Abstract
Hearing loss is the most common sensory disorder and because of its high genetic heterogeneity, implementation of Massively Parallel Sequencing (MPS) in diagnostic laboratories is greatly improving the possibilities of offering optimal care to patients. We present the results of a two-year period of molecular diagnosis that included 207 French families referred for non-syndromic hearing loss. Our multi-step strategy involved (i) DFNB1 locus analysis, (ii) MPS of 74 genes, and (iii) additional approaches including Copy Number Variations, in silico analyses, minigene studies coupled when appropriate with complete gene sequencing, and a specific assay for STRC. This comprehensive screening yielded an overall diagnostic rate of 48%, equally distributed between DFNB1 (24%) and the other genes (24%). Pathogenic genotypes were identified in 19 different genes, with a high prevalence of GJB2, STRC, MYO15A, OTOF, TMC1, MYO7A and USH2A. Involvement of an Usher gene was reported in 16% of the genotyped cohort. Four de novo variants were identified. This study highlights the need to develop several molecular approaches for efficient molecular diagnosis of hearing loss, as this is crucial for genetic counselling, audiological rehabilitation and the detection of syndromic forms.
Similar content being viewed by others
Introduction
Hearing loss (HL) is the most common congenital sensory impairment in humans, and it affects approximately 1 in 600 newborns1. It is estimated that half of the cases have a genetic origin. HL can be non-syndromic (NSHL) and not associated with other clinical signs, or it can present as one of the symptoms in syndromic forms. In addition, some non-syndromic forms can evolve to syndromic forms later in life and are then defined as NSHL mimics2. The most common example is Usher syndrome (USH), which alters hearing and in some cases balance early in life, whereas it is only after the first decade that clinical signs of retinitis pigmentosa (RP) will affect the patient’s vision3.
Over 100 genes have been associated with NSHL and more still with syndromic HL4. Simultaneous screening of multiple genes is now possible with the advent of massively parallel sequencing (MPS). This approach offers the possibility of identifying the aetiology of the HL and thus providing proper genetic counselling to the families. Gene testing also impacts the clinical management of patients, as identifying the pathogenic alterations in syndromic genes of patients referred for NSHL will require a change or adaptation in care, as in the case of Usher syndrome5. Genetic findings can also indicate the need for additional clinical evaluation that may detect subtle syndromic features not necessarily related to the associated syndrome6. MPS improves diagnostic rates7,8,9. Although several approaches can be used, i.e. exome or gene panels, the latter have thus far shown the best compromise between the mutation detected rate and cost2. Studies of large cohorts of different origins have highlighted that the mutation detection rate depends on clinical characteristics and ethnicity2,9. Yet no data on the prevalence of HL genes and positive rates for diagnostic purposes are available for French patients. We have therefore tested a gene-panel approach over a two-year recruiting period for 207 families referred for NSHL from 14 centres distributed all over France. We present in this study an efficient decision-making process that identifies the genetic HL aetiology in 48% of patients.
Results
We analysed a total of 207 index cases over a two-year period following the decision-making tree presented below.
Decision-making tree
Analyses were performed following the tree presented on Fig. 1 (and Supplementary Table 1 and Supplementary Figure 1).
Decision-making tree for molecular diagnosis of isolated hearing loss. *74 HL genes, see Supplementary Table 1. **See Supplementary Figure 1. $CNV: Copy Number Variation, deletions/duplications involving at least one exon and occurring in an isoform involved in hearing. $$aCGH: array Comparative Genomic Hybridization; QMPSF: Quantitative Multiplex PCR of Short Fluorescent fragments. All SNVs and small indels are confirmed by Sanger sequencing or long-range PCRs followed by Sanger sequencing for STRC variants.
DFNB1 analysis was performed for all cases with the exception of those patients presenting with a family history of dominant transmission. Although the GJB2 and GJB6 genes are included in the panel, DFNB1 locus screening can be performed at reduced cost with a fast turn-around time. Patients with suspicion of Pendred/DFNB4 disease underwent the same strategy, even though SLC26A4 can be prioritized according to scan data.
DFNB1 genotypes
Fifty-five patients were identified with pathogenic DFNB1 variants (Supplementary Tables 2 and 3). Six patients carrying a single pathogenic variant were further analysed on the gene panel. Patient S1760, who was homozygous for the p.Met34Thr variant, was also included for further studies because the clinical data were not coherent with the identified genotype (profound HL and enlarged vestibular aqueduct: EVA).
The 49 DFNB1 pathogenic genotypes included 20 different variants: although c.35delG represented 58% (57/98) of the pathogenic alleles, genetic heterogeneity was notable as most of the other variants were encountered only once or twice.
