Abstract
There is some evidence that children born post-term (≥42 weeks of gestation) have metabolic abnormalities that may be associated with an increased risk of adverse health outcomes in adulthood. However, there are no data as to whether adolescents born post-term display alterations in aerobic capacity or cardiovascular function. We studied 48 adolescents (56% males) in Auckland (New Zealand) with a mean age of 14.3 years (SD = 1.7): 25 born post-term and 23 born at term (37–41 weeks of gestation). Assessments included metabolic markers in blood, whole body DXA scans, 24-hour ambulatory blood pressure monitoring, maximal exercise capacity, as well as cardiac MRI scan at rest and during submaximal exercise. Exercise capacity was lower in the post-term than in control participants (44.5 vs 47.8 ml/kgffm/min; p = 0.04). There were no differences in left ventricular volumes at rest and during exercise between groups. The 24-hour ambulatory blood pressure monitoring also showed no differences between the two groups. Being born post-term was associated with reduced exercise capacity, but with no observed differences in central cardiac function. We speculate that the reduction in exercise capacity may be due to changes in the peripheral vascular system.
Similar content being viewed by others
Introduction
Post-term birth, defined as birth on or after 42 weeks of gestation, has been shown to be associated with an increased risk of morbidity and mortality, both in the short- and long-term1,2. As the risk of delivery complications and neonatal morbidity climb rapidly once pregnancies go past 41 weeks of gestation, there are universal international recommendations to avoid post-term delivery3. Although obstetric practice has changed, the rates of post-term birth vary considerably worldwide, ranging from 0.4% of live births in Austria to as many as 8.1% in Denmark4.
Currently, there is little to no research involving the later impact of being born post-term. The vast majority of the research has focused on a narrow window, i.e. on the increase risk of perinatal complications1,5. Only relatively recently post-term birth has been identified as a risk factor for adverse metabolic outcomes in childhood and adolescence6,7. Post-term children displayed similar metabolic and body composition abnormalities compared to preterm and small for gestational age (SGA) children with reduced insulin sensitivity, higher fat mass, and higher blood pressure6,7. In addition, Derraik et al. showed that post-term birth was associated with greater BMI and increased risk of overweight and obesity in adult women, particularly among those born ≥43 weeks gestation8. There are a number of factors that may be associated with an increased risk of post-term delivery5. Some of these are potentially modifiable, notably maternal obesity9.
Previous studies investigating growth and metabolism in children born post-term6,7 showed a 34% reduction in insulin sensitivity in pre-pubertal children and an increased risk of obesity in adolescence males. These abnormalities are similar to those observed in children and adults born preterm or SGA, suggesting post-term children may have similar metabolic sequelae10,11,12,13. Children born SGA and preterm have also been shown to have impairments in cardiovascular function and aerobic capacity2,14,15,16,17,18. Preterm children have reduced aerobic capacity compared to term counterparts with higher rates of hypertension and increased left ventricular mass and volume2,14,15,16,17,18. SGA infants also display cardiovascular changes with more globular hearts, impaired relaxation, increased vascular wall thickness, and higher blood pressure compared to controls born of appropriate weight-for-age19. Adults born both preterm and SGA have increased risk of cardiovascular disease suggesting that abnormalities detected in childhood progress to clinically relevant conditions over time14. Thus, childhood alterations in cardiac structure and function may be indicative of later adult disease.
There is currently no information regarding the impact of being born post-term on metabolic and cardiovascular health later in life, particularly during adolescence. This is important considering that a reduction in insulin sensitivity has been shown to impact cardiac function and exercise capacity in young individuals20. Insulin resistance and hyperinsulinemia are universal in all adolescents during puberty, and are primarily a result of the rise in sex steroid and growth hormone levels21. Increased insulin secretion is required to maintain euglycaemia22. If this is transient there are no sequelae and indeed it may promote anabolism during puberty. However, a persistent reduction in insulin sensitivity leads to insulin resistance and an increase in cardiovascular risk, stroke and type 2 diabetes mellitus in adulthood23. The heart is an insulin-responsive organ, and the presence of insulin resistance and obesity (as previously demonstrated in post-term children) could underpin the altered cardiac function. A study with 25,618 adults born post-term24 showed no differences in maximal exercise capacity in adulthood compared to subjects born at term, but data from the study were not adjusted for metabolic tissue (lean mass) and did not include cardiac function assessments. Therefore, we aimed to assess whether aerobic capacity and cardiovascular function in healthy adolescents born post-term was altered compared to those born at term.
