Abstract
In-vitro studies suggest that vitamin D reduces inflammation by inhibiting nuclear factor kappa-B (NFκB) activity. Yet, no trials have examined the effects of vitamin D supplementation on NFκB activity in-vivo in humans. We conducted a double-blind randomized trial (RCT) examining effects of vitamin D supplementation on inflammatory markers and NFκB activity in peripheral blood mononuclear cells (PBMCs). Sixty-five overweight/obese, vitamin D-deficient (25-hydroxyvitamin D [25(OH)D] ≤ 50 nmol/L) adults were randomized to a single 100,000 IU bolus followed by 4,000 IU daily cholecalciferol or matching placebo for 16 weeks. We measured BMI, % body fat, serum 25(OH)D, high-sensitivity C-reactive protein (hsCRP), tumour necrosis factor (TNF), monocyte chemoattractant protein-1 (MCP-1), interferon-gamma (IFN-γ), several interleukins, and NFκB activity in PBMCs. Fifty-four participants completed the study. Serum 25(OH)D concentrations increased with vitamin D supplementation compared to placebo (p < 0.001). Vitamin D and placebo groups did not differ in any inflammatory markers or NFκB activity (all p > 0.05). Results remained non-significant after adjustment for age, sex, and % body fat, and after further adjustment for sun exposure, physical activity, and dietary vitamin D intake. Although in-vitro studies report anti-inflammatory effects of vitamin D, our RCT data show no effect of vitamin D supplementation on inflammatory markers or NFκB activity in-vivo in humans.
Similar content being viewed by others
Introduction
Chronic low-grade inflammation is common in obesity and plays an important role in the etiology of many chronic diseases including type 2 diabetes and cardiovascular disease1. While reducing obesity is effective in reducing inflammation and delaying disease onset and progression, weight-loss strategies on a population scale are difficult to achieve and maintain in the long-term1. Identification of additional easily modifiable risk factors is therefore needed to mitigate risk factors associated with chronic diseases, including inflammation.
Increasing evidence suggests that vitamin D may have a role in inflammation and immunoregulation2. Yet, vitamin D deficiency remains prevalent worldwide3. Most experts define vitamin D deficiency as 25-hydroxyvitamin D (25(OH)D) concentrations ≤ 50 nmol/L; however optimal concentrations required for extra-skeletal health are debated3. Nevertheless, it is of concern that 20–60% and 10–40% of the UK and US populations, respectively, have vitamin D levels < 50 nmol/L4. An adequate vitamin D status is achieved primarily via cutaneous synthesis upon exposure to ultraviolet (UV) B radiation, and can also be derived from diet and supplements5. However, the rising prevalence of obesity, sedentary indoor lifestyles, and current sun exposure restrictions to prevent skin cancer have made it difficult to obtain adequate vitamin D through sun exposure, and few foods are naturally high in vitamin D or vitamin D fortified6.
A role for vitamin D in influencing inflammatory and immune responses is supported by the presence of the nuclear vitamin D receptor (VDR) in most immune cells including monocytes, macrophages, and activated T and B lymphocytes [10]. In vitro studies report that active vitamin D (1,25-dihydroxyvitamin D3 [1,25(OH)2D3]) inhibits pro-inflammatory cytokine expression, promotes anti-inflammatory cytokine production, and regulates immune cell activity7,8,9. These effects are believed to occur partly via inhibition of the nuclear factor kappa-B (NFκB) pathway10,11,12,13,14, a transcription factor with a critical role in inflammatory and immune responses, as well as in the pathophysiology of several chronic diseases such as cancer, asthma, and diabetes15,16. In vivo administration of 1,25(OH) 2D3 in mice has been shown to modulate chemokine and cytokine expression and to prevent the development of inflammatory arthritis, type 1 diabetes, and autoimmune encephalomyelitis17. Animal models also show that treatment with 1,25(OH)2D3 reduces NFκB signaling by increasing VDR expression18,19.
Human studies are limited. To the best of our knowledge, no previous observational studies or randomized trials have examined the effect of vitamin D on NFκB activity in vivo in humans. For inflammatory markers, some20,21,22,23, but not all24,25, cross-sectional studies have reported inverse associations between 25(OH)D concentrations and C-reactive protein (CRP), tumor necrosis factor (TNF), and interleukin-6 (IL-6) in healthy21,22,23 or overweight/obese populations20. However, few good-quality randomized controlled trials (RCTs) have examined the effects of vitamin D supplementation on inflammatory markers. A recent systematic review of RCTs in overweight and obese individuals highlighted important limitations in existing trials including low supplementation doses, variability in participants’ vitamin D status, lack of assessment of lifestyle factors (diet, exercise, sun exposure), and the use of co-interventions including calcium and/or weight reduction programs in most trials26. These limitations have made it difficult to interpret findings.
We aimed to address these limitations by examining the effects of vitamin D supplementation on inflammatory markers and NFκB activity in overweight or obese but otherwise healthy individuals, with vitamin D-deficiency (25(OH)D ≤ 50 nmol/L), who receive a sufficient dose (≥4000 IU daily) of supplementation, in a clinical trial without co-interventions and with rigorous methods to minimize confounding by lifestyle factors. We hypothesized that vitamin D supplementation would decrease pro-inflammatory markers and NFκB activity and increase anti-inflammatory markers, compared with placebo.
Methods
Study Design and Participants
This study was part of a parallel-group, double-blind, randomized, placebo-controlled trial designed to assess the effects of vitamin D supplementation on insulin sensitivity and secretion, and a detailed trial protocol is published27. Briefly, 65 overweight/obese but otherwise healthy adults were recruited over a two-year period from the local community in Melbourne, Australia, via posters, flyers, email newsletters and online social media and community websites. Participants were screened and those with serum 25(OH)D concentrations ≤ 50 nmol/L on screening were recruited if they met the following inclusion criteria: aged 18–60 years, generally healthy on medical screening, overweight or obese (body mass index (BMI) ≥ 25 kg/m2; weight < 159 kg due to facility restrictions), with a stable weight (<5 kg change in the preceding year) and no intention to lose weight or change their diet or physical activity for the trial duration. Exclusion criteria included: smoking or high alcohol use (>4 and >2 standard drinks/week for men and women, respectively); use of medication, vitamins, or supplements; hypercalcemia or allergies; diabetes (previously diagnosed or based on oral glucose tolerance test [OGTT]) or any major diseases including active cancer in the preceding 5 years; or presence of acute inflammation (based on medical history or physical or laboratory examination). Women who were pregnant, lactating, or experiencing menopause were excluded.