Other NSHL genotypes
In 50 additional patients, pathogenic genotypes were identified in 18 different genes using MPS strategy (Supplementary Table 2). The data were categorized according to genotypes identified in truly non-syndromic genes and in those of the NSHL mimics (i.e. USH genes).
STRC pathogenic genotypes were identified in nine patients presenting with mild to moderate HL. Of these 18 mutant alleles, 12 consisted of large deletions of the gene. Although it was not possible to characterize the deletion breakpoints with our approach, we identified deletions of different sizes. The most frequent type encompassed the entire STRC gene and its 5′ sequence (c.(?_−78)_(*109_?)del), whereas the others maintained the 5′ end of the gene.
Patient S1537 was first identified as a heterozygous carrier of two alterations, a short deletion in intron 23 (c.4545+2_4545+6del) and a potential copy number variation (CNV) in exon 28. However, visual inspection of the aligned sequence reads using the Integrative Genomics Viewer (IGV) highlighted a reduced depth of coverage of STRC-exon 28 associated with an increased depth of ψSTRC-exon 28 reads, in comparison with control (Supplementary Figure 2). Therefore, this CNV was more likely to be a software alignment artefact. Sanger analysis with STRC-specific long-range PCR followed by nested PCR (LR/nested PCR) that was focused on exon 28 then identified the heterozygous substitution c.5125A > G in this patient S1537. This variant was already reported as pathogenic by Vona et al.10 and corresponds to an existing divergent nucleotide in the pseudogene sequence, explaining the misalignment of sequence reads observed with MPS technology.
IGV analyses were also conducted for S1511 and S1516, both of whom presented with moderate HL and carried a heterozygous deletion encompassing the STRC gene. In both cases, a substitution in intron 11 was identified by visual inspection (c.3100-2A > T for S1511 and c.3100-18G > A for S1516). Results of the LR/nested PCRs confirmed the STRC localization of the variants and minigene assays revealed in both cases complete skipping of exon 12, resulting in the loss of 13 amino acids (Fig. 2A).
Minigene analysis to assess the impact of variants on splicing. Full-length gels showing: (A) STRC variants c.3100-2 A > T and c.3100-18 G > A leading to complete skipping of exon 12. The lower band observed for the wild-type construction may be due to an artefact of the minigene system or alternative splicing of exon 12; (B) TJP2 c.2880G > A leading to complete skipping of exon 19; (C) USH2A variant c.14134-3169 A > G leading to the inclusion of a pseudoexon between native exons 63 and 64 (PE64); bold: termination codon.
MYO15A, OTOF and TMC1 mutations were also identified in four, three and two patients, respectively. All identified mutations were associated with severe to profound HL.
All together more than a third (18/50) of the patients carrying a pathogenic genotype detected by MPS screening carried mutations in one of the four following genes: STRC, MYO15A, OTOF and TMC1.
Variants in rarely involved genes were also identified, such as the de novo c.2880G > A alteration in TJP2 (patient S1324). Interestingly, this variant, located at the last nucleotide of exon 19, was not predicted to alter the protein sequence; the MaxEnt algorithm predicted a potential impact on splicing. Our minigene analysis confirmed that c.2880G > A indeed led to skipping of in-frame exon 19, which resulted in the deletion of amino acid residues 890–960 in the protein (Fig. 2B).
Pathogenic genotypes in Usher genes
The 10 genes responsible for USH were included in our design. Sixteen of the 99 patients carried a pathogenic genotype in an Usher gene, the most frequent genes being MYO7A and USH2A in five and four patients, respectively. All patients, with the exception of two carrying mutations in MYO7A, were under 15 years and had been referred for NSHL, which is consistent with the onset of RP after the first decade.
Interestingly, MPS analysis on patient S1679 revealed a single USH2A heterozygous c.4645C > T nonsense variant. According to our diagnostic strategy, USH2A-whole-gene screening was then performed and detected a deep intronic substitution likely to affect splicing. This variation, c.14134-3169A > G in intron 64, predicted the creation of a strong donor splice site (MaxEntScan score: 7.64) potentially leading to a new pseudoexon (PE) activation. Minigene assay confirmed the pathogenic effect of this intronic variant on the splicing process and defined the size of the inserted pseudoexon PE64 (Fig. 2C). The latter, composed of 52 nucleotides, carried a premature termination codon (PTC) leading to a truncated protein if synthetized.