Methods
Participants
Potential post-term participants (≥42 weeks gestation) born between 2000 and 2001 were identified from the obstetrics database at the National Women’s Health (Auckland City Hospital), which contains data on births from three hospitals throughout the Auckland area (New Zealand). Each recruited participant born post-term was asked to invite up to two friends or family members who were born at term (38–41 weeks of gestation), so that participants in both groups were approximately matched for age, sex, and lifestyle, as well as reported physical activity levels. Participants self-reported as being physically active or not, which was defined as ≥3 hours a week of guided exercise. This information was obtained using a questionnaire6,25,26.
Participants were included if they were able to perform vigorous exercise on a bike, did not suffer from claustrophia, and able to perform exercise while lying down during the MRI scan. Participants were excluded if they had evidence or history of musculoskeletal, cardiovascular or pulmonary disease; used cardiovascular or hypertensive medication; had any contraindication for MRI scanning including pacemakers, metal fragments in the eye, spinal column stimulators, aneurysm clips in the brain; were pregnant; were born small for gestational age (birth weight below the 10th percentile for the gestational age and sex), large for gestational age (birth weight above the 90th percentile for that gestational age and sex), or following assisted reproduction; if born from mothers that had gestational diabetes, pre-eclampsia, gestational or pre-existing hypertension, chronic illness, or maternal recreational drug use during pregnancy (including tobacco).
Socioeconomic status was assessed using the overall Index of Multiple Deprivation (IMD), which encompasses deprivation across seven domains (i.e. Access, Crime, Education, Employment, Health, Housing, and Income)27.
Ethics approval was given by the Health and Disability Ethics Committees (Ministry of Health, New Zealand – 14/NTA/15). All participants provided written and verbal informed consents, while those aged <16 years also needed written parental consent. This study was performed in accordance with all appropriate institutional and international guidelines and regulations for medical research, in line with the principles of the Declaration of Helsinki.
Assessments
All clinical assessments were carried out at the Liggins Institute in two separate visits to our clinic. In the first visit, height was measured to nearest mm using a Harpenden stadiometer. Weight and body composition were determined by dual-energy x-ray absorptiometry (DXA; Lunar Prodigy 2000; General Electric, Madison, USA). Physical activity was assessed by questionnaire, while birth weight was extracted from hospital records. Fasting venous blood samples were drawn to assess lipid profile, as well as glucose, insulin, IGF-I, IGFBP-1, leptin, and adiponectin concentrations. Lipids and glucose were measured on a Roche Hitachi 902 autoanalyser (Hitachi High Technologies Corporation, Tokyo, Japan) and insulin on a Roche Elecsys 2010. Commercially available ELISA kits were used to analyse leptin, adiponectin, IGF-I, and IGFBP-1.
For the assessment of Maximal Exercise Capacity (\(\dot{{\rm{V}}}\)O2max), participants pedaled to exhaustion on an electronically-braked upright cycle ergometer (Schiller CG6340 BAAR-Switzerland) with simultaneous measurements of inspired O2 and CO2 volumes (ParvoMedics TrueOne 2400 Metabolic Measurement system, Parvomedics; Sandy, Utah, USA), based on an established protocol using an upright cycle ergometer26,28. In brief, participant’s initial workload was set at 55 watts, with an increase of 15 watts every minute. Participants were advised to remain at or above 60 revolutions per minute throughout the test. The protocol was designed to last a maximum of 15 minutes, and the test was terminated when the participant was unable to continue due to exhaustion, pain, discomfort, or technical error. The participant’s maximum exercise capacity was determined by the average of the two highest consecutive VO2 values. Heart rate and workload for each participant was recorded every minute during the test. Blood pressure was measured after resting for 5 minutes prior to the test and immediately after the test termination.