Ethics
All participants provided written informed consent prior to commencing the trial. The trial was conducted according to the principles of the Declaration of Helsinki28 and received ethical approval from the Monash University Human Research Ethics Committee and Monash Health (Protocol ID: CF13/3874-2013001988). The trial was registered on clinicaltrials.gov (ID: NCT02112721) on April 10th, 2014.
Intervention and Randomization
Participants were randomized to either the vitamin D group receiving an initial bolus dose of 100,000 IU (in 2 capsules) followed by 4,000 IU (in 4 capsules) of cholecalciferol daily, or the placebo group receiving an equivalent number of identical placebo capsules, which were continued daily for a period of 16 weeks. The bolus dose selected is well below levels associated with toxicity or adverse effects29. All participants were instructed to consume the four capsules daily, and maintain their usual diet and exercise habits.
Randomization was performed as described previously27. Briefly, randomization was performed by an independent researcher not involved in the trial using a computerized random sequence generation program. Participants were randomized in blocks of four by sex and time of study entry (seasons) to ensure balance between sexes in each test group and to control for the effect of seasonal change. All capsules were identical and tasteless to maintain blinding, and all participants, investigators, and outcome assessors remained blinded until data analysis was complete. Compliance was assessed by the empty pill containers returned by participants at the final follow up visit and by post-intervention 25(OH)D concentrations.
Data Collection and Analyses
Detailed descriptions of data collection and outcome measures are reported in our published protocol27. Outcome measures were obtained at baseline prior to the initial bolus of vitamin D and repeated after 16 weeks of supplementation. Participants who were eligible on phone screening attended a medical screening which included medical history, physical examination (including anthropometry, and pregnancy tests for women), measurement of serum 25(OH)D concentrations to document vitamin D status, and an OGTT to exclude diabetes according to World Health Organization guidelines30.
Anthropometric and Lifestyle Assessments
Body composition was measured by dual X-ray absorptiometry (DEXA) (Monash Health Radiology, Melbourne). Body mass index (BMI) was calculated from weight (kg)/square of height (m), and waist and hip circumferences were used to establish the waist-to-hip ratio (WHR) as an additional index for body fat distribution (waist (mm)/hip (mm) = WHR). Participants completed validated questionnaires assessing self-reported sun exposure habits, physical activity (International Physical Activity Questionnaire)31, and diet (3-day food record; Foodworks 8.0 Professional, Xyris Software, Australia).
Blood Sample Analyses
Fasting venous blood samples were drawn at baseline, and then after the 16-week intervention using standard phlebotomy techniques into serum separation vacutainers for measurement of serum inflammatory markers and 25(OH)D concentrations, respectively. All blood samples were analyzed under blinded conditions using standard quality control systems (all results within ± 2 SD). Serum 25(OH)D and hsCRP were measured by an accredited and quality-assured laboratory (Monash Health Pathology, Melbourne). Serum 25(OH)D was measured using direct competitive chemiluminescent immunoassays (CLIA) (DiaSorin Inc., MN, USA) with inter- and intra-assay coefficients of variation (CVs) of <10% and <4%, respectively. Plasma high sensitivity CRP (hsCRP) was measured by highly sensitive near-infrared particle immunoassay rate methodology on a Synchron LX system analyzer (Beckman Coulter Inc., Sydney, Australia) according to manufacturer’s instructions. Inter- and intra-assay CVs for hsCRP were <3% and <5%, respectively. Inflammatory markers were measured using a bead-based multi-analyte assay (LEGENDplex™ Human Inflammation panel, Cat. 740118; Biolegend, San Diego, CA). This panel was used to simultaneously quantify 13 pro- and anti- inflammatory cytokines/chemokines including TNF, monocyte chemoattractant protein-1 (MCP-1), interferon alpha (IFN-α) and gamma (IFN- γ), and IL-1β, IL-6, IL-8, IL-10, IL-12, IL-17A, IL-18, IL-23, and IL-33. Serum samples were diluted 2-fold based on preliminary testing and the kit-specified protocol was performed on 96-well v-bottom plates as follows. Sonicated pre-mixed beads, serum samples, cytokine standards, and detection antibodies were added to each well and then plates were incubated (in the dark) on a plate shaker at 600 rpm for 2 hours at room temperature (RT). Following incubation, streptavidin–phycoerythrin (SA-PE) solution was added to each well and further incubated on a plate shaker for 30 min at RT. The conjugated beads were then centrifuged (1000 g, 5 minutes, RT) and pelleted, and resuspended in wash buffer before being transferred to mini FACS tubes for data acquisition. Data acquisition was performed using the BD™ LSR II flow cytometer and FACS DIVA software set up as per manufacturer’s instructions (Becton Dickinson, San Diego, CA). Data were analyzed using the LEGENDplex™ Data Analysis Software (BioLegend, San Diego, CA) with standard curves generated from 0 to 50,000 pg/ml, and samples adjusted for the dilution factors. Inter- and intra-assay CVs for all analytes were <8% and <9%, respectively. Of the 13 cytokines, two were undetectable (IFN-γ and IL17A) in most of the samples and were therefore excluded from the analysis.