Additional pathogenic variants
Ten of the 50 patients genotyped by MPS carried an additional pathogenic variant in another gene (Supplementary Table 2). Four corresponded to the GJB2 heterozygotes initially screened at the DFNB1 locus. Unsurprisingly, one of them was a c.35delG heterozygote and two other patients carried the p.(Met34Thr) alteration. This reflects the carrier frequencies of these two variants in the general population11. Two of the patients were MYO15A heterozygotes and another carried a deletion in STRC; again, these being two frequently involved genes in NSHL.
3D analysis
In order to better characterize the missense variants, we performed a structural analysis using 3D modelling. This approach requires the availability of a crystallographic structure or a reliable model. For example, patient S1542 carried the POU4F3 p.(Phe322Ser) variant and the structure showed a localization in helix 3 of the homeodomain in the model Oct-1 (POU2F1) protein. This helix directly binds DNA and the highly conserved Phe is part of the hydrophobic core involving helices 1 and 2 that stabilizes the helix-turn-helix conformation of the domain and allows the binding with DNA. Replacement of Phe with the small polar Ser would very likely destabilize the region and might impact DNA binding (Fig. 3). Familial segregation of this variant was further confirmed over a three-generation family that included six affected patients.
3D analysis of POU4F3 p.(Phe322Ser) using homolog Oct-1 PDB structure 1E3O. The homeodomain directly binds DNA through helix 3 and the positively charged amino acids Arg or Lys (red). The equivalent of Phe 322 (A, white), also located in helix 3, is involved in the hydrophobic core with helices 1 and 2 stabilizing the region. Introduction of a small polar amino acid (Ser, B, white) is likely to modify the hydrophobic core and the domain and might modify DNA binding properties of the protein.
Discussion
Diagnostic rate
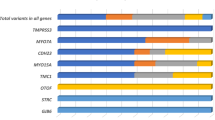
In order to establish an unbiased diagnostic rate, we included all patients referred for NSHL over a two-year period, without selecting the degree of HL or familial history. Following the described strategy, we were able to unambiguously genotype 48% of the patients (Fig. 4). Use of MPS drastically improved the diagnostic rate, by doubling the number of patients diagnosed with a confirmed genetic origin in our cohort. Nineteen different genes were causally implicated, but among them only five contributed to 71% of the cases: GJB2 (DFNB1 locus), STRC, MYO7A, MYO15A and USH2A (Fig. 4).
Two other studies testing large cohorts with different HL gene panels have recently reported diagnostic rates varying from 327 to 56%12. However, in the latter, the high positive rate was explained by an enrichment of familial cases. Sloan-Heggen et al. provided data on the largest cohort (n = 1,119) with an overall rate of 39%. They showed that the positive rate varies greatly with degree of HL, inheritance of HL and ethnicity2. Comparing rates across different studies is difficult as many different parameters can interfere, from the composition of the cohort to the genes analysed.
One hundred and eight patients remained undiagnosed with no or a single identified pathogenic variant. For an unknown proportion of these patients, the HL is not of genetic origin. However, to improve the diagnostic rate of genetic cases, additional genes mimicking NSHL and involved in syndromic forms could be included in the panel2. Recently, Abou Tayoun et al. suggested that only genes with strong gene-disease association criteria should be included in diagnostics, and these authors provided a valuable core panel13. However, if genes with weak gene-disease association are not included, additional clues that a gene is indeed involved in HL might be missed. As an example, not including TJP2 would have missed the diagnosis for patient S1324.
Using the gene panel approach for diagnostic purposes is still valuable as the quality remains superior to clinical exome or whole exome sequencing14. Therefore, a reasonable approach would be to include HL genes as well as candidates in a larger gene panel and to define a core diagnostic list following recommendations13. Diagnosis would be established quite confidently for genes included in the core list, whereas interpretation would be more cautious for the other genes.
STRC analysis pitfalls
In accordance with other studies2,7, our results show that STRC was prone to large deletions and was clearly the second most frequent gene to be involved in NSHL. Tandem repeats are genomic features prone to instability and, as expected, large CNVs were the major mutational event for the STRC gene, with an estimated heterozygous deletion frequency of 1% to 1.6%15. In line with this high carrier rate, S1689 was identified as a heterozygous carrier of a STRC deletion (Supplementary Table 3). STRC was most likely not involved as this patient presented with profound hearing loss.
Overall, because of the high sequence homology between STRC and ψSTRC, specific approaches need to be developed to detect the large rearrangements as well as the point mutations that can be masked by the ψSTRC sequences.