At the end of the first visit, participants were fitted with an oscillometric device (Spacelabs 90217; Spacelabs Medical Inc, Redmond, Washington, USA) on the non-dominant arm for the 24-hour ambulatory blood pressure monitoring. Blood pressure was measured every 20 minutes 07:00–22:00 and every 30 minutes 22:00–07:00 over 24 hours.
Participants returned to our clinic within a week of the first visit, when cardiac structure and function were measured using MRI scans. Left ventricular (LV) structure and function were assessed at rest and during submaximal exercise using a 1.5 Telsa Magnetom Avanto MRI scanner (Siemens Erlangen, Germany), and an MRI-compatible cycle ergometer as previously described26,29. In brief, cardiac MRI images were obtained during mid expiration breath-hold for 5–10 seconds. Six short-axis slices from the base to the apex of the LV and 3 long-axis slices were obtained with 2 slices per breath-hold with a 6-mm slice thickness. Images were acquired at rest and during submaximal exercise. The latter were obtained once one minute of targeted steady-state heart rate (110 ± 5 beats per minute) was reached. The power (watts) or work rate at which the subject exercised was also recorded. Cardiac MRI images were analysed using the Cardiac Image Moddeller software (Auckland, New Zealand) to obtain left ventricular mass, ejection fraction, stroke volume, and end-diastolic and end-systolic volumes. Cardiac output was calculated by multiplying heart rate by stroke volume. Parameters were also indexed for participant’s fat-free mass.
Sample size and power calculation
Power calculation was based on data from a previous study on non-obese adolescents aged 16.7 years on average, with a standard deviation for VO2max indexed for fat-free mass of 1.426. A study with 20 controls and 20 adolescents born post-term was powered to detect a difference in VO2max indexed for fat-free mass of ±1.27 ml/kg FFM/min between groups, with 80% power and α = 0.05. To account for an approximate 10% loss due to dropouts, we aimed to recruit 22 participants per group. The same sample size (i.e. 20 per group) was powered to detect differences in stroke volume and left ventricular mass of ±0.06 ml/kg FFM and ±0.07 g/kg FFM between groups, respectively.
Statistical Analysis
Demographic data for post-term and control groups were compared with one-way analyses of variance and Chi-square tests. Outcomes were compared using linear mixed regression models, adjusting for sex and age, and including family as a random factor to account for sibling clusters. Statistical analyses were carried out in Minitab v.16 (Pennsylvania State University, State College, PA, USA) and SAS v.9.4 (SAS Institute, Cary, NC, USA). All statistical tests were two-tailed and maintained at a 5% significance level.
Results
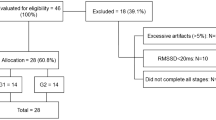
There were fifty post-term adolescents in the National Woman’s Health database who met the study criteria. From those, 17 declined to participate and 11 were unable to be contacted. Three post-term participants had post-term siblings/family members that met our inclusion criteria and were also invited to the study. Hence we recruited 25 post-term participants. Twenty six term-born control adolescents were also recruited. Seven were term born siblings of post-term participants and 19 from the community, family and friends (Fig. 1). There were no observed differences in levels of socioeconomic deprivation or of self-reported physical activity (data not shown).
Participant’s age, birth weight SDS, gender ratio, ethnic composition, blood variables and BMI were similar between groups (Table 1). Birth weight in grams was higher in the post-term group compared to term born group (Table 1). Percentage body fat and fat-free mass assessed by DXA scan showed no differences between the two groups (Table 1). Maximal exercise capacity parameters including heart rate, systolic and diastolic blood pressure, workload and \(\dot{{\rm{V}}}\)O2max (L/min) showed no differences between groups. However, when normalized for fat-free mass the post-term group showed lower exercise capacity (Table 2). Cardiac parameters assessed at rest and during submaximal exercise using MRI technology revealed no differences between groups (Table 3). The 24-hour ambulatory blood pressure monitoring are shown on Table 4. Again, there were no differences between groups.