Measurement of NFκB activity
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifuging fasting whole blood samples collected in BD Vacutainer® CPT™ cell preparation tubes with sodium citrate. The harvested PBMC pellet was resuspended in fetal bovine serum (FBS) with 10% dimethylsulphoxide (DMSO) and stored at −80 °C. Immediately prior to analysis, PBMC samples were thawed and washed in excess phosphate buffered saline (PBS) and centrifuged (400 g, 5 minutes, 4 °C) to remove residual FBS and DMSO. The supernatant was discarded and the pellets resuspended in 100 μL of triple detergent buffer. These were then sonicated for 1 minute and then centrifuged (400 g, 3 minutes, 4 °C). Resulting supernatant was used in the NFκB assay. Protein concentration of all protein isolates was determined using the Pierce Bicinchoninic Acid protein assay (Pierce, Rochford, IL), performed according to manufacturer’s instructions. The TransAM NFκB DNA-binding activity assay (Active Motif, Carlsbad, CA) was used to detect and quantify NFκB transcription factor activation, specifically of the p65 subunit. Nuclear extracts obtained from PBMC samples (30 μg protein per well) were analyzed for their binding capacity to an NFκB consensus sequence in labelled DNA in a 96 well plate format. The assays were performed according to manufacturer’s instructions and absorbance was measured on a Victor3V Multilabel plate reader (Perkin Elmer, Wellesley, MA). Results are expressed as pg p65 activity per μg protein (pg/μg protein).
Statistical Analyses
The sample size calculation has been reported elsewhere27, and was based on the main study where insulin sensitivity was the primary outcome. Analyses were performed on a per-protocol basis using Stata V.12.1 (StatCorp LP, USA). Shapiro-Wilk tests and visual inspection of histograms and scatterplots were used to assess normality with the assistance of an experienced biostatistician. Baseline characteristics are presented as mean ± SD and frequencies (%), or median (interquartile range [IQR]) for skewed variables. Due to the skewed distributions of the inflammatory markers and NFκB activity, all results were analyzed using non-parametric tests. Spearman correlations and Mann-Whitney U-tests were used to examine associations at baseline between 25(OH)D concentrations and continuous and categorical variables, respectively. Within-group differences were assessed using Wilcoxon signed-rank tests. Differences between treatments groups were assessed using Mann-Whitney U-tests and chi-squared tests for continuous and categorical variables, respectively. Efficacy of the intervention on the outcomes (between-group differences) were analyzed using changes in outcome variables and quantile multiple regression (to examine difference in medians). In quantile multiple regression, we adjusted for those variables which were significantly associated with the outcome measure based on Spearman correlations, as well as other clinically relevant variables including baseline values, age, sex, and % body fat, and factors which may affect vitamin D status including season, sun exposure, and dietary vitamin D intake. Pre-specified subgroups including those with baseline 25(OH)D ≤ 30 nmol/L were assessed as well as obese (BMI ≥ 30 kg/m2) subgroups. Further exploratory analyses were conducted in subgroups of participants with high baseline concentrations (≥median values) of selected inflammatory markers. All tests were two-sided and Bonferroni correction was used to adjust for multiple testing (p = 0.05/total number of tests (n = 13) = 0.004). Statistical significance was therefore set at p < 0.004.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Results
Sample and baseline characteristics
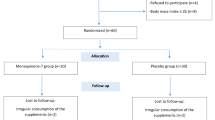
The participant flowchart is presented in Fig. 1. Of 1,072 participants screened for eligibility, 132 attended medical screening and 65 were successfully randomized between September 2014 and July 2016 (33 and 32 in the vitamin D and placebo groups, respectively) (Fig. 1). By the end of the study, nine participants had dropped out and two were withdrawn (one due to protocol violation and one due to an adverse event of thrombophlebitis after an intravenous glucose tolerance test). The remaining 54 participants (vitamin D group = 28 and placebo group = 26) completed the study and were analyzed in a blinded per-protocol fashion. Baseline demographic, anthropometric, and biochemical characteristics of both groups are presented in Table 1. Baseline characteristics did not differ between drop outs and non-drop outs (all p > 0.05) (data not shown).
Participant Flowchart: Numbers of participants who were recruited, randomized, dropped out, and analyzed during the trial. aMajority of interested participants did not meet criteria due to taking medication/supplements; not being overweight/obese; or not being interested after receiving a detailed description of study procedures. Abbreviations: 25(OH)D, 25-hydroxyvitamin D; OGTT, oral glucose tolerance test; PCOS, polycystic ovary syndrome; SSRIs, selective serotonin reuptake inhibitors.
Thirty-five male and 19 female participants aged 30 (25–36) years (median [IQR]) with a BMI of 30.1 (27.7–33.2) kg/m2, and % body fat of 39.6 ± 8.7% (mean ± SD) completed the study. Mean baseline serum 25(OH)D concentration was 32.7 ± 11.4 nmol/L (range = 9–50 nmol/L), with 43% of participants (n = 23) having a 25(OH)D concentration ≤ 30 nmol/L. Demographic, anthropometric and biochemical measures did not differ between the treatment groups at baseline, nor did physical activity, dietary intake of vitamin D (Table 1), or diet composition comprising of total energy, protein, fiber, fat, and carbohydrate intake (data not shown). Baseline 25(OH)D concentrations were not associated with age or anthropometric measures or with baseline values or change scores for any of the outcome measures (all p > 0.1; data not shown). CRP levels at baseline were positively associated with total % body fat (r = 0.40, p = 0.002) and fat mass (r = 0.30, p = 0.03) and both CRP and IL-33 were negatively associated with fat-free mass (r = −0.33, p = 0.01 and r = −0.27, p = 0.04, respectively). Patient characteristics including age and anthropometric variables were not associated with other inflammatory markers or NFκB activity at baseline (all p > 0.05; data not shown). NFκB activity at baseline was inversely associated with baseline levels of TNF, IL10, IL-1β, IL-8, IL-12, IL-23, and IL-33 (all p < 0.05; data not shown).
Changes in serum 25(OH)D concentrations
As previously reported32, mean serum 25(OH)D concentration after the 16-week intervention was 63.2 ± 32.1 (range = 9–147 nmol/L). Serum 25(OH)D concentrations increased significantly in the vitamin D group, from 31.4 ± 12.6 at baseline to 88.4 ± 21.0 nmol/L at 16 weeks (p < 0.001), with no change in the placebo group for the same time-period (34.2 ± 10.0 and 36.1 ± 15.3 nmol/L, respectively; p = 0.5) (Table 2). All but one participant in the vitamin D group achieved a 25(OH)D concentration ≥ 60 nmol/L, with 82% (n = 23/28) and 64% (n = 18/28) having 25(OH)D concentrations >70 and >75 nmol/L at follow-up, respectively.
