with a unique code, measured their heights, and recorded whether they were fed on by primates (gorillas and/or golden guenons). We concluded there was consumption by primates when we identified particular bite marks on bamboo shoots in combination with other signs (e.g., evidence of shoot pealing or foot prints). To ensure accurate identification of consumption, we drew from our collective—as an organization—extensive history studying primate feeding behaviour in the field18.
The Rwanda Meteorology Agency provided climatic data, specifically monthly rainfall (mm), and maximum and minimum temperatures for the years 2013–2018, from a weather station in the town of Musanze (1°29′S, 29°37′E, 1878 m a.s.l.) approximately 8–10 km from the nearest study plot and 732 metres lower than the lowest of our 82 study plots. During one site visit, in 2017, we measured various additional environmental variables in each plot. First, we looked at topographic factors known to influence the growth of bamboo, at least that of other species44,45. We calculated slope steepness (in percent) at each plot using a person-to-person method and a clinometer. Steepness of slope is known to influence the growth and regeneration of other species of bamboo, as it is a proxy for both soil depth (i.e., levelled slopes will generally have deeper soil profiles than steep slopes) and reception of solar radiation44. Finally, we calculated the mean soil pH in each plot, by averaging measurements (using an Oakton pH 150 meter) of soil samples taken at two random locations within each plot.
We also measured vegetation characteristics in an extension (14 m × 14 m) of each plot. First, we used a fisheye hemispherical lens to photograph the canopy at each corner of an expanded plot, analysed these circular images with Gap Light Analyzer v. 2.046, and calculated the mean canopy cover at each plot. Canopy cover is known to influence light conditions in bamboo forests, which subsequently influences bamboo shoot regeneration, at least in other bamboo species45. We calculated canopy height, another factor influencing light conditions, at each plot, by averaging two measurements taken with a clinometer at two sides of each plot. Light conditions at ground level might moreover be influenced by the density and structure of strata below the canopy47. We therefore estimated vertical vegetation structure of the understory shrub layer, which—being predominantly made up of bamboo—indirectly provided us with a proxy for bamboo culm density. For this, we photographed the understory shrub layer against a background consisting of a black sheet (1 m × 1 m) arranged perpendicularly to the ground. We used a normal 65 mm lens on a camera positioned approximately 3 m from the background, and took photographs at two corners of a plot, looking into the centre of the plot. From these photographs, we calculated vertical vegetation density using Sidelook v. 1.148. Next, we estimated ground cover in two 50 cm × 50 cm quadrats placed 2 m from the centre point of each plot. We visually estimated the percentage of ground covered by vegetation in each of these quadrants. Finally, as vegetative regrowth of bamboo might be triggered by the death of bamboo culms, we counted the number of dead bamboo culms found in each plot.
Data analyses
Our data analyses consisted of: (1) time-series analyses to assess trends in bamboo shoot regeneration, bamboo consumption by primates, and bamboo shoot growth rates; (2) regression modelling of the trends in climatic factors over the study period; (3) analyses of the relationship between primate herbivory and bamboo shoot regeneration; and 4) tests to assess potential relationships between environmental factors and patterns in bamboo shoot regeneration. We conducted all analyses using packages “lme4”49, “lmtest”50, “nlme”51, “tseries”52, “gam”53, “MASS”54, “mgcv”55 and base packages in R v. 3.5.056.
To test for temporal trends in bamboo shoot regeneration and consumption, we first assessed temporal autocorrelation in our data. Initial data exploration using autocorrelation and partial autocorrelation function (ACF and PACF) plots revealed significant temporal autocorrelation between subsequent seasons. In addition, local regression analyses (LOESS) (Fig. 2) indicated that trends in bamboo shoot regeneration and bamboo shoot consumption over time were likely non-linear and that there were substantial differences between trends for the early and late growing seasons. Given these observations, we opted to fit generalized additive mixed models (GAMMs) to our data with an AR1 temporal autocorrelation term, which allow for effective modelling of nonlinear trends unlike alternative models such as seasonal autoregressive integrated moving average models (SARIMAs) (for similar uses see, e.g.57,58,59). Residuals of our AR1 models showed no significant autocorrelation.