Discussion
This is the first study to describe the cardiac function and exercise capacity of adolescents born post-term, who displayed a reduction in exercise capacity but no differences in cardiac function compared to term counterparts. This reduction in exercise capacity was not explained by changes in body composition, baseline physical activity, or left ventricular function.
As previously described, pre-pubertal children born post-term displayed reduced insulin sensitivity compared to those born at term6. Reduced insulin sensitivity has been linked to alterations in cardiac and peripheral cardiovascular function including altered endothelial function, hypertension and dyslipidemia, as well as an increased risk of cardiovascular pathology in later life23,30. Both SGA and preterm adolescents and young adults displayed reduced exercise capacity, and this has been correlated to changes in both cardiac and peripheral cardiovascular function15,16,17, in particular increased vascular thickness and left ventricular mass14. The reduction in exercise capacity noted in this study in subjects born post-term suggests there may be differences in cardiac function, peripheral vascular function, and/or altered muscle function. The lack of change in cardiac function indicates these changes are not central or cardiac, and we speculate that this may reflect an alteration in peripheral vascular or muscle function.
VO2max is dependent not only on cardiac function but also on adequate oxygen diffusion and effective uptake of oxygen peripherally by the metabolically active tissues31. During exercise, there is increased systemic blood flow and hence capillary recruitment to improve oxygen extraction by skeletal muscle32. This is regulated by the endothelium and in particular nitric oxide33. Insulin is involved in activating the production of nitric oxide and reductions in insulin sensitivity reduce capillary vasodilation and recruitment, thereby reducing peripheral oxygen extraction34. As a reduction in insulin sensitivity in post-term pre-pubertal children has been documented6, this could be a factor reducing peripheral vascular perfusion in our older adolescent population and further investigations are required.
A study by Nadeau et al. has recently showed that insulin resistance may play an important role on the decreased exercise capacity in non-obese type 1 diabetic youth20. We did not directly measure insulin resistance in this cohort, but a previous study using intravenous glucose tolerant test and the minimal model methodology showed reduced insulin sensitivity in prepubertal children born post-term6. The lack of any observed difference in fasting insulin levels (which primarily measures hepatic insulin sensitivity) in this study is still consistent with a peripheral reduction in insulin sensitivity. Indeed, normal fasting insulin levels have been reported in preterm children who had evidence of a 40% reduction in insulin sensitivity using the minimal model approach10.
These finding potentially could be explained by a reduction in peripheral insulin sensitivity. As previously described, pre-pubertal children born post-term displayed at 34% reduction in peripheral insulin sensitivity compared to those born at term using intravenous glucose tolerance tests and the minimal model6. Importantly, fasting insulin levels (a measure primarily in hepatic insulin sensitivity) were the same in both post-term and term cohorts6. The similar fasting insulin levels are consistent with the current study. We understand this was a limitation of our study and future research looking into peripheral insulin sensitivity and cardiac function in this population should be explored.
Our relatively small sample size is a limitation of our study as small differences in outcomes might have been missed. However, we contend that cardiac MRI with submaximal exercise provides extremely precise and reproducible data, and it is unlikely any clinically-relevant differences were missed. The control group was also heterogeneous with approximately 30% being siblings of post-term participants. Self-reported activity is less reliable than quantified measures of physical activity (e.g. accelerometers), but this was the only approach available for this study. While this is a limitation, the adopted questionnaire does provide an estimate of physical activity levels, and this method has been used previously in published clinical studies6,25,26.
While the current clinical relevance of the lower exercise capacity observed in adolescents born post-term is unclear, it is well established that lifestyle and aging can lead to a more marked reduction in VO2max35. Therefore, over time this difference in VO2max may become greater (as lifestyle tend to change into adulthood), being potentially associated with an increased risk of diseases linked to a sedentary lifestyle. Thus, we suggest that the maintenance of an active lifestyle may be particularly important for those born post-term.
In summary, our study has provided some evidence of a reduction in exercise capacity in adolescents born post-term despite similar central cardiac function as those born at term. This difference in exercise capacity could be attributed to differences in peripheral vascular function and suggest further studies testing peripheral blood flow and compliance need to be conducted.
