Abstract
The grass goldfish appeared early in the evolutionary history of goldfish, and shows heritable stability in the development of the caudal fin. The twin-tail phenotype is extremely rare, however, some twin-tail individuals were produced in the process of breeding for ornamental value. From mutations in the twin-tail goldfish genome, we identified two kinds of Tgf2 transposons. One type was completely sequenced Tgf2 and the other type was ΔTgf2, which had 858 bp missing. We speculate that the bifurcation of the axial skeletal system in goldfish may be caused by an endogenous ΔTgf2 insertion mutation in Chordin A, as ΔTgf2 has no transposition activity and blocks the expression of Chordin A. The twin-tail showed doubled caudal fin and accumulation of red blood cells in the tail. In addition, in situ hybridization revealed that ventral embryonic tissue markers (eve1, sizzled, and bmp4) were more widely and strongly expressed in the twin-tail than in the wild-type embryos during the gastrula stage, and bmp4 showed bifurcated expression patterns in the posterior region of the twin-tail embryos. These results provide new insights into the artificial breeding of genetically stable twin-tail grass goldfish families.
Similar content being viewed by others
Introduction
Goldfish (Carassius auratus) are one of the three famous ornamental fish, and are known as the “water fairy.” Goldfish evolved from wild crucian carp and mutated into different species (grass goldfish, wen goldfish, dragon-eye goldfish, and oval goldfish). Each species has unique characteristics, but the most characteristic phenotype is the bifurcated caudal fin1. The caudal fin of aquatic animals is mainly divided into two types (wild-type and twin-tail) and goldfish are representative species of the twin-tail. The goldfish tail is supported by the caudal axial skeleton, but the twin-tail has bifurcated fin folds that differ from the wild-type. There are many factors that caused bifurcation of the goldfish caudal fin, such as growth environment, genetic factors, and epigenetic effects. Previous reports showed that the development of the goldfish caudal fin is closely related with regulatory factors in the ooplasm2,3. In addition, a single nucleotide mutation at the 127th amino acid base in ChordinA restricted its transcription regulation, which generated the twin-tail phenotype4.
Tgf2, the second transposon in vertebrates, belongs to the hAT family and is also found in goldfish. Tgf2 plays an important role in transgenesis and gene trapping with independent transposition activity5,6. Previous research on transgenesis was carried out by coinjecting the vector plasmid and transposase mRNA into zebrafish, grass carp, and goldfish. The results showed that the eGFP reporter gene was transferred from the vector plasmid into grass carp and goldfish genomes, with an incorporation efficiency of 96% and 37%, respectively. In addition, the Tgf2 transposon in goldfish has three other copies that belong to the goldfish Tgf2-Ds group (ΔTgf2) and have no independent transposition capability5,7,8.
In this study, we obtained the twin-tail phenotype induced by the endogenous ΔTgf2 transposon insertional mutation and conducted pedigree breeding to obtain a genetically stable twin-tail goldfish strain. We also conducted functional assays and analyzed the expression patterns in ventral embryonic tissue markers.
Results
The discrimination of wild-type and twin-tail goldfish
In the 10 families (Tgf2 family-1–4 and ΔTgf2 family-1–6), all the goldfish from the Tgf2 family-1 and -2 were wild-type, more than 95% of goldfish from family-3 and -4 were wild-type, and only a few individuals were twin-tail (Fig. 1a). Similarly, all the goldfish from ΔTgf2 family-1 and -3 were wild-type, more than 92% of goldfish from family-4 and -5 were wild-type, and only a few were twin-tail (Fig. 1d). In contrast, 89% and 100% goldfish from ΔTgf2 family-2 and -6 were twin-tailed, respectively. As such, we inferred that endogenous ΔTgf2 may participate in the generation of, but not necessarily induce, the twin-tail phenotype.
Test of the insertion site of the ΔTgf2 transposon in grass goldfish. Different families with or without the Tgf2 transposon were established to analyze the frequency of wild-type and twin-tail grass goldfish, respectively. (a,d) Observed Tgf2 and ΔTgf2 transposon insertion copies (b,e), and the position of ChordinA insertion (c,f).