De novo occurrence of variants
De novo variants were observed in several cases involving both DFNA or DFNB/USH genes. The ACTG1 variant (c.434C > T, p.(Ser145Phe)) arose de novo in S1572. This variant was confirmed to be absent in both parent samples. Mutations in ACTG1 can be associated with either Baraister-Winter syndrome16 or NSHL17. In the present case, the young patient presented only NSHL with normal facial appearance, although MRI was not performed. At birth, otoacoustic emissions (OAEs) were recordable and hearing loss was moderate but evolved into profound within three years. De novo variants of this gene have already been described in several Baraister-Winter cases18.
A de novo TJP2 variant (c.2880G > A) was also identified in patient S1324. Minigene analysis demonstrated an effect on the splicing process (Fig. 2B), and the implication of TJP2 in NSHL remains questionable. The progression of HL was noted over 15 years, altering mainly high frequencies. The de novo occurrence of the variant together with the alteration in TJP2 splicing reinforces the hypothesis that this gene is indeed involved in NSHL.
Familial segregation performed for S1759 revealed that neither parent was a carrier of the TECTA p.(Pro2079Leu) variant, suggesting a de novo occurrence. The residue Pro was perfectly conserved among 89 orthologs (UCSC MultiZ alignment, not shown) and was located within a highly-conserved region of the protein. The patient presented with a moderate congenital HL with a flat configuration; as he was under 2 years of age, an evolution in HL cannot be excluded.
Last, patient S701 was found to carry two alterations in the CDH23 gene. But as the c.6254-2A > G variant arose de novo, it was not possible to determine whether the two alterations are in trans. At this date, a question remains about a possible Usher syndrome.
All patients were initially sent for isolated, sporadic NSHL and these findings pinpoint the importance of systematic segregation analyses as identification of de novo variants in dominant forms of NSHL will have a direct impact on transmission risk and further genetic counselling.
Correlating genotypes with phenotypes for NSHL
This study confirms that TMPRSS3 is involved in progressive HL. The patient audiograms had a characteristic ski-slope configuration as previously shown19. The MYO15A and TMC1 mutations are mainly associated with severe to profound HL, whereas STRC alterations lead to mild to moderate hearing loss. All patients carrying OTOF mutations presented with profound HL. Most of the variants led to PTC and loss of functional protein. Recent data suggest that some missense variants can act as hypomorphic alleles and can be associated with moderate HL20. It is likely that in the future we will also extend the phenotypic spectrum associated with OTOF variants.
Questions linked to the identification of pathogenic genotypes in genes mimicking NSHL
We identified a USH pathogenic genotype in 16% of the patients. All patients were initially referred for NSHL. After genotyping, two groups should be distinguished based on age, i.e. those with NSHL mimics and those with true NSHL.
CDH23, MYO7A and USH1C have long been known to be involved in USH as well as non-syndromic hearing loss21,22,23. Therefore, it is possible that not all patients with mutations in these genes will develop a typical Usher syndrome. For example, patient S1692 is 27 years old and the electroretinogram (ERG) performed after the molecular results was found to be subnormal. Consequently, this patient is not expected to develop a typical Usher syndrome and signs of RP may remain very subtle throughout his lifetime. Similarly, S1741, S1763 and S1799 underwent further clinical examination after the molecular results and it is suspected that all will develop Usher syndrome. The awareness of future development of RP will have an impact on audiological rehabilitation and the genetic counselling for the families. In several cases, prenatal diagnosis could thus be offered for the next pregnancies. Last, patients carrying USH1C (S1536, S1707), USH2A (S1682, S1679, S1752 and S1786) and ADGRV1 variants (S1338 and S1601) were referred to ophthalmology clinics in order to establish their visual function.
Additional tools are required for optimized diagnostic service
In this study, we provide a rather comprehensive approach through the development of interpretation tools. Indeed, when possible, 3D analysis can provide additional clues to classify a variant, which is shown with the example of the POU4F3 p.(Phe322Ser) variant (Fig. 3).
An additional step for assessing missense pathogenicity would be to perform in vitro functional tests, whose feasibility has been demonstrated for genes such as SLC26A4 24.
We previously showed the usefulness of integrating minigene analysis for detailed study of the consequences of predicted splicing alterations25,26. Again, four variants identified in this cohort could be confirmed as affecting splicing by minigene analysis (Fig. 2).
In addition, because several USH2A deep intronic mutations have already been identified26,27, we recommend performing either transcript analysis or whole USH2A gene sequencing for patients carrying a single USH2A pathogenic variant. This allowed the identification of a novel deep intronic mutation in intron 64, leading to a new PE insertion. This mutation has since been identified in another patient presenting with Usher type 2 (unpublished results). All together, this is the fifth deep intronic mutation resulting in PE insertion identified in USH2A, thus confirming the particularly high rate of this type of alteration in the most frequent USH gene.