Data availability statement
The clinical data cannot be made available in a public repository due to strict conditions of the ethics approval of our study. Nonetheless, anonymized and unidentifiable data would be made available to other investigators upon request. For this purpose, anyone interested should contact the senior author (P.L.H.).
References
Divon, M. Y., Haglund, B., Nisell, H., Otterblad, P. O. & Westgren, M. Fetal and neonatal mortality in the postterm pregnancy: the impact of gestational age and fetal growth restriction. American journal of obstetrics and gynecology 178, 726–731 (1998).
Crump, C., Sundquist, K., Sundquist, J. & Winkleby, M. A. Gestational age at birth and mortality in young adulthood. JAMA 306, 1233–1240, https://doi.org/10.1001/jama.2011.1331 (2011).
American College of, O. & Gynecologists. Practice bulletin no. 146. Management of late-term and postterm pregnancies. Obstetrics and gynecology 124, 390–396, https://doi.org/10.1097/01.AOG.0000452744.06088.48 (2014).
Zeitlin, J., Blondel, B., Alexander, S., Breart, G. & Group, P. Variation in rates of postterm birth in Europe: reality or artefact? BJOG: an international journal of obstetrics and gynaecology 114, 1097–1103, https://doi.org/10.1111/j.1471-0528.2007.01328.x (2007).
Ayyavoo, A., Derraik, J. G., Hofman, P. L. & Cutfield, W. S. Postterm births: are prolonged pregnancies too long? J Pediatr 164, 647–651, https://doi.org/10.1016/j.jpeds.2013.11.010 (2014).
Ayyavoo, A. et al. Pre-pubertal children born post-term have reduced insulin sensitivity and other markers of the metabolic syndrome. PLoS One 8, e67966, https://doi.org/10.1371/journal.pone.0067966 (2013).
Beltrand, J. et al. Post-term birth is associated with greater risk of obesity in adolescent males. J Pediatr 160, 769–773, https://doi.org/10.1016/j.jpeds.2011.10.030 (2012).
Derraik, J. G., Lundgren, M., Cutfield, W. S. & Ahlsson, F. Body Mass Index, Overweight, and Obesity in Swedish Women Born Post-term. Paediatric and perinatal epidemiology 30, 320–324, https://doi.org/10.1111/ppe.12292 (2016).
Roos, N., Sahlin, L., Ekman-Ordeberg, G., Kieler, H. & Stephansson, O. Maternal risk factors for postterm pregnancy and cesarean delivery following labor induction. Acta obstetricia et gynecologica Scandinavica 89, 1003–1010, https://doi.org/10.3109/00016349.2010.500009 (2010).
Hofman, P. L. et al. Premature birth and later insulin resistance. N Engl J Med 351, 2179–2186, https://doi.org/10.1056/NEJMoa042275 (2004).
Mathai, S. et al. Insulin sensitivity and beta-cell function in adults born preterm and their children. Diabetes 61, 2479–2483, https://doi.org/10.2337/db11-1672 (2012).
Mathai, S. et al. Increased adiposity in adults born preterm and their children. PLoS One 8, e81840, https://doi.org/10.1371/journal.pone.0081840 (2013).
Derraik, J. G., Lundgren, M., Cutfield, W. S. & Ahlsson, F. Association Between Preterm Birth and Lower Adult Height in Women. Am J Epidemiol 185, 48–53, https://doi.org/10.1093/aje/kww116 (2017).
Lewandowski, A. J. et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 127, 197–206, https://doi.org/10.1161/CIRCULATIONAHA.112.126920 (2013).
Welsh, L. et al. The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax 65, 165–172, https://doi.org/10.1136/thx.2008.107474 (2010).
Smith, L. J., van Asperen, P. P., McKay, K. O., Selvadurai, H. & Fitzgerald, D. A. Reduced exercise capacity in children born very preterm. Pediatrics 122, e287–293, https://doi.org/10.1542/peds.2007-3657 (2008).