To examine how the endogenous ΔTgf2 contributed to the twin-tail phenotype, we amplified and sequenced cDNA from wild-type and twin-tail goldfish. In the twin-tail goldfish, ΔTgf2 was found inserted into 401 nt in the 2nd exon of ChordinA. We named the gene as ChordinAΔTgf2 and described the homozygous genotype as ChordinAΔTgf2/ΔTgf2 (Fig. 1f). Conversely, in the wild-type goldfish, no insert was found in ChordinA and we named the gene ChordinA+, and described the homozygous genotype as ChordinA+/+ (Fig. 1c). A previous report showed that ChordinA plays an important role in the differentiation of the caudal fin in goldfish4, and we speculate that the insertion of endogenous ΔTgf2 leads to mutagenesis in ChordinA, which results in bifurcation of the caudal fin.
Breeding of twin-tail goldfish strains based on the ΔTgf2 transposon
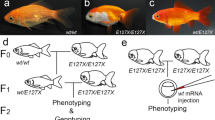
When ChordinA+/+ individuals were crossed with ChordinAΔTgf2/ΔTgf2, the F1 group (~375 individuals) were vent group(ChordinA+/ΔTgf2), and the F2 group (~846 individuals) was separated into three types: wild-type (wt) larvae from wild-type (chordinA+/+) embryos (~589 individuals), single caudal fin (vent) larvae from weakly-ventralized (chordinA+/ΔTgf2) or bifurcated caudal fin fold (chordinAΔTgf2/ΔTgf2) embryos (~21 individuals), and bifurcated caudal fin (twin) larvae from bifurcated fin fold (chordinAΔTgf2/ΔTgf2) embryos (~236 individuals) (Fig. 2a, Table 1). At 2 dpf, the wt phenotype showed the single tail phenotype. The vent phenotype with the incomplete forked fin folds was characterized by a single tail and the twin phenotype with completely divided fin folds showed twin-tail phenotype. However, the vent and twin individuals have a common characteristic that they have the accumulation of red blood cells in the tail (Fig. 2b). The F3 group, which included 317 twin individuals (93%) and 23 vent individuals (7%), suggested that the vent phenotype may be atavism due to the external environment and/or other genetic factors9,10.
Family breeding design and embryonic observations of the twin-tail grass goldfish. The breeding strain of the twin-tail grass goldfish was obtained using hybridization and selfing. (a) Triangles indicate the wild-type phenotype. Square indicates the twin-tail phenotype. Gray, filled triangles indicate the variation in wild-type. In the F2 generation, three phenotypes were observed. (b) Arrows point to the red blood cell aggregation and bifurcated caudal fin.
Comparison between wild-type and twin-tail goldfish
We compared embryonic and adult fish to distinguish between wild-type and twin-tail goldfish. We observed several different characteristics between the two strains of goldfish. Morphologically, wild-type goldfish share characteristics with wild-type crucian carp (Carassius auratus) and have a traditional dovetail-shape tail lobe (Fig. 3a). Meanwhile, in the evolutionary history of the grass goldfish, a twin-tail phenotype appeared with bifurcated fin folds11. Alizarin red staining of axial skeletons is a practical method to analyze the anatomical structure of goldfish tails12,13,14. Caudal axial skeletal elements were identified according to previous reports15. The results showed that the tail nerve bone of both wild-type and twin-tail goldfish were completely mineralized and formed the caudal fin. The caudal fin were attached to the Ep (epural bone), Hy (hypural bone), and Ph (paranasal bone of the hypural bone), however, the quantification data of Nc (notochord), Ph, and Hy1~6 in the wild-type and twin-tail goldfish showed no obvious difference (Table 2). The number of caudal fin rays in wild-type goldfish was 20 to 24, arranged in a double-leaf type shape (Fig. 3b,c), while that of twin-tail goldfish was 39 to 47, arranged in a four-leaf type shape (Fig. 3f,g). Histologically, the characteristics of the axial skeleton demonstrated that the origin of the twin-tail phenotype was attributable to double germination of Hy and Ph (Fig. 3h), which were developed non-redundantly in the wild-type goldfish (Fig. 3d). Embryological results showed that accumulation of red blood cells in bifurcated fin folds only occurred in twin-tail goldfish, and not in wild-type goldfish (Figs 2b and 4). This was consistent with previous reports4.