We first fitted a full GAMM with a negative binomial error distribution, to handle overdispersion, to the number of newly developed shoots. As predictor variables we included year, elevation, and growing season, with a smoothing spline to capture possible non-linear effects of the variable year58. We also included an interaction term between elevation and growing season, given that field observations suggest differences in the regeneration rates at low and high elevations in the two seasons, and a corAR1 autocorrelation function to account for temporal correlations of measurements made within plots.
Next, we created GAMMs with the number of shoots consumed (negative binomial distribution to account for overdispersion), the proportion of shoots consumed (quasibinomial error distribution for proportions), and growth rates of the shoots (loglinear Gaussian error distribution, given positive skewness) as response variables. We again included year (smoother), elevation, and growing season as predictor variables and a temporal autocorrelation term (corAR1). We dropped non-significant terms from these full models before interpreting results. As our models indicated that trends in bamboo shoot regeneration differed between growing seasons and across elevations (see Results), we also provide summary statistics for both growing seasons and models for low and high elevations separately. For this, we split our data into a low and a high-elevation subset using the median elevation of our plots (2879 m a.s.l.) as cut-off.
To understand how climatic drivers might have changed over the years, we also looked at temporal trends in the average amount of rainfall (in mm) and maximum and minimum temperatures. We again used GAMMs (Gaussian error distributions after checks for normal distributions) with year and season as predictor variables, a smoothing spline for variable year, and a term to address temporal autocorrelation (corAR1). We decided not to explore statistical relationships between these climatic data and shoot regeneration and consumption variables as we did not have climatic data for the different plots or across elevations, but data from one single source at a relatively low elevation. In addition, we did not want to overqualify a possible similarity in temporal trends in climatic conditions and bamboo shoot regeneration, given we were unable to distinguish possible causative from correlative relationships.
We used a generalized linear model (GLM), with a negative binomial error distribution to address overdispersion, to test whether there was a relationship between the number of bamboo shoots that came up in a given growing season and the total number of bamboo shoots that showed signs of feeding by primates in the same time period. We repeated this exercise (with a quasibinomial error distribution for proportions) for the relationship between the bamboo shoot regeneration and the proportion of bamboo shoots consumed. Our next step was to test whether shoot consumption in a plot in one growing season had an effect on regeneration in the subsequent season. For this, we created a GAMM (Gaussian error distribution, normal distribution) with the number of shoots consumed as predictor variable, the change in bamboo shoot regeneration in the next season (i.e., the number of shoots, Ni+1, produced in growing season i + 1 minus the number of shoots produced in the previous season, Ni) as dependent variable, a corAR1 term to address temporal autocorrelation, and season as random effect.
Finally, we addressed possible links between a select number of environmental factors and spatial variation in bamboo shoot regeneration. For this, we created a generalized linear mixed model (GLMM; negative binomial error distribution) with the number of shoots that regenerated between 2013 and 2018 as the response variable. As predictor variables we included degree of slope (rescaled), soil pH (rescaled), vertical understory density (rescaled zero-mean and unit-variance), percentage of ground cover consisting of live vegetation (rescaled), canopy height (rescaled), and canopy cover (rescaled) of each plot. To address the effects of having plots nested within transects, and thus potentially less variation between plots within transects than between transects, we added transect as a random effect. We used variance inflation factors (VIFs) to test for collinearity among predictor variables that could potentially bias model outcomes and found no problematic correlations (VIFs were <2 for all variables, below the threshold at which collinearity becomes problematic60). From this full model we dropped non-significant terms before interpreting results.
Data Availability
Original raw data on bamboo shoot regeneration and consumption are available in Supplementary Dataset 1.
References
Liese, W. & Kohl, M. Bamboo. The plant and its uses. Switzerland (2015).
Sheil, D. et al. Bamboo for people, mountain gorillas, and golden monkeys: Evaluating harvest and conservation trade-offs and synergies in the Virunga Volcanoes. Forest ecology and management 267, 163–171 (2012).