In conclusion, this study based on the genetic screening of 207 patients referred for NSHL shows that MPS clearly improves the molecular diagnosis of NSHL in the French population, as already shown in other populations. However, this diagnostic rate of 48% was achieved using MPS plus other approaches aimed at characterizing the impact of variants or confirming their actual existence (in silico and minigenes studies, CNVs analysis), or identifying them in the case of STRC variants and USH2A deep intronic variants (customized whole gene screening). In addition, a high proportion of our cohort presented with pathogenic genotypes in USH genes, which should thus definitely be included in NSHL screening as their involvement immediately modifies the medical care for both patients and their families.
Methods
Patient recruitment
All patients included in this study were referred from medical genetics or ENT departments for apparent NSHL. They or their parents responded to a clinical questionnaire for family history and audiograms were performed to evaluate their degree of HL.
Informed consent to genetic testing was obtained from adult probands or parents in the case of minors after explanation of the nature and its possible implications for the patient and his family. This study was performed in accordance with the French law on bioethics: ‘loi de bioéthique’, revised 7 July 2011, number 2011-814. The experimental protocol is approved by the Montpellier University Hospital (CHU Montpellier) as part of the molecular diagnostic activity. The authorization number given by the Agence Régionale de la Santé (ARS) is LR/2013-N°190. Nominative licensing in the name of Dr. Roux was delivered by Agence de la Biomédecine.
Hearing loss assessment
Audiometric data were collected from the various departments and obtained using calibrated audiometers according to the International Organization for Standardization ISO28. In adults and children of at least 6 years old, bilateral air and bone conduction hearing thresholds (dB HL) were obtained for 0.25, 0.5, 1, 2, 4 and 8 kHz frequencies. For young children unable to undergo bilateral evaluation, open-field air and bone conduction thresholds were assessed using age-appropriate procedures (behavioural observation audiometry, visual reinforced audiometry or play audiometry). An electrophysiological assessment completed the audiological evaluation if necessary (i.e. auditory brainstem response thresholds, auditory steady state response, acoustic otoemission). Data from universal newborn screening were collected for the youngest patients.
Hearing loss degree was analysed according to the International Bureau for Audiophonology audiometric classification (https://www.biap.org/en/recommandations/recommendations/tc-02-classification/213-rec-02-1-en-audiometric-classification-of-hearing-impairments/file), whereas other phenotype features were analysed according to the recommendations for the description of non-syndromic hearing loss29. Sensorineural hearing loss was found in all cases.
DFNB1 locus screening
The screening was performed as recommended by the EMQN DFNB1 guidelines30 and included Sanger sequencing of GJB2 exons 1 and 2 and their flanking intronic regions and multiplex PCR as designed by del Castillo et al.31 to detect the two large deletions encompassing part of the GJB6 gene, del(GJB6-D13S1830) and del(GJB6-D13S1854).
Sequences were compared with the reference sequence using the Seqscape 2.5 software.
Gene-panel sequencing and bioinformatics
All patients underwent MPS gene-panel testing if DFNB1 locus screening showed no evidence of a pathogenic genotype (Fig. 1). Two panels were used. The first one included 65 genes and was designed with Illumina® Nextera Rapid Capture Custom Enrichment technology. An updated design of 74 DFN genes was performed and used for most of the patients (Supplementary Table 1) with NimbleGen SeqCap EZ Choice technology. All patients negative for the first panel were analysed with the updated 74 NSHL gene panel. The pros and cons of both enrichment technologies are detailed in32. Both designs were targeting the exons referenced in RefSeq or Ensembl (coding and non-coding) with 50 bp surroundings. Sequencing was performed on either an Illumina MiSeq instrument (using version 2 chemistry) or an Illumina MiniSeq system. As the two instruments have a similar output, the number of samples per run was the same (12 samples). The secondary analysis (mainly alignment and variant calling) was performed using the commercial software MiSeqReporter (v2.5) for the MiSeq runs and LocalRunManager (v1.3.1) for the MiniSeq runs, these workflows being very similar. Variant Calling Files (VCFs) were automatically included in our in-house database system (USHVaM2), which also handled variant annotation. In addition, when the pathogenic genotype was unclear, the samples were re-analysed using an in-house pipeline (Nenufaar, https://github.com/mobidic/nenufaar), which performed the secondary analysis and annotation of the variants. In particular, Nenufaar uses more recent software than the commercial pipelines and is better at retrieving small indels. Last, variants of interest were all confirmed by Sanger sequencing.