Expression patterns of Tgf2 and ΔTgf2 transposon
In order to detect the effect of Tgf2 and ΔTgf2 on grass goldfish, we performed in situ hybridization of Tgf2 in wide-type (single-tail) and ΔTgf2 embryos (twin-tail). The result showed that the expression patterns of Tgf2 and ΔTgf2 were different. Tgf2 transposase mRNA was detected in tail cells in the wide-type embryos (Fig. 5b). However, Tgf2 transposase mRNA was not detected in ΔTgf2 embryos (Fig. 5c).
Dorsal-Ventral (DV) axis development of twin-tail goldfish embryos
The previous research showed that dino regulating dorsal development in the zebrafish, the tail is enlarged in dino mutant embryos. The zebrafish mutant ogon (also called short tail) displays ventralized phenotypes similar to the dino mutant. In these mutants, the ventral embryonic tissue markers (eve1, sizzled) are expressed in ventrolateral marginal cells showed different expression patterns. Krox20 is a marker gene, mainly expressed in the hindbrain, which is usually used as a positive control for other ventral marker genes in the DV axis. Bmps also function in skeletal development and the dorsal-ventral axis of vertebrate embryos is thought to be specified by a gradient of bone morphogenetic protein (bmp) activity16,17,18. To determine how the insertional mutation in ChordinA that was mediated by endogenous ΔTgf2 contributed to DV patterning in twin-tail goldfish embryos, we examined the expression patterns of ChordinA, ventral embryonic tissue markers (eve1, sizzled, and bmp4), and a hindbrain marker (krox20) in the embryos of wild-type and twin-tail goldfish (F3). The results showed that the expression patterns were different between wild-type and twin-tail embryos. During the gastrula stage (80% epiboly), ChordinA was expressed in the outward germ layer and showed wider expression patterns in wild-type (Fig. 6a–c) than in twin-tail embryos (Fig. 6a’–c’). Similarly, during the segmentation stage, ChordinA was mainly expressed in the tailbud and showed obviously wider expression patterns in wild-type (Fig. 6d,e) than in twin-tail embryos (Fig. 6d’,e’). A few reports have suggested that ChordinA plays an important role in the development of the DV axis and can control the caudal fin phenotype of goldfish19,20,21,22. On the contrary, during the gastrula stage, eve1 and sizzled showed stronger signal and wider expression patterns in twin-tail (Fig. 6f’–h’) than in wild-type embryos (Fig. 6f–h) in the posterior-ventral regions. Bmp4 is one of the important determining factors for ventral embryonic development23,24. During the late gastrula stage (40% epiboly), the expression of bmp4 (Fig. 6i’) was stronger in twin-tail than in wild-type embryos (Fig. 6i). In addition, bmp4 expression patterns in wild-type and twin-tail embryos showed obvious differences during the segmentation stage. As shown in Fig. 6k’,l’, bmp4 showed bifurcated expression patterns in the posterior region of twin-tail embryos, which were not observed in the wild-type embryos (Fig. 6k,l). This indicated that the axial skeleton became bifurcated and doubled during the segmentation stage in the twin-tail goldfish. The ventral embryonic tissue marker expression patterns were consistent with a previous study of dino zebrafish mutants and other chordin-deficient embryos16,17,25,26,27,28,29. The results showed that krox20 expression patterns were not different between wild-type and twin-tail embryos4, which differed from previous reports of significantly reduced in krox20 expression patterns in chordin-deficient vertebrate embryos17,25,27,29,30. This indicated that the formation of the DV axis played an important regulatory role in the differentiation of tail-patterns in goldfish.
Discussion
The generation of diversified caudal fins in goldfish is caused by a mutation. Previous studies have found that Chordin mutants generate bifurcated caudal axial skeletons2,3,4,31. This natural mutation has been produced and inherited consistently through artificial selection since the song dynasty of China32.