Tuanmu, M.-N. et al. Climate-change impacts on understorey bamboo species and giant pandas in China’s Qinling Mountains. Nature Climate Change 3, 249 (2013).
Li, R. et al. Climate change-induced decline in bamboo habitats and species diversity: implications for giant panda conservation. Diversity and Distributions 21, 379–391 (2015).
Hedberg, O. Vegetation belts of East African mountains. Svensk Botanisk Tidakrift 45, 140–195 (1951).
Bussmann, R. W. The forests of Mount Kenya (Kenya): vegetation, ecology, destruction and management of a tropical mountain forest ecosystem. PhD. Thesis, Universität Bayreuth (1994).
Wimbush, S. The African alpine bamboo. Empire Forestry Journal, 33–39 (1945).
Agnew, A. D. Cyclic sequences of vegetation in the plant communities of the Aberdares Mountains, Kenya. (East Africa Natural History Soc., 1985).
Bussmann, R. W. Regeneration and succession patterns in African, Andean and Pacific tropical mountain forests: the role of natural and anthropogenic disturbance. Lyonia 6, 93–111 (2004).
Tabarelli, M. & Mantovani, W. Gap-phase regeneration in a tropical montane forest: the effects of gap structure and bamboo species. Plant Ecology 148, 149–155 (2000).
Keeley, J. E. & Bond, W. J. Mast flowering and semelparity in bamboos: the bamboo fire cycle hypothesis. The American Naturalist 154, 383–391 (1999).
Wimbush, S. The African alpine bamboo. The East African Agricultural Journal 13, 56–60 (1947).
McNaughton, S. Compensatory plant growth as a response to herbivory. Oikos, 329–336 (1983).
Shaw, P. & Shewry, M. Population density and habitat associations of restricted-range bird species at Ruhija, Bwindi Impenetrable Forest, Uganda. Bird Conservation International 11, 161–174 (2001).
Watts, D. P. Composition and variability of mountain gorilla diets in the central Virungas. American Journal of Primatology 7, 323–356 (1984).
McNeilage, A. Diet and habitat use of two mountain gorilla groups in contrasting habitats in the Virunga in Mountain gorillas: three decades of research at Karisoke (eds Robbins, M. M. et al.) 265–292 (Cambridge University Press, 2001).
Twinomugisha, D. & Chapman, C. A. Golden monkey ranging in relation to spatial and temporal variation in food availability. African Journal of Ecology 46, 585–593 (2008).
Fossey, D. Feeding ecology of free-ranging mountain gorillas (Gorilla gorilla beringei) in Primate ecology: Studies of feeding and ranging behavior in lemurs, monkeys and apes (ed. Clutton-Brock, T. H.) 415–447 (Academic Press, 1977).
Vedder, A. L. Movement patterns of a group of free-ranging mountain gorillas (Gorilla gorilla beringei) and their relation to food availability. American Journal of Primatology 7, 73–88 (1984).
Grueter, C. C. et al. Long-term temporal and spatial dynamics of food availability for endangered mountain gorillas in Volcanoes National Park, Rwanda. American Journal of Primatology 75, 267–280 (2013).
Plumptre, A. J. Plant-herbivore dynamics in the Birungas (University of Bristol, 1991).
Bitariho, R. & McNeilage, A. Population structure of montane bamboo and causes of its decline in Echuya Central Forest Reserve, South West Uganda. African Journal of Ecology 46, 325–332 (2008).
Huberman, M. Bamboo silviculture. Unasylva 13, 36–43 (1959).
Hull, V. et al. The impact of giant panda foraging on bamboo dynamics in an isolated environment. Plant Ecology 212, 43–54 (2011).
Watts, D. P. Long-term habitat use by mountain gorillas (Gorilla gorilla beringei). 2. Reuse of foraging areas in relation to resource abundance, quality, and depletion. International Journal of Primatology 19, 681–702 (1998).