When STRC variants were identified, confirmation was performed by nested PCR. Briefly, STRC nested PCRs consist of a first long-range amplification (LR-PCR) excluding the ψSTRC pseudogene followed by nested amplifications targeting exons of interest and exon 20 to confirm pseudogene exclusion. The GoTaqLong PCR Promega kit (Promega) LR-PCR was used for LR-PCR according to the manufacturer’s recommendations and with primers described lesewhere33. LR-PCR products were diluted 1:1000 and then used for the nested PCRs. Primer sequences and reaction conditions for nested PCR are available on request.
Whole USH2A sequencing
Whole USH2A sequencing was performed for S1679 as already described by Liquori et al.26. Following the defined thresholds, a single variant susceptible to alter splicing was tested by minigene analysis.
Detection of copy number variants (CNVs)
In order to highlight the potential CNVs, which are not retrieved by the described pipelines, we used an in-house spreadsheet that computes the inter-sample normalized depth of coverage per exon in a given run. Potential CNVs were validated by array CGH using a Sure Print G3 CGH personal 4*180K custom design for 26 HL genes labelled with the SureTag Complete labelling kit. The array was analysed on an Agilent DNA microarray scanner C. For the remaining genes, quantitative multiplex PCR of short fluorescent fragments (QMPSF) designed with specific labelled primers was used when necessary.
In particular, suspected large deletions of STRC were confirmed by QMPSF using four sets of primers targeting specific STRC sequences (5′ sequence: forward: 5′-TAGCTGGGATTACAGGTGC-3′, reverse: FAM5′-CATTCACTACCGGGCGTAG; 3′ sequence: forward: 5′-GTTGCACCAGCTCCACCTAAG-3′, reverse: FAM5′-TGAGATCCTAAGGGATTAGGAC; Intron 16: forward: 5′-AGTGTTTGGTCCATTGTAAAGTC-3′, reverse: FAM5′-CCATTGTTCTTCTAATGTGGGTG; Intron 22: forward: 5′-TGGGTTCTACATGTGCTCTTCC-3′, reverse: FAM5′-TACAGAATTCTAGAACTACAAGAGG-3′) and one additional set screening exon 18 of the USH2A gene, as a control (forward: 5′-AAGTAACCCCTTTGTCTGATGAGT-3′, reverse: FAM5′-AAGACTCTGAACTCATACTTGGTG-3′).
For potential short STRC CNVs, binary alignment map (BAM) files were loaded into the integrative genomics viewer (IGV) software (v2.3.55)34 in order to globally inspect the aligned sequence reads between the STRC gene and the ψSTRC pseudogene.
Point mutations were validated with a long-range PCR assay specific to the STRC gene (specificity of the amplification was ascertained by the absence of the divergent c.4057C > T base pair) followed by nested PCRs.
Variant classification
Variants were classified using a fully described method35 (Supplementary Figure 1) that includes six classes from neutral to pathogenic and four classes of variants of unknown clinical significance. Variants predicting the inclusion of a PTC or located at canonical splicing positions (−2,−1, +1, +2 positions around exons) of isoforms described in pathology were considered a priori pathogenic. The main characteristics for missense classification were the familial segregation of the variant, its frequency in public databases, evolutionary conservation throughout orthologs and paralogs (domain conservation) and, when available, the impact on the 3D structure.
3D analysis of NP_002691.1: p.(Phe322Ser) was performed using human Oct-1 homeodomain encoded by POU2F1 (Protein Data Bank ID: 1E3O) binding DNA as the model. 1E3O shares 56% amino acids identity with pou4f3 through residues 182–332 and 66% (12/18) for residues surrounding pou4f3 position 322 in helix 3 (residues 141–158). The pictures were built using the PyMOL software (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC).
The potential impact on splicing for rare missense, isosemantic and intronic variants was also assessed using a local implementation of the MaxEnt algorithm36 and, when available, using dbscSNV results37. Minigene analysis was performed to experimentally verify the impact of selected variants on the splicing process, as described alsewhere38.
Data Availability
All DNA variants identified during the course of this study have been deposited into the public LOVD3 shared genetic database: https://databases.lovd.nl/shared/ or the public LOVD2 “Retinal and hearing impairment genetic mutation database” (https://grenada.lumc.nl/LOVD2/Usher_montpellier/).
References
Morton, C. C. & Nance, W. E. Newborn hearing screening–a silent revolution. N Engl J Med 354, 2151–2164, https://doi.org/10.1056/NEJMra050700 (2006).
Sloan-Heggen, C. M. et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum Genet 135, 441–450, https://doi.org/10.1007/s00439-016-1648-8 (2016).
Saihan, Z., Webster, A. R., Luxon, L. & Bitner-Glindzicz, M. Update on Usher syndrome. Curr Opin Neurol 22, 19–27 (2009).