Effect of vitamin D supplementation on serum pro- and anti- inflammatory markers and NFκB activity in PBMCs
There were no differences between vitamin D and placebo groups in change in hsCRP (−0.1 ± 1.4 versus −0.4 ± 2.3 mg/L, p = 0.9), TNF (−8.0 ± 109.4 versus −2.9 ± 103.9 pg/ml, p = 0.9), MCP-1 (−234.8 ± 1071.9 versus −58.8 ± 819.4 pg/ml, p = 0.6) or IFN-α (11.1 ± 85.8 versus −1.3 ± 22.7 pg/ml, p = 0.9). Similarly, no differences between vitamin D and placebo groups were found for any of the interleukins (all p > 0.1; Table 2). Changes in pro-/anti-inflammatory marker ratios including hsCRP/IL-10, TNF/IL-10 and IL-6/IL-10 ratios also did not differ between groups (data not shown). Change in NFκB activity in PBMCs did not differ between vitamin D and placebo groups (−17.8 ± 31.9 versus −13.2 ± 32.4 pg/μg protein, p = 0.7; Table 2); however change in NFκB activity within the vitamin D group (between baseline and follow up) trended toward significance (p = 0.05; Table 2).
Results for all inflammatory markers and NFκB activity remained non-significant in multiple regression analyses adjusted for baseline values, age, sex, ethnicity, and season of blood collection (all p > 0.1; Table 3, Model 1). Replacing ethnicity and season of blood collection in the multivariable model with factors which may affect vitamin D status including dietary vitamin D intake, sun exposure, and physical activity did not alter the results (all p > 0.1; Table 3, Model 2). Results remained non-significant in a third model adjusting for baseline values and factors which are clinically relevant to both vitamin D status and inflammation including change in % body fat, dietary vitamin D intake, diet composition (as fat/carbohydrate ratio), physical activity, and sun exposure (all p > 0.1; Table 3, Model 3). Addition of protein and/or fiber intake to the models as well as replacing % body fat with fat mass only or with BMI and WHR did not change the results (data not shown).
Sub-group Analyses
Results of the subgroup analyses are presented in Table 4. Pre-specified subgroup analysis was conducted in participants with 25(OH)D concentrations ≤30 nmol/L (n = 23; 15 males/8 females; age = 32.0 ± 7.6 years). In this subgroup, baseline 25(OH)D concentration was 21.7 ± 5.7 nmol/L (range = 9–29 nmol/L), with a mean BMI of 30.5 ± 4.2 kg/m2 and % body fat of 40.3 ± 8.7%. Baseline demographic, anthropometric, and biochemical characteristics did not differ between groups (data not shown). Serum 25(OH)D concentrations increased in the vitamin D group compared to placebo (59.9 ± 20.2 versus 6.9 ± 18.4 nmol/L, p < 0.001). There were no significant differences between vitamin D and placebo groups in any of the pro- or anti-inflammatory markers measured (all p > 0.1; Table 4). NFκB activity also did not differ between vitamin D and placebo groups in this subgroup (p = 0.3; Table 4).
Subgroup analysis was also conducted to explore the effects of vitamin D supplementation in obese participants (BMI ≥ 30 kg/m2) (n = 28; 13 males/15 females; age = 33.7 ± 9.0 years). Mean baseline 25(OH)D concentration in this subgroup was 34.6 ± 11.1 nmol/L (range = 9–50 nmol/L), with a BMI of 34.1 ± 3.8 kg/m2 and % body fat of 44.2 ± 8.4%. There were no differences between groups in demographic, anthropometric or biochemical characteristics at baseline. Changes in pro- and anti-inflammatory markers and NFκB activity did not differ between vitamin D and placebo groups in this subgroup of obese participants (Table 4). Further exploratory analyses were conducted to examine the effects of vitamin D supplementation in subgroups of participants with high baseline concentrations (≥median values) of selected inflammatory markers and NFκB activity. Changes in inflammatory markers and NFκB activity did not differ between vitamin D and placebo groups in subgroups of participants with high CRP (≥2.0 mg/L), IL-6 (≥21.0 pg/ml), TNF (≥28.0 pg/ml) or NFκB activity (≥37.0 pg/μg protein) (data not shown). Finally, we compared those in the vitamin D group who reached a 25(OH)D concentration >70 nmol/L (n = 23) or >75 nmol/L (n = 18) at follow up with the placebo group (n = 23 with 25(OH)D < 50 nmol/L at follow up) and found no differences in any of the parameters measured (all p > 0.05; data not shown).
Discussion
This randomized placebo-controlled trial examined the effect of oral cholecalciferol (100,000 IU bolus followed by 4,000 IU daily) supplementation for 16 weeks on pro- and anti-inflammatory markers and NFκB activity in overweight or obese but otherwise healthy individuals with vitamin D deficiency (25(OH)D ≤ 50 nmol/L). Despite a significant increase in 25(OH)D concentration in the vitamin D group, we found no differences between vitamin D and placebo groups in any of the pro- or anti- inflammatory markers measured, including hsCRP, TNF, MCP-1, IFN-α, and IL-1β, -6, -8, -10, -12, -18, -23, and -33, as well as no difference in NFκB activity in PBMCs. Adjustment for potential confounders did not alter the results and similar results were found in subgroup analyses of individuals with 25(OH)D ≤ 30 nmol/L, obese individuals (BMI > 30 kg/m2), or individuals with higher inflammatory marker or NFκB concentrations at baseline (concentrations ≥ median for hsCRP, TNF, IL-6, and NFκB activity).
Previous RCTs in overweight and obese but otherwise healthy adults have reported similar findings. A recent meta-analysis of 13 RCTs26 including 1,995 overweight and obese participants reported that vitamin D supplementation had no effect on plasma CRP, TNF, or IL-6 concentrations. With the exception of three trials33,34,35, all the included RCTs reported no effect of vitamin D supplementation on several pro- and anti-inflammatory markers including but not limited to hsCRP, TNF, MCP-1, and several interleukins (IL-2, -4, -5, -6, -8, -10, -12, -13, and -17)26. Of the three studies which found a beneficial effect, one had co-supplemented vitamin D with calcium33, and another combined vitamin D with a weight reduction program35. This makes results difficult to interpret since both calcium and weight loss have been shown to influence levels of circulating inflammatory markers36,37. The third study reporting that vitamin D supplementation improved inflammatory markers was limited to South American women34. This may explain their discrepant finding since polymorphisms in the VDR and metabolizing enzymes which affect the absorption and genetic functions of vitamin D are known to differ by ethnicity34,38.