Watts, D. Effects of mountain gorilla foraging activities on the productivity of their food plant species. African Journal of Ecology 25, 155–163 (1987).
Hemp, A. Vegetation of Kilimanjaro: hidden endemics and missing bamboo. African Journal of Ecology 44, 305–328 (2006).
Nsanzurwimo, A. Etude analytique des communautes de bambous a Sinarundinaria alpina en rapport avec leur milieu ecologique (cas de du Parc National des Volcans) Bachelor thesis, Unversite Nationale du Rwanda (2004).
Kleinhenz, V. & Midmore, D. J. Aspects of bamboo agronomy. Advances in Agronomy 74, 99–145 (2001).
Campos, F. A. et al. Does climate variability influence the demography of wild primates? Evidence from long-term life-history data in seven species. Global change biology 23, 4907–4921 (2017).
Grimshaw, J. M. The afromontane bamboo, Yushania alpina, on Kilimanjaro. Journal of East African Natural History 88, 79–83 (1999).
Lind, E. M., Morrison, M. E. & Hamilton, A. East African vegetation. (Longman Group Limited., 1974).
Winiger, M. Bodentemperaturen und niederschlag als indikatoren einer klimatischökologischen gliederung tropischer gebirgsräume. Methodische aspekte und anwendbarkeit, diskutiert am beispiel des Mount Kenya (Ostafrika)(Soil temperatures and precipation as indicators of a climatic-ecologic zonation of tropical high mountains). Geomethodica 4, 121–150 (1979).
Ayebare, S., Plumptre, A., Kujirakwinja, D. & Segan, D. Conservation of the endemic species of the Albertine Rift under future climate change. Biological Conservation 220, 67–75 (2018).
Gray, M. et al. Genetic census reveals increased but uneven growth of a critically endangered mountain gorilla population. Biological Conservation 158, 230–238 (2013).
Li, Y. et al. Effects of elevated CO2 and temperature on photosynthesis and leaf traits of an understory dwarf bamboo in subalpine forest zone, China. Physiologia Plantarum 148, 261–272 (2013).
Mulatu, Y. & Fetene, M. Stand structure, growth and biomass of Arundinaria alpina (highland bamboo) along topographic gradient in the Choke Mountain, northwestern Ethiopia. Ethiopian Journal of Biological Sciences 12, 1–23 (2013).
Vázquez-López, J. M. et al. Effects of harvesting on the structure of a neotropical woody bamboo (Otatea: Guaduinae) populations. Interciencia 29, 207–211 (2004).
Grueter, C. C. et al. Elevated activity in adult mountain gorillas is related to consumption of bamboo shoots. Journal of Mammalogy 97, 1663–1670 (2016).
Twinomugisha, D., Chapman, C. A., Lawes, M. J., Worman, C. O. D. & Danish, L. M. How does the golden monkey of the Virungas cope in a fruit scarce environment? in Primates of Western Uganda (Newton-Fisher, N. et al.) 45–60 (Springer, 2006).
Kayiranga, A. et al. Analysis of climate and topography impacts on the spatial distribution of vegetation in the Virunga volcanoes massif of east-Central. Africa. Geosciences 7, 17 (2017).
Marchesini, V. A., Sala, O. E. & Austin, A. T. Ecological consequences of a massive flowering event of bamboo (Chusquea culeou) in a temperate forest of Patagonia, Argentina. Journal of Vegetation Science 20, 424–432 (2009).
Kayitete, L., van der Hoek, Y., Nyirambangutse, B. & Derhé, M. A. Observations on regeneration of the keystone plant species Hagenia abyssinica in Volcanoes National Park, Rwanda. African Journal of Ecology 57, 274–278 (2019).
Wei, F., Feng, Z., Wang, Z. & Liu, J. Association between environmental factors and growth of bamboo species Bashania spanostachya, the food of giant and red pandas. Acta Ecologica Sinica 19, 710–714 (1999).
Wang, Y. J., Tao, J. P. & Zhong, Z. C. Factors influencing the distribution and growth of dwarf bamboo, Fargesia nitida, in a subalpine forest in Wolong Nature Reserve, southwest China. Ecological Research 24, 1013–1021 (2009).