The Tgf2 transposon has not been observed in wild crucian carp, but occurs in grass goldfish as well as other goldfish strains. In the grass goldfish genome, the complete sequence type (Tgf2) and the multiple missing pieces type (ΔTgf2) have been detected, which indirectly indicated that grass goldfish is the ancestor of many other strains7. The Tgf2 transposon relies on its encoded transposase to mediate translocation. Under the artificial selection, those goldfish whose morphological variation caused by transposon were preserved, this may be because the Tgf2 transposon retained their independent transposition activity through long-term evolution33. Generally, there are large differences between goldfish parents and progeny in terms of color and proportions, which could be due to the insertion mutation of the Tgf2 transposon5,33. However, the ΔTgf2 transposon are unable to produce transposase and has no independent transposition capability. According to Mendel’s law, ΔTgf2 homozygote will be generated when the goldfish with ΔTgf2 was selfed. Therefore, the phenotype caused by the ΔTgf2 insertion mutation might be genetically stable5,7,8. A limited number of individuals with the mutation might have survived. Therefore, we speculated that such a large number of twin-tail goldfish from only 10 families were not directly produced by the insertion mutation, but from several generations of hybridization of insertion mutation individuals. In previous studies, in situ hybridization of Tgf2 showed that it has different expression patterns in different embryos and was detected in the head and tail6. In our study, Tgf2 can be detected in the tail. Our result is consistent with previous reports of Tgf2 expression patterns34. This may be caused by the Tgf2 has independent transposition capability. The insertion site in the goldfish genome affects the expression of Tgf2 transposase. However, no transposase mRNA was detected in ΔTgf2 embryos confirming the ΔTgf2 with large deletion lost its independent transposition capability. In this study, ΔTgf2 transposon was found inserted in the 2nd exon of ChordinA in the twin-tail goldfish. And it is precisely because ΔTgf2 has no independent transposition capability, insertional mutagenesis in ChordinA inhibited the expression of the 2nd to 4th exons of ChordinA. Previous studies showed that a single nucleotide mutation in the 127th amino acid of ChordinA (on the 2nd exon, prior to the ΔTgf2 insertion site) generated a stop codon and produced the twin-tail phenotype, we speculate that the bifurcation of the axial skeletal system in goldfish may be caused by an endogenous ΔTgf2 insertion mutation in ChordinA. However, the relation between the insertional mutation mediated by the ΔTgf2 transposon and a single nucleotide mutation in ChordinA require further study.
Alizarin red staining showed that the tail nerve bones forming the caudal fin of both wild-type and twin-tail goldfish were completely mineralized. The difference was that the elements of the axial skeleton system of twin-tail goldfish were doubled with bifurcated fin folds. In situ hybridization indicated that the expression patterns of the two goldfish strains were different during the gastrula stage. The expression of ventral embryonic tissue genes increased significantly due to the ΔTgf2 insertional mutation in ChordinA. This indicated that the ventral tissue had proliferated, which generated the double caudal fin35. In addition, ChordinA showed wider expression patterns in wild-type goldfish than in twin-tail goldfish embryos, during both the gastrula and segmentation stages. However, the reduced expression of ChordinA can also lead to the generation of the twin-tail phenotype. Therefore, we speculate that the insertional mutation in ChordinA mediated by ΔTgf2 contributed to the generation of twin-tail by suppressing the inhibitory effect of ChordinA.
Insertional mutagenesis induced by the ΔTgf2 transposon has mainly been detected in goldfish, so we speculate that this insertional mutation has a special regulatory role in the generation of twin-tail goldfish5,7,36. Based on this study, we infer that insertional mutagenesis mediated by the ΔTgf2 transposon was the main cause of twin-tail; however, it was also affected by other genetic and/or environment factors. By exploring the mechanism of ΔTgf2 insertional mutagenesis and the generation of the goldfish caudal fin, these results provide a theoretical basis for artificial breeding of genetically stable twin-tail grass goldfish families.