Overall, existing RCTs in overweight and obese populations are limited by several important factors. First, most RCTs did not take into account participant smoking status, season, sun exposure, or dietary vitamin D intake – factors which may have influenced their results39,40. Second, 80% of existing RCTs were not in vitamin D-deficient individuals and this group has been proposed to benefit most from vitamin D supplementation41. Although we found no effect in our subgroup analysis of participants with 25(OH)D concentrations ≤ 30 nmol/L, this may be due to the small sample size. Importantly, the two largest RCTs33,42 (n = 928 and n = 332) in overweight and obese adults were in participants without vitamin D deficiency (baseline 25(OH)D range = 56–136 nmol/L), and both trials reported increased CRP concentrations after one year of supplementation with 20,000–40,000 IU of cholecalciferol weekly33,42. This suggests that high doses of vitamin D in non-deficient participants may be detrimental43, and highlights the need for similar large-scale studies that are restricted to individuals with vitamin D deficiency. Third, it has been suggested that at least 4,000 IU daily is required to raise 25(OH)D concentrations to optimal levels within 2–3 months44,45. Yet, more than half of the RCTs supplemented low doses (700–2000 IU daily) which may have been insufficient to adequately raise 25(OH)D concentrations. Here, we address these knowledge gaps by showing that vitamin D supplementation, provided in a sufficient dose to vitamin D-deficient individuals, did not improve circulating pro- or anti-inflammatory marker concentrations, which persisted after adjusting for multiple confounders. Based on our findings and those from most other studies, current evidence does not support the use of vitamin D supplementation for improving inflammation in overweight or obese, but otherwise healthy individuals.
However, vitamin D supplementation may be beneficial in other population groups including those with existing chronic or inflammatory diseases. RCTs and systematic reviews of RCTs have shown that vitamin D supplementation improved inflammatory marker concentrations in patients with chronic heart failure46, systemic lupus erythematosus47, inflammatory bowel disease48, and chronic obstructive pulmonary disease49. Moreover, although RCTs in prediabetes have shown no effect of vitamin D supplementation on inflammatory markers50,51,52, a meta- analysis by our group in patients with type 2 diabetes found that vitamin D-supplemented groups had lower levels of CRP and TNF compared to placebo (Mousa, unpublished data). It has been suggested that the effects of vitamin D on the immune system are more prominent when the immune system is impaired or stimulated53. Differences between studies may therefore be due to the presence of acute or chronic inflammation in participants with established diseases compared to their healthy counterparts. This is supported by a meta-analysis of mixed population groups (healthy and with existing diseases; n = 924)54, which found that vitamin D supplementation had a significantly greater effect on inflammation in patients with higher CRP levels (>5 mg/L) at baseline, compared to normal ranges. Subgroup analysis of participants with CRP levels >5 mg/L was not possible in our study due to the small number of participants with elevated CRP (n = 11). Moreover, although we found no differences between vitamin D and placebo groups in our subgroup analyses of participants with baseline concentrations > the median for CRP, TNF, IL-6, and NFκB activity, our sample size may have been too small to observe effects in these subgroups (n = 27). Future studies in healthy individuals should consider including large samples of vitamin D-deficient participants with a wide range of inflammatory marker concentrations at baseline in order to confirm whether vitamin D supplementation improves pro- and anti- inflammatory profiles in this population group.
We found no effect of vitamin D supplementation on NFκB activity in PBMCs. To the best of our knowledge, this is the first study to investigate the effects of vitamin D supplementation on NFκB activity in vivo in humans. Only experimental human studies and animal models have investigated this relationship to date, most of which reported that vitamin D inhibits NFκB activity in several human cell types including macrophages, fibroblasts, keratinocytes, pancreatic islet cells, and adipocytes10,11,12,13,14. These in vitro studies have consistently shown that treatment with active vitamin D (1,25(OH)2D3) increases mRNA and protein levels of IκBα (an inhibitor of nuclear translocation of NFκB), which in turn decreases NFκB activity and DNA-binding capacity, and reduces nuclear translocation of the NFκB p65 subunit10,11,12,13,14. However, some studies showed that 1,25(OH)2D3 can both stimulate and inhibit NFκB in different types of myeloid leukemic cells55,56. This suggests that vitamin D may influence NFκB activity in a cell- and tissue- specific manner. Similarly, in vivo animal models have shown that the VDR directly regulates the NFκB pathway57, and that treatment with active 1,25(OH)2D3 or analogs (eg: paricalcitol) increases VDR expression, with concomitant reductions in the NFκB signaling cascade18,19. Our finding of no effect of vitamin D supplementation on NFκB activity in PBMCs seems to conflict with the widely reported inhibitory effect of vitamin D in experimental and animal models. However, experimental studies have explored the active form of vitamin D, 1,25(OH)2D3, and have utilized different stimuli including bacteria, viruses, or agents like lipopolysaccharide (LPS) that activate transcription factors including NFκB58. Because our study measured 25(OH)D rather than 1,25(OH)2D3, and NFκB activity was measured in unstimulated PBMCs, these factors may partly explain the discrepancy between our findings and those from experimental studies. Moreover, vitamin D and NFκB may function differently in the natural biological environment in humans (ie: in vivo) compared to what is observed in animals or under experimental conditions. For instance, a study by Wamberg et al.14 in obese individuals found that 1,25(OH)2D3 treatment inhibited cytokine expression in vitro; however no effect was observed after in vivo treatment with 1,25(OH)2D3 in the same population. This suggests that in some circumstances, extrapolation of in vitro findings to the in vivo context may not be appropriate. Here, we show for the first time that vitamin D supplementation has no effect on in vivo NFκB activity in vitamin-deficient and overweight or obese but otherwise healthy individuals. Further studies are needed to corroborate our finding and to examine whether these effects may differ in other cell types or among other populations including those with existing inflammatory or chronic diseases.