Fraser, G., Canham, C. & Lertzman, K. Gap light analyser (GLA): imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. (Simon Fraser University and the Institute of Ecosystem Studies, 1999).
Montgomery, R. A. Effects of understory foliage on patterns of light attenuation near the forest floor. Biotropica 36, 33–39 (2004).
Nobis, M. SideLook 1.1- Imaging software for the analysis of vegetation structure with true-colour photographs, http://www.appleco.ch/ (2005).
Bates, D., Maechler, M., Bolker, B. & Walker, S. lme4: linear mixed-effects models using Eigen and S4, https://cran.r-project.org/web/packages/lme4 (2018).
Hothorn, T. et al. lmtest: testing linear regression models, https://cran.r-project.org/web/packages/lmtest (2018).
Pinheiro, J., Bates, D., DebRoy, S. & Sarkar, D. nlme: linear and nonlinear mixed effects models, https://cran.r-project.org/web/packages/nmle (2018).
Trapletti, A., Hornik, K. & LeBaron, B. tseries: time series analysis and computational finance, https://cran.r-project.org/web/packages/tseries (2018).
Hastie, T. gam: generalized additive models, https://cran.r-project.org/web/packages/gam (2018).
Ripley, B. et al. MASS: Support Functions and Datasets for Venables and Ripley’s MASS, https://cran.r-project.org/web/packages/MASS (2018).
Wood, S. mgcv: mixed GAM computation vehicle with automatic smoothness estimation, https://cran.r-project.org/web/packages/mcgv (2018).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/ (2018).
MacKenzie, B. R. & Schiedek, D. Daily ocean monitoring since the 1860s shows record warming of northern European seas. Global change biology 13, 1335–1347 (2007).
Yee, T. W. & Mitchell, N. D. Generalized additive models in plant ecology. Journal of vegetation science 2, 587–602 (1991).
Cheung, W. W., Watson, R. & Pauly, D. Signature of ocean warming in global fisheries catch. Nature 497, 365 (2013).
Hair, J. F., Ringle, C. M. & Sarstedt, M. PLS-SEM: Indeed a silver bullet. Journal of Marketing theory and Practice 19, 139–152 (2011).
Acknowledgements
We thank the Rwanda Development Board (RDB) for granting us access to conduct fieldwork in in Volcanoes National Park. We are very grateful to Biodiversity Program field team of The Dian Fossey Gorilla Fund’s Karisoke Research Center for their hard work in the long-term bamboo regeneration monitoring program. We thank an anonymous reviewer for constructive comments.
Author information
Authors and Affiliations
Contributions
Y.H. performed data analyses, interpreted analyses, and helped draft the manuscript; F.E. collected data, and helped draft the manuscript; I.K. collected data and helped draft the manuscript; W.E. provided assistance with data formatting, analysis, and interpretation as well as drafting the manuscript; MAD contributed to the design of the study, data analysis and interpretation, and helped draft the manuscript; T.S. interpreted data analyses, and helped drafted the final manuscript; D.C. conceived of parts of the study, contributed to the design of the study, data interpretation and manuscript writing; D.T. conceived of parts of the study, designed the study, collected the data, and helped draft the manuscript. All authors provided comments and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Hoek, Y., Emmanuel, F., Eckardt, W. et al. Recent decline in vegetative regeneration of bamboo (Yushania alpina), a key food plant for primates in Volcanoes National Park, Rwanda. Sci Rep 9, 13041 (2019). https://doi.org/10.1038/s41598-019-49519-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49519-w
This article is cited by
-
Human-Wildlife Conflict in Golden Monkeys (Cercopithecus mitis kandti) of the Volcanoes National Park, Rwanda
International Journal of Primatology (2023)
-
On the effects of spatial resolution on effective distance measurement in digital landscapes
Ecological Processes (2021)
-
Daily defecation outputs of mountain gorillas (Gorilla beringei beringei) in the Volcanoes National Park, Rwanda
Primates (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.