Van Camp, G & Smith, R. J. H. Hereditary Hearing Loss Homepage. http://webhost.ua.ac.be/hhh/.
Kimberling, W. J. et al. Frequency of Usher syndrome in two pediatric populations: Implications for genetic screening of deaf and hard of hearing children. Genet Med 12, 512–516, https://doi.org/10.1097/GIM.0b013e3181e5afb8 (2010).
Bademci, G. et al. Variations in Multiple Syndromic Deafness Genes Mimic Non-syndromic Hearing Loss. Sci Rep 6, 31622, https://doi.org/10.1038/srep31622 (2016).
Sommen, M. et al. DNA Diagnostics of Hereditary Hearing Loss: A Targeted Resequencing Approach Combined with a Mutation Classification System. Hum Mutat 37, 812–819, https://doi.org/10.1002/humu.22999 (2016).
Mehta, D. et al. Outcomes of evaluation and testing of 660 individuals with hearing loss in a pediatric genetics of hearing loss clinic. Am J Med Genet A 170, 2523–2530, https://doi.org/10.1002/ajmg.a.37855 (2016).
Yan, D. et al. Spectrum of DNA variants for non-syndromic deafness in a large cohort from multiple continents. Hum Genet 135, 953–961, https://doi.org/10.1007/s00439-016-1697-z (2016).
Vona, B. et al. DFNB16 is a frequent cause of congenital hearing impairment: implementation of STRC mutation analysis in routine diagnostics. Clin Genet 87, 49–55, https://doi.org/10.1111/cge.12332 (2015).
Roux, A. F. et al. Molecular epidemiology of DFNB1 deafness in France. BMC Med Genet 5, 5–15 (2004).
Bademci, G. et al. Comprehensive analysis via exome sequencing uncovers genetic etiology in autosomal recessive nonsyndromic deafness in a large multiethnic cohort. Genet Med 18, 364–371, https://doi.org/10.1038/gim.2015.89 (2016).
Abou Tayoun, A. N. et al. Improving hearing loss gene testing: a systematic review of gene evidence toward more efficient next-generation sequencing-based diagnostic testing and interpretation. Genet Med 18, 545–553, https://doi.org/10.1038/gim.2015.141 (2016).
Consugar, M. B. et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genet Med 17, 253–261, https://doi.org/10.1038/gim.2014.172 (2015).
Hoppman, N. et al. Genetic testing for hearing loss in the United States should include deletion/duplication analysis for the deafness/infertility locus at 15q15.3. Mol Cytogenet 6, 19, https://doi.org/10.1186/1755-8166-6-19 (2013).
Di Donato, N. et al. Update on the ACTG1-associated Baraitser-Winter cerebrofrontofacial syndrome. Am J Med Genet A 170, 2644–2651, https://doi.org/10.1002/ajmg.a.37771 (2016).
Zhu, M. et al. Mutations in the gamma-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26). Am J Hum Genet 73, 1082–1091, https://doi.org/10.1086/379286 (2003).
Riviere, J. B. et al. De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat Genet 44, 440–444, S441–442, https://doi.org/10.1038/ng.1091 (2012).
Weegerink, N. J. et al. Genotype-phenotype correlation in DFNB8/10 families with TMPRSS3 mutations. J Assoc Res Otolaryngol 12, 753–766, https://doi.org/10.1007/s10162-011-0282-3 (2011).
Yildirim-Baylan, M. et al. Evidence for genotype-phenotype correlation for OTOF mutations. Int J Pediatr Otorhinolaryngol 78, 950–953, https://doi.org/10.1016/j.ijporl.2014.03.022 (2014).
Astuto, L. M. et al. CDH23 mutation and phenotype heterogeneity: a profile of 107 diverse families with Usher syndrome and nonsyndromic deafness. Am J Hum Genet 71, 262–275 (2002).
Liu, X. Z. et al. Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nat Genet 16, 188–190 (1997).
Ouyang, X. M. et al. Mutations in the alternatively spliced exons of USH1C cause non- syndromic recessive deafness. Hum Genet 111, 26–30 (2002).
Pera, A. et al. Functional assessment of allelic variants in the SLC26A4 gene involved in Pendred syndrome and nonsyndromic EVA. Proc Natl Acad Sci USA 105, 18608–18613, https://doi.org/10.1073/pnas.0805831105 (2008).
Le Guédard-Mereuze, S. et al. Ex vivo splicing assays of mutations at non-canonical positions of splice sites in USHER genes. Hum Mutat 31, 347–355 (2010).
Liquori, A. et al. Whole USH2A Gene Sequencing Identifies Several New Deep Intronic Mutations. Hum Mutat 37, 184–193, https://doi.org/10.1002/humu.22926 (2016).