Materials and Methods
Experimental goldfish and embryos
P0 generation of wild-type and twin-tail goldfish were obtained from the Genetics and Breeding Center of Shanghai Ocean University, Shanghai, China. Embryos were generated by artificial insemination. Fertilized eggs (~150) were placed in petri dishes (10 cm in diameter) and development at room temperature (22 ± 1 °C). Petri dish with well-aerated water to maintain normal dissolved oxygen (DO) levels during embryogenesis and replaced every 4 hours. Embryos used for RNA extraction were stored by immersion in RNA Store (ABigen, Beijing, China) and kept in 4 °C overnight and then −80 °C until used. Embryos collected for in situ hybridization analysis were kept in embryonic medium supplemented with 0.003% (w/v) 2-phenylthiourea to prevent pigmentation, then fixed overnight at 4 °C in 4% paraformaldehyde (PFA) and then −20 °C in methyl alcohol. Juvenile goldfish used for anatomical and histological analyses were sacrificed by immersion in MS-222 (tricaine methanesulfonate, Sigma, St. Louis, MO) for 5 min. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Advisory Committee for Laboratory Animal Research and conducted following the guidelines approved by the Shanghai Ocean University Committee on the Use and Care of Animals.
Family construction and breeding of goldfish
In breeding experiments, we observed that the twin-tail phenotype occurred in the wild-type family. From the mutation in the twin-tail goldfish genome, we identified two kinds of Tgf2 transposon, one that was completely sequenced (Tgf2), and one with multiple pieces missing (ΔTgf2) (Fig. 7). To detect the relationship between the Tgf2 transposon and the differentiation in the caudal fin, we constructed 10 families (Tgf2 family-1–4 and ΔTgf2 family-1–6) for analysis. Families were divided based on the results of Tgf2 sequencing and each family contained 100 fishes. Primer set Tgf2-F1/-R1 (designed base on accession number HM146132.1, Table 3) was used to amplification Tgf2/ΔTgf2 sequence.
To obtain a genetically stable twin-tail goldfish strain, we crossed a wild-type goldfish (ChordinA+/+, ♂) with a mutant twin-tail goldfish (ChordinAΔTgf2/ΔTgf2, ♀) to obtain the F1 hybrid ChordinA+/ΔTgf2. The F1 hybrid individuals were selfed to obtain F2 offspring. Twin-tail strains (ChordinAΔTgf2/ΔTgf2) selected from the F2 were selfed to obtain F3 individuals. The pedigree structure is shown in Fig. 2a. The phenotypes of goldfish strains were based on the embryonic morphology at 2–3 dpf (days post fertilization) following previous studies16,28. All embryos were observed using a Nikon ECLIPSE 80i stereoscopic microscope (Tokyo, Japan). A total of 823 specimens were phenotyped at the embryonic stage.
Anatomical and histological analyses of axial skeletons
For anatomical analysis, we referred to previous methods with appropriate improvements where necessary37. Juvenile goldfish specimens were fixed in 4% PFA solution and subsequently scaled and rinsed 3 times (for 30 min each time) using diethylpyrocarbonate (DEPC)-treated water. To eliminate the residual PFA stationary liquid and degrease, the specimens were rinsed twice (for 20 min each time) using Tris Buffered Saline Tween (TBST). In order to avoid pigmentation, the specimens were immersed in 1% KOH solution (potassium hydroxide with 1% H2O2) and exposed to strong high-lights. After being digested with trypsin, the specimens were rinsed and soaked with 1% KOH, then stained overnight with alizarin red solution (0.1% alizarin red in 95% ethanol). Finally, specimens were successively rinsed with mixed solutions (1% KOH:glycerinum at 3:1, 1:1, and 1:3).
For histological analysis, juvenile goldfish samples were fixed in Bouin’s solution. The specimens were dehydrated using a graded ethanol series, and made transparent using dimethylbenzene, embedded in paraffin, and then sliced into 3-μm sections using a slicer (RM2265, Leica). Finally, sections were stained using hematoxylin and eosin. All anatomical and histological samples were examined using a Nikon ECLIPSE 80i stereoscopic microscope (Tokyo, Japan).
Probe preparation and sequencing
The probes of ΔTgf2, Tgf2, chordinA, eve1, sizzled, bmp4 and krox20 were prepared by using PCR. Total RNA was isolated from goldfish embryos at 44 hpf using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and subsequently treated with DNase (Promega, Madison, WI, USA) to eliminate contaminating genomic DNA. First-strand cDNA was reverse-transcribed from the total RNA using Reverse Transcriptase M-MLV (TaKaRa, Tokyo, Japan) with oligo-dT primers according to the manufacturer’s instructions. Primer sets Tgf2-F1/-R1, Tgf2-F2/-R2 (designed base on accession number HM146132.1), chordinA-F1/-R1 (designed base on accession number AB874473), eve1-F/-R, sizzled-F/-R, bmp4-F/-R and krox20-F/-R (refer to Gembu Abe) (Table 3) were used to amplification probes sequence4. PCR products were gel-purified, ligated into the T/A cloning vector pGEM-T (Promega, Madison, WI, USA) and transformed into Escherichia coli DH5α. Positive clones were examined by PCR and direct sequencing.