Our study has several strengths, particularly the use of rigorous methodology and a double-blind randomized controlled design. This was the first study investigating the effects of vitamin D supplementation on NFκB activity in vivo in humans. We studied overweight or obese participants with vitamin D deficiency who were more likely to be at increased risk of inflammation and who have been proposed to benefit most from vitamin D supplementation. Compliance to study medication was high, with 82% of participants in the intervention group achieving 25(OH)D concentrations >70 nmol/L at follow-up. Our study sample comprised of a well-characterized cohort of healthy individuals where there was no confounding by disease status or medication use. We were able to adjust for important confounders including season, sun exposure, and dietary vitamin D intake- factors which have seldom been considered in previous studies.
Some limitations should be noted. Pro- and anti- inflammatory markers and NFκB activity were secondary outcomes of the main trial where the sample size calculation was based on changes in insulin sensitivity27. Our sample size was therefore not powered to detect differences in these outcomes or to draw valid conclusions from subgroup analyses. Moreover, most participants had hsCRP levels within a normal range (<5 mg/L) and narrow ranges for most of the other inflammatory markers. This may have contributed to our null findings since any changes in inflammatory markers would have been minimal, thereby requiring a much larger sample size in order to detect small effect sizes. Additionally, although 82% of participants in the vitamin D group reached a serum 25(OH)D level >70 nmol/L at follow up, it has been suggested that 25(OH)D concentrations as high as 80–100 nmol/L may be required for optimal immune function59, which may explain our lack of findings. Because our sample comprised of healthy young individuals, our results may not be generalizable to other populations including those with existing diseases. We measured NFκB activity in PBMCs, thus our results may not apply to other cell or tissue types. Potential contributions by genetic polymorphisms in the VDR or metabolizing enzymes were not explored in this study. Although in vitro data suggest that the biologically active vitamin D (1,25(OH)2D3) is involved in immunoregulation, we did not measure this form of vitamin D because circulating levels are tightly regulated60. Due to resource constraints, we were not able to measure 25(OH)D using the gold-standard liquid chromatography-mass spectrometry method, and instead used Diasorin assays which capture both 25(OH)D2 and 25(OH)D3 concentrations.
In summary, the present study addresses current knowledge gaps by providing high-doses of vitamin D supplementation to vitamin D-deficient individuals, without co-interventions, and with adjustment for important confounders – all of which are factors that have not been adequately addressed in previous trials. We found no beneficial effect of vitamin D supplementation on circulating pro- and anti-inflammatory markers or NFκB activity in overweight or obese but otherwise healthy individuals. Our results suggest that vitamin D may function differently in the human in vivo context compared to the inhibitory effects reported in in vitro and animal models. However, further trials in healthy individuals are needed to corroborate our findings, with the inclusion of: (1) large sample sizes with sufficient power to detect small changes; (2) participants with vitamin D-deficiency and a wider range of baseline inflammatory marker concentrations; and (3) assessment of whether vitamin D may affect in vivo NFκB activity in other cell and tissue types.
References
Pittas, A., Lau, J., Hu, F. & Dawson-Hughes, B. The role of Vitamin D and calcium in type 2 diabetes: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism 92, 2017–2029 (2007).
Hansdottir, S. & Monick, M. M. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm 86, 217–237, https://doi.org/10.1016/B978-0-12-386960-9.00009-5 (2011).
Holick, M. F. & Chen, T. C. Vitamin D deficiency: a worldwide problem with health consequences. The American journal of clinical nutrition 87, 1080S–1086S (2008).
Prentice, A. Vitamin D deficiency: a global perspective. Nutr Rev 66, S153–164, https://doi.org/10.1111/j.1753-4887.2008.00100.x (2008).
Wang, C. Role of vitamin d in cardiometabolic diseases. Journal of diabetes research 2013, 243934, https://doi.org/10.1155/2013/243934 (2013).
van Schoor, N. M. & Lips, P. Worldwide vitamin D status. Best practice & research. Clinical endocrinology & metabolism 25, 671–680, https://doi.org/10.1016/j.beem.2011.06.007 (2011).
Guillot, X., Semerano, L., Saidenberg-Kermanac’h, N., Falgarone, G. & Boissier, M. C. Vitamin D and inflammation. Joint, bone, spine: revue du rhumatisme 77, 552–557, https://doi.org/10.1016/j.jbspin.2010.09.018 (2010).
Lemire, J. M. Immunomodulatory role of 1,25-dihydroxyvitamin D3. Journal of cellular biochemistry 49, 26–31, https://doi.org/10.1002/jcb.240490106 (1992).
Muller, K., Odum, N. & Bendtzen, K. 1,25-dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol Lett 35, 177–182 (1993).
Cohen-Lahav, M., Shany, S., Tobvin, D., Chaimovitz, C. & Douvdevani, A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 21, 889–897, https://doi.org/10.1093/ndt/gfi254 (2006).
Giarratana, N. et al. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. Journal of immunology (Baltimore, Md.: 1950) 173, 2280–2287 (2004).
Harant, H., Wolff, B. & Lindley, I. J. 1Alpha,25-dihydroxyvitamin D3 decreases DNA binding of nuclear factor-kappaB in human fibroblasts. FEBS letters 436, 329–334 (1998).
Riis, J. L. et al. 1alpha,25(OH)(2)D(3) regulates NF-kappaB DNA binding activity in cultured normal human keratinocytes through an increase in IkappaBalpha expression. Archives of dermatological research 296, 195–202, https://doi.org/10.1007/s00403-004-0509-9 (2004).
Wamberg, L., Cullberg, K. B., Rejnmark, L., Richelsen, B. & Pedersen, S. B. Investigations of the anti-inflammatory effects of vitamin D in adipose tissue: results from an in vitro study and a randomized controlled trial. Hormone and metabolic research=Hormon- und Stoffwechselforschung=Hormones et metabolisme 45, 456–462, https://doi.org/10.1055/s-0032-1331746 (2013).
Harant, H., Andrew, P. J., Reddy, G. S., Foglar, E. & Lindley, I. J. 1alpha,25-dihydroxyvitamin D3 and a variety of its natural metabolites transcriptionally repress nuclear-factor-kappaB-mediated interleukin-8 gene expression. European journal of biochemistry/FEBS 250, 63–71 (1997).