Vaché, C. et al. Usher syndrome type 2 caused by activation of an USH2A pseudoexon: Implications for diagnosis and therapy. Hum mutat 33, 104–108, https://doi.org/10.1002/humu.21634 (2012).
Preferred test conditions for determining hearing thresholds for standardization. ISO/TC 43/WG 1 Threshold of hearing. International Organization for Standardization Technical Committee 43. Scand Audiol 25, 45–52 (1996).
Mazzoli, M. et al. Recommendations for the description of genetic and audiological data for families with nonsyndromic hereditary hearing impairment. Audiol Medicine 1, 148–150, https://doi.org/10.1080/16513860301713 (2003).
Hoefsloot, L. H., Roux, A. F. & Bitner-Glindzicz, M. EMQN Best Practice guidelines for diagnostic testing of mutations causing non-syndromic hearing impairment at the DFNB1 locus. Eur J of Hum Genet 21, 1325–1329, https://doi.org/10.1038/ejhg.2013.83 (2013).
del Castillo, F. J. et al. A novel deletion involving the connexin-30 gene, del(GJB6-d13s1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 non-syndromic hearing impairment. J Med Genet 42, 588–594 (2005).
Garcia-Garcia, G. et al. Assessment of the latest NGS enrichment capture methods in clinical context. Sci Rep 6, 20948, https://doi.org/10.1038/srep20948 (2016).
Mandelker, D. et al. Comprehensive diagnostic testing for stereocilin: an approach for analyzing medically important genes with high homology. J Mol Diagn 16, 639–647, https://doi.org/10.1016/j.jmoldx.2014.06.003 (2014).
Thorvaldsdottir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14, 178–192, https://doi.org/10.1093/bib/bbs017 (2013).
Baux, D., Vaché, C., Malcolm, S., Claustres, M. & Roux, A.-F. In Inner Ear Development and Hearing Loss (eds S. Riazuddin & Z. M. Ahmed) Ch. 10, 159–174 (Nova Science Publishers, Inc., 2013).
Yeo, G. & Burge, C. B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol 11, 377–394, https://doi.org/10.1089/1066527041410418 (2004).
Jian, X. & Liu, X. In Silico Prediction of Deleteriousness for Nonsynonymous and Splice-Altering Single Nucleotide Variants in the Human Genome. Methods Mol Biol 1498, 191–197, https://doi.org/10.1007/978-1-4939-6472-7_13 (2017).
Guédard-Méreuze, S. L. et al. Sequence contexts that determine the pathogenicity of base substitutions at position +3 of donor splice-sites. Hum Mutat 30, 1329–1339 (2009).
Acknowledgements
The authors are grateful to the patients and families. The authors acknowledge the clinicians Dr. Khau Van kien, Dr. Dollfus, Dr. Perthus, Dr. Boucher, Dr. Leheup, Dr. Moutton, Dr. Drumare and Dr. Collignon, for referring patients. GGG was a recipient of the E-rare “EURUSH” project at the time of the study.
Author information
Authors and Affiliations
Contributions
A.-F.R., M.K., M.C., D.B. and C.V. conceived the experiments. C.Ba., M.Moc., V.F., G.G.-G. and Z.A. performed the experiments. A.-F.R., C.V., D.B., C.Ba., M.Moc., V.F., G.G.-G. and Z.A. analysed the data. A.-F.R., C.V. and D.B. discussed the results and wrote the manuscript. C.Bl., M.W., R.T., B.I., D.-D.D., M.N., M.V., S.M., L.L., Y.P., F.G., C.C. and M.Mon. provided the clinical data and interpreted the phenotypes. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baux, D., Vaché, C., Blanchet, C. et al. Combined genetic approaches yield a 48% diagnostic rate in a large cohort of French hearing-impaired patients. Sci Rep 7, 16783 (2017). https://doi.org/10.1038/s41598-017-16846-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16846-9
This article is cited by
-
The prevalence and clinical features of MYO7A-related hearing loss including DFNA11, DFNB2 and USH1B
Scientific Reports (2024)
-
Identification and in vivo functional investigation of a HOMER2 nonstop variant causing hearing loss
European Journal of Human Genetics (2023)
-
Allelic hierarchy for USH2A influences auditory and visual phenotypes in South Korean patients
Scientific Reports (2023)
-
The natural history, clinical outcomes, and genotype–phenotype relationship of otoferlin-related hearing loss: a systematic, quantitative literature review
Human Genetics (2023)
-
Genome sequencing identifies coding and non-coding variants for non-syndromic hearing loss
Journal of Human Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.