Whole-mount in situ hybridization
Fixed goldfish embryos were washed briefly in PBS containing 0.1% Tween-20, then transferred to 100% methanol, and stored at −20 °C for a minimum of 24 h. Whole-mount in situ hybridization using digoxigenin (DIG)-labeled RNA riboprobes was performed as reported previously, with modifications38. Embryos were photographed using a Nikon SMZ1500 fluorescence microscope (Tokyo, Japan).
References
Fang, G. A., Shi, Z. N. & Wang, L. H. Grass goldfish breeding technology and offspring screening. Scientific Fish. Farming. 6, 77–78 (2013).
Cai, N. E. Studies on the formation of single caudal fin in the goldfish, carassius auratus (The effect of polyethyleneglycol and ultraviolet rays on the development of caudal fin). Oceanologia et limnologia sinica. 24, 242–247 (1993).
Cai, N. E. Studies on the formation of single caudal fin in the goldfish, carassius auratus (The effect of the cytoplasm on the development of caudal). Oceanologia et limnologia sinica. 20, 453–459 (1989).
Abe, G. et al. The origin of the bifurcated axial skeletal system in the twin-tail goldfish. Nat Commun. 5, 3360, https://doi.org/10.1038/ncomms4360 (2014).
Zou, S. M., Du, X. D., Yuan, J. & Jiang, X. Y. Cloning of goldfish hAT transposon Tgf2 and its structure. Hereditas. 32, 1263–1268 (2010).
Jiang, X. Y. et al. Goldfish transposase Tgf2 presumably from recent horizontal transfer is active. Faseb J. 26, 2743–2752 (2012).
Du, X. D. Research on goldfish Tgf2 transposon and its amplification in transgenesis. Shanghai Ocean University (2011).
Koga, A. & Hori, H. The Tol2 transposable element of the medaka fish: an active DNA-based element naturally occurring in a vertebrate genome. Genes Genet Syst. 76, 1–8 (2001).
Qin, B. The construction transgenic P0 grass carp of reovious and molecular cloning of Chordin gene in fish and its impact on tail type in goldfish. Shanghai Ocean University (2015).
Xu, K. et al. Development and application of biological technologies in fish genetic breeding. Sci China Life Sci. 58, 187–201 (2015).
Tian, S. X. Ten kinds of goldfish tail. Aquarium 05, 138–147 (2010).
Darias, M. J. et al. Double staining protocol for developing European sea bass (Dicentrarchus labrax) larvae. J Appl Ichthyol. 26, 280–285 (2010).
Darias, M. J. et al. Dietary vitamin D3 affects digestive system ontogenesis and ossification in European sea bass (Dicentrachus labrax, Linnaeus, 1758). Aquaculture 298, 300–307 (2010).
Gregory, C. A., Gunn, W. G., Peister, A. & Prockop, D. J. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 329, 77–84 (2004).
Schultze, H. P. & Arratia, G. The composition of the caudal skeleton of teleosts (Actinopterygil_ Osteichthyes). Zool Jlinn Soc-Lond. 97, 189–231 (1989).
Hammerschmidt, M. et al. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123, 95–102 (1996).
Miller-Bertoglio, V. et al. Maternal and zygotic activity of the zebrafish ogon locus antagonizes BMP signaling. Dev Biol. 214, 72–86 (1999).
Yabe, T. et al. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development 130, 2705–2716 (2003).
Bradford, Y. et al. ZFIN: enhancements and updates to the Zebrafish Model Organism Database. Nucleic Acids Res. 39, D822–829 (2011).
Mabee, P. M. et al. Connecting evolutionary morphology to genomics using ontologies: a case study from Cypriniformes including zebrafish. J Exp Zool B Mol Dev Evol. 308, 655–668 (2007).