Patel, S. & Santani, D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacological reports: PR 61, 595–603 (2009).
Gysemans, C. A. et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 146, 1956–1964, https://doi.org/10.1210/en.2004-1322 (2005).
Choi, M., Park, H., Cho, S. & Lee, M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine 63, 27–35, https://doi.org/10.1016/j.cyto.2013.03.018 (2013).
Tan, X., Wen, X. & Liu, Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. Journal of the American Society of Nephrology: JASN 19, 1741–1752, https://doi.org/10.1681/asn.2007060666 (2008).
Bellia, A. et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Internal and emergency medicine 8, 33–40, https://doi.org/10.1007/s11739-011-0559-x (2013).
Jablonski, K. L., Chonchol, M., Pierce, G. L., Walker, A. E. & Seals, D. R. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57, 63–69, https://doi.org/10.1161/hypertensionaha.110.160929 (2011).
Laird, E. et al. Vitamin D Deficiency Is Associated With Inflammation in Older Irish Adults. The Journal of clinical endocrinology and metabolism, jc20133507, https://doi.org/10.1210/jc.2013-3507 (2014).
Timms, P. M. et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? Qjm 95, 787–796 (2002).
Azizieh, F., Alyahya, K. O. & Raghupathy, R. Association between levels of vitamin D and inflammatory markers in healthy women. Journal of Inflammation Research 9, 51–57, https://doi.org/10.2147/JIR.S103298 (2016).
Peterson, C. A. & Heffernan, M. E. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. Journal of inflammation (London, England) 5, 10, https://doi.org/10.1186/1476-9255-5-10 (2008).
Jamka, M. et al. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: a systematic review with meta-analysis. European journal of nutrition 55, 2163–2176, https://doi.org/10.1007/s00394-015-1089-5 (2016).
de Courten, B. et al. Vitamin D supplementation for the prevention of type 2 diabetes in overweight adults: study protocol for a randomized controlled trial. Trials 16, 335, https://doi.org/10.1186/s13063-015-0851-6 (2015).
WMA. World Medical Association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA: the journal of the American Medical Association 310, 2191–2194, https://doi.org/10.1001/jama.2013.281053 (2013).
Hathcock, J. N., Shao, A., Vieth, R. & Heaney, R. Risk assessment for vitamin D. The American journal of clinical nutrition 85, 6–18 (2007).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic medicine: a journal of the British Diabetic Association 15, 539–553, https://doi.org/10.1002/(sici)1096-9136(199807)15:7<539::aid-dia668>3.0.co;2-s (1998).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise 35, 1381–1395, https://doi.org/10.1249/01.mss.0000078924.61453.fb (2003).
Mousa, A. et al. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: a randomized placebo-controlled trial. American Journal of Clinical Nutrition (2017).
Beilfuss, J., Berg, V., Sneve, M., Jorde, R. & Kamycheva, E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine 60, 870–874, https://doi.org/10.1016/j.cyto.2012.07.032 (2012).
de Medeiros Cavalcante, I. G. et al. Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: Vitamin D3 megadose reduces inflammatory markers. Exp Gerontol 66, 10–16, https://doi.org/10.1016/j.exger.2015.03.011 (2015).
Zittermann, A. et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. The American journal of clinical nutrition 89, 1321–1327, https://doi.org/10.3945/ajcn.2008.27004 (2009).
Huang, J. H., Tsai, L. C., Chang, Y. C. & Cheng, F. C. High or low calcium intake increases cardiovascular disease risks in older patients with type 2 diabetes. Cardiovascular diabetology 13, 120, https://doi.org/10.1186/s12933-014-0120-0 (2014).
Peake, J. M., Kukuljan, S., Nowson, C. A., Sanders, K. & Daly, R. M. Inflammatory cytokine responses to progressive resistance training and supplementation with fortified milk in men aged 50+ years: an 18-month randomized controlled trial. European journal of applied physiology 111, 3079–3088, https://doi.org/10.1007/s00421-011-1942-z (2011).
Mackawy, A. M. H. & Badawi, M. E. H. Association of vitamin D and vitamin D receptor gene polymorphisms with chronic inflammation, insulin resistance and metabolic syndrome components in type 2 diabetic Egyptian patients. Meta gene 2, 540–556, https://doi.org/10.1016/j.mgene.2014.07.002 (2014).
Chandler, P. D. et al. Impact of vitamin D supplementation on inflammatory markers in African Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer prevention research (Philadelphia, Pa.) 7, 218–225, https://doi.org/10.1158/1940-6207.capr-13-0338-t (2014).
Ziff, O. J., Penny, H., Frame, S., Cronin, A. & Goldsmith, D. Impact of seasonality on the dynamics of native Vitamin D repletion in long-term renal transplant patients. Clinical Kidney Journal, sfw136 (2017).
Pittas, A. G. et al. Systematic review: Vitamin D and cardiometabolic outcomes. Annals of internal medicine 152, 307–314, https://doi.org/10.7326/0003-4819-152-5-201003020-00009 (2010).
Jorde, R., Strand Hutchinson, M., Kjaergaard, M., Sneve, M. & Grimnes, G. Supplementation with High Doses of Vitamin D to Subjects without Vitamin D Deficiency May Have Negative Effects: Pooled Data from Four Intervention Trials in Tromso. ISRN endocrinology 2013, 348705, https://doi.org/10.1155/2013/348705 (2013).
Jorde, R., Hutchinson, M. S., Kjærgaard, M., Sneve, M. & Grimnes, G. Supplementation with High Doses of Vitamin D to Subjects without Vitamin D Deficiency May Have Negative Effects: Pooled Data from Four Intervention Trials in Tromsø. ISRN Endocrinology, 1–7 7p, doi:2013/348705 (2013).
Ilahi, M., Armas, L. A. & Heaney, R. P. Pharmacokinetics of a single, large dose of cholecalciferol. The American journal of clinical nutrition 87, 688–691 (2008).
Vieth, R., Chan, P. C. & MacFarlane, G. D. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. The American journal of clinical nutrition 73, 288–294 (2001).