Vrijlandt, E. J., Gerritsen, J., Boezen, H. M., Grevink, R. G. & Duiverman, E. J. Lung function and exercise capacity in young adults born prematurely. American journal of respiratory and critical care medicine 173, 890–896, https://doi.org/10.1164/rccm.200507-1140OC (2006).
Crump, C., Winkleby, M. A., Sundquist, K. & Sundquist, J. Risk of hypertension among young adults who were born preterm: a Swedish national study of 636,000 births. Am J Epidemiol 173, 797–803, https://doi.org/10.1093/aje/kwq440 (2011).
Crispi, F. et al. Cardiovascular programming in children born small for gestational age and relationship with prenatal signs of severity. American journal of obstetrics and gynecology 207, 121 e121–129, https://doi.org/10.1016/j.ajog.2012.05.011 (2012).
Nadeau, K. J. et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 95, 513–521, https://doi.org/10.1210/jc.2009-1756 (2010).
Amiel, S. A., Sherwin, R. S., Simonson, D. C., Lauritano, A. A. & Tamborlane, W. V. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 315, 215–219, https://doi.org/10.1056/NEJM198607243150402 (1986).
Amiel, S. A. et al. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab 72, 277–282, https://doi.org/10.1210/jcem-72-2-277 (1991).
DeFronzo, R. A. & Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14, 173–194 (1991).
Svedenkrans, J., Henckel, E., Kowalski, J., Norman, M. & Bohlin, K. Long-Term Impact of Preterm Birth on Exercise Capacity in Healthy Young Men: A National Population-Based Cohort Study. Plos One 8, ARTN e80869 10.1371/journal.pone.0080869 (2013).
Pinto, T. E. et al. Systolic and diastolic abnormalities reduce the cardiac response to exercise in adolescents with type 2 diabetes. Diabetes Care 37, 1439–1446, https://doi.org/10.2337/dc13-2031 (2014).
Gusso, S. et al. Diastolic function is reduced in adolescents with type 1 diabetes in response to exercise. Diabetes Care 35, 2089–2094, https://doi.org/10.2337/dc11-2331 (2012).
Exeter, D. J., Zhao, J., Crengle, S., Lee, A. & Browne, M. The New Zealand Indices of Multiple Deprivation (IMD): A new suite of indicators for social and health research in Aotearoa, New Zealand. PLoS One 12, e0181260, https://doi.org/10.1371/journal.pone.0181260 (2017).
Gusso, S. et al. Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diabetologia 51, 1317–1320 (2008).
Gusso, S. et al. Design and testing of an MRI-compatible cycle ergometer for non-invasive cardiac assessments during exercise. Biomed Eng Online 11, 13, https://doi.org/10.1186/1475-925X-11-131475-925X-11-13 (2012).
Yki-Jarvinen, H. Insulin resistance and endothelial dysfunction. Best Pract Res Clin Endocrinol Metab 17, 411–430 (2003).
Rowell, L. B. Human Circulation Regulation During Physical Stress. (Oxford University Press, 1986).
Maiorana, A., O’Driscoll, G., Taylor, R. & Green, D. Exercise and the nitric oxide vasodilator system. Sports Med 33, 1013–1035 (2003).
Vogel, R. A. Measurement of endothelial function by brachial artery flow-mediated vasodilation. Am J Cardiol 88, 31E–34E (2001).
Baron, A. D. & Brechtel, G. Insulin differentially regulates systemic and skeletal muscle vascular resistance. Am J Physiol 265, E61–67 (1993).
Ogawa, T. et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86, 494–503 (1992).
Acknowledgements
This study was funded by a University of Auckland Faculty Research Development Fund.
Author information
Authors and Affiliations
Contributions
S.G., P.L.H., T.H., M.M. and W.S.C. conceived and designed the study; M.M. coordinated and performed the assessments; J.G.B.D. carried out the statistical analyses; S.G., M.M., P.L.H. and J.G.B.D. wrote the manuscript with input from all other authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murali, M., Hofman, P.L., Derraik, J.G.B. et al. Exercise capacity and cardiac function in adolescents born post-term. Sci Rep 8, 12963 (2018). https://doi.org/10.1038/s41598-018-31343-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31343-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.