Tsai, H. Y., Chang, M., Liu, S. C., Abe, G. & Ota, K. G. Embryonic development of goldfish (Carassius auratus): a model for the study of evolutionary change in developmental mechanisms by artificial selection. Dev Dyn. 242, 1262–1283 (2013).
van Eeden, F. J. et al. Genetic analysis of fin formation in the zebrafish, Danio rerio. Development 123, 255–262 (1996).
Hu, S. N., Yan, Y. C. & Li, Y. P. The blastocysts transition and back abdomen axis specialized regulation of Zebrafish. Chinese. Journal of Cell Biology. 32, 337–342 (2010).
Ma, Z. S., Hu, Y. Y. & Wang., Z. Demonstration and Localization of Bone Morphogenetic Protein-4(BMP4)in Fracture Healing. Chinese Journal of Orthopaedics. 17, 517–519 (1997).
De Robertis, E. M. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. 126, 925–941 (2009).
Fisher, S. & Halpern, M. E. Patterning the zebrafish axial skeleton requires early chordin function. Nat Genet. 23, 442–446 (1999).
Oelgeschlager, M., Kuroda, H., Reversade, B. & Robertis, E. M. D. Chordin Is Required for the Spemann Organizer Transplantation Phenomenon in Xenopus Embryos. Dev Cell. 4, 219–230 (2003).
Schulte-Merker, S., Lee, K. J., McMahon, A. P. & Hammerschmidt, M. The zebrafish organizer requires chordino. Nature 387, 862–863 (1997).
Takashima, S. et al. Phenotypic analysis of a novel chordin mutant in medaka. Dev Dyn. 236, 2298–2310 (2007).
Fisher, S., Amacher, S. L. & Halpern, M. E. Loss of cerebum function ventralizes the zebrafish embryo. Development 124, 1301–1311 (1997).
Cai, N. E. Studies on the formation of single caudal fin in the goldfish, carassius auratus (The effect of carp egg-mRNA on the development of the goldfish embryo). Oceanologia et limnologia sinica. 20, 508–513 (1989).
Gao, L. Y. Why the godfish is twin-tail. Science Express. 26, 71 (2014).
Wang, C. Y. & Li, Y. L. Taxonomy and phylogeny of different varieties of the goldfish (carassius auratus) in china. Acta Zoologica Sinica. 29, 267–277 (1983).
Zou, S. M. The invention relates to a method for screening the parents of goldfish carrying missing Tgf2 transposon to breed offspring with high yield. CN 105409882 B (2018).
Saina, M., Genikhovich, G., Renfer, E. & Technau, U. BMPs and chordin regulate patterning of the directive axis in a sea anemone. Proc Natl Acad Sci USA 106, 18592–18597 (2009).
Wang, X. M. & Guo, L. Progress of studies on the origin and phylogenesis of goldfish. Journal of Tianjin Agricultural College. 6, 27–30 (1999).
Jiang, Y. S. & Jiang, Y. X. A simple staining method of fetal rats. Occupational Medicine. 15, 41 (1988).
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 3, 59–69 (2008).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31572220), the Key Technologies Research and Development Program of China (2012BAD26B02), the Shanghai University Knowledge Service Platform (ZF1206) and the Capacity Building Plan of Shanghai Local Colleges and Universities (18050501900).
Author information
Authors and Affiliations
Contributions
Y.W.S., D.D.G. and S.M.Z. wrote the manusucript. S.M.Z. designed the experiments. Y.W.S. conducted pedigree breeding. D.D.G. conducted the embryonic observations during the incubation period. Y.W.S., W.T.C., H.H.G., S.K.D. and J.C. conducted functional assays and analyzed the expression patterns in ventral embryonic tissue markers. All authors contributed to finalizing and approving the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, DD., Sun, YW., Cui, WT. et al. Insertional mutagenesis in ChordinA induced by endogenous ΔTgf2 transposon leads to bifurcation of axial skeletal systems in grass goldfish. Sci Rep 9, 4098 (2019). https://doi.org/10.1038/s41598-019-40651-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40651-1
This article is cited by
-
Exploring the origin of a unique mutant allele in twin-tail goldfish using CRISPR/Cas9 mutants
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.