Jiang, W. L. et al. Vitamin D Supplementation in the Treatment of Chronic Heart Failure: A Meta-analysis of Randomized Controlled Trials. Clin Cardiol 39, 56–61, https://doi.org/10.1002/clc.22473 (2016).
Abou-Raya, A., Abou-Raya, S. & Helmii, M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol 40, 265–272, https://doi.org/10.3899/jrheum.111594 (2013).
Nicholson, I., Dalzell, A. M. & El-Matary, W. Vitamin D as a therapy for colitis: a systematic review. J Crohns Colitis 6, 405–411, https://doi.org/10.1016/j.crohns.2012.01.007 (2012).
Rezk, N. A. S. A., Aly, N. Y. A. & Hewidy, A. A. H. Effect of vitamin D replacement in chronic obstructive pulmonary disease patients with vitamin D deficiency. Egyptian Journal of Chest Diseases and Tuberculosis 64, 353–357, https://doi.org/10.1016/j.ejcdt.2015.01.002 (2015).
Sinha-Hikim, I. et al. Effect of long term vitamin D supplementation on biomarkers of inflammation in Latino and African-American subjects with pre-diabetes and hypovitaminosis D. Horm Metab Res 47, 280–283, https://doi.org/10.1055/s-0034-1383652 (2015).
Sollid, S. T. et al. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care 37, 2123–2131, https://doi.org/10.2337/dc14-0218 (2014).
Tuomainen, T. P. et al. Glucose Metabolism Effects of Vitamin D in Prediabetes: The VitDmet Randomized Placebo-Controlled Supplementation Study. J Diabetes Res 2015, 672653, https://doi.org/10.1155/2015/672653 (2015).
Jorde, R. et al. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine 50, 175–180, https://doi.org/10.1016/j.cyto.2009.12.006 (2010).
Chen, N. et al. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients 6, 2206–2216, https://doi.org/10.3390/nu6062206 (2014).
Hughes, P. J., Lee, J. S., Reiner, N. E. & Brown, G. The vitamin D receptor-mediated activation of phosphatidylinositol 3-kinase (PI3Kalpha) plays a role in the 1alpha,25-dihydroxyvitamin D3-stimulated increase in steroid sulphatase activity in myeloid leukaemic cell lines. Journal of cellular biochemistry 103, 1551–1572, https://doi.org/10.1002/jcb.21545 (2008).
Tse, A. K.-W. et al. 1,25-Dihydroxyvitamin D3 induces biphasic NF-κB responses during HL-60 leukemia cells differentiation through protein induction and PI3K/Akt-dependent phosphorylation/degradation of IκB. Experimental Cell Research 313, 1722–1734, https://doi.org/10.1016/j.yexcr.2007.02.022 (2007).
Wu, S. et al. Vitamin D Receptor Negatively Regulates Bacterial-Stimulated NF-κB Activity in Intestine. The American Journal of Pathology 177, 686–697, https://doi.org/10.2353/ajpath.2010.090998 (2010).
Wöbke, T. K., Sorg, B. L. & Steinhilber, D. Vitamin D in inflammatory diseases. Frontiers in physiology 5, 244, https://doi.org/10.3389/fphys.2014.00244 (2014).
Daly, R. M. in Nutritional Influences on Bone Health: 8th International Symposium (ed P. Burckhardt, Dawson-Hughes, B., Weaver, C.M.) (Springer-Verlag, London, 2013).
Shea, M. K. et al. Vitamin K and Vitamin D Status: Associations with Inflammatory Markers in the Framingham Offspring Study. American Journal of Epidemiology 167, 313–320, https://doi.org/10.1093/aje/kwm306 (2008).
Acknowledgements
We thank all volunteers who participated in the trial. We thank our research assistants Mrs. Nicole Ng and Mrs. Rebecca Chandra for their assistance in running the trial; Dr. Eveline Jona and Dr. Melanie Gibson-Helm for the random assignment of participants; and Prof. Helena Teede for her support during the trial. AM and NN are recipients of the Australian Postgraduate Award Scholarships provided by Monash University. BdC is supported by National Heart Foundation Future Leader Fellowship (100864) and is a recipient of the National Health and Medical Research Council (NHMRC) grant which funded this trial (APP1047897). MP is recipient of a NHMRC Senior Research Fellowship (APP490977).
Author information
Authors and Affiliations
Contributions
A.M. conducted the research, analyzed the data, and wrote the first draft of the paper. N.N. conducted the research and contributed to data interpretation and writing and editing the manuscript. J.J., K.S., K.W. and M.P. performed the laboratory analyses and contributed to writing and editing the manuscript. R.S. and M.d.C. contributed to study design, obtained funding for the trial, contributed to data interpretation, and writing and editing the manuscript. B.d.C. is the chief investigator of the trial and planned and designed the study, obtained funding, oversaw data collection and analysis, and supervised data interpretation and writing and editing of the manuscript. B.d.C. is the guarantor of this work and takes responsibility for data integrity and accuracy. All authors meet the ICMJE criteria for authorship, and have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mousa, A., Naderpoor, N., Johnson, J. et al. Effect of vitamin D supplementation on inflammation and nuclear factor kappa-B activity in overweight/obese adults: a randomized placebo-controlled trial. Sci Rep 7, 15154 (2017). https://doi.org/10.1038/s41598-017-15264-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15264-1
This article is cited by
-
Signaling pathways of dental implants’ osseointegration: a narrative review on two of the most relevant; NF-κB and Wnt pathways
BDJ Open (2024)
-
1α,25-Dihydroxyvitamin D3 (VD3) Shows a Neuroprotective Action Against Rotenone Toxicity on PC12 Cells: An In Vitro Model of Parkinson’s Disease
Neurochemical Research (2023)
-
Vitamin D supplementation and cardiac tissue inflammation in obese rats
BMC Nutrition (2022)
-
Vitamin D3 decreases TNF-α-induced inflammation in lung epithelial cells through a reduction in mitochondrial fission and mitophagy
Cell Biology and Toxicology (2022)
-
The Role of Nutrition on Meta-inflammation: Insights and Potential Targets in Communicable and Chronic Disease Management
Current Obesity Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.