Abstract
We developed a novel type of Meju starter culture using single and combined extracts of Allium sativum (garlic clove), Nelumbo nucifera (lotus leaves), and Ginkgo biloba (ginkgo leaves) to improve the quality and functionality of Meju-based fermented products. Meju samples fermented with plant extracts (10 mg/ml) showed phenolic contents of 11.4–31.6 mg/g (gallic acid equivalents). Samples of extracts (garlic clove, lotus leaves, ginkgo leaves and their combination) fermented with Meju strongly inhibited tyrosinase, α-glucosidase, and elastase activities by 36.43–64.34%, 45.08–48.02%, and 4.52–10.90%, respectively. Specifically, ginkgo leaves extract added to fermented Meju samples at different concentrations (1% and 10%) strongly inhibited tyrosinase, α-glucosidase, and elastase activities and exhibited a potent antibacterial effect against Bacillus cereus with a significant reduction in bacterial counts compared with the effects observed for garlic clove and lotus leaf added to Meju samples. Scanning electron microscopy revealed severe morphological alterations of the B. cereus cell wall in response to ginkgo leaf extracts. Gas chromatographic mass spectroscopic analysis of plant extract-supplemented Meju samples and control Meju samples identified 113 bioactive compounds representing 98.44–99.98% total extract. The proposed approach may be useful for the development of various fermented functional foods at traditional and commercial levels.
Similar content being viewed by others
Introduction
Korean soy foods are becoming increasingly widespread in the global market. Kochujang (fermented red pepper paste with soybean, flour, and glutinous rice) and fermented soybean paste products (Doenjang and Chungkukjang) were registered in the Codex Alimentarius in July 2009 and are now internationally accepted foods1. Meju is a Korean traditional starter culture used to ferment various Korean traditional sauces, such as Doenjang. Meju is prepared from cooked soybean blocks as the main ingredient. During the natural drying period, live microorganisms, such as bacteria and fungi (e.g., yeast) with enzymatic activities, are cultivated2, 3. Although Meju manufactured by traditional methods has a favorable taste and adds functionality to fermented soybean foods, its safety remains controversial because of the presence of naturally occurring microorganisms associated with traditional Meju fermentation. Therefore, several attempts have been made to develop Meju manufacturing methods ensuring improved food safety by utilizing effective and beneficial microorganisms as the starter culture4.
Many synthetic antioxidants such as butylated hydroxyl anisole and butylated hydroxyl toluene are effective and are used for industrial processing. However, these synthetic chemicals possess toxic properties that pose potential risks to human health and should be replaced with natural antioxidants5. Additionally, exposing the skin to ultraviolet (UV) light results in the activation of reactive oxygen species (ROS) such as singlet oxygen and the superoxide anion radical, which can attack tissues in the dermis or epidermis, thus causing skin pigmentation5. Elastin is an extracellular-matrix protein providing elasticity to connective tissues6. Elastase is a proteinase enzyme that catalyzes the degradation of elastin7. Therefore, inhibition of elastase activity may be used as a method for protecting against skin aging8. Because accumulation of excessive epidermal pigmentation leads to various dermatological disorders, such as melasma associated with aging, freckling, age spots, and sites of actinic damage, tyrosinase inhibitors have become increasingly popular as medications and cosmetics for preventing hyperpigmentation through inhibition of enzymatic oxidation9. Hence, compounds derived from natural sources capable of protecting against ROS-mediated damage may have potential applicability in the prevention and/or treatment of skin diseases. Thus, there is a need to identify compounds that inhibit tyrosinase activity. Phenolic and flavonoid compounds derived from herb spices have been reported to be associated with biological activities such as tyrosinase inhibitory and anti-cholinesterase effects10. α-Glucosidase inhibitors reduce the rate of carbohydrate digestion and delay carbohydrate absorption in the digestive tract. Therefore, α-glucosidase inhibitors have the potential to prevent the development of type 2 diabetes by lowering after-meal glucose levels11. Plants and microorganisms are known as rich sources of α-glucosidase inhibitors11.
Bacillus cereus (B. cereus) is commonly found in contaminated foods containing fermented soybeans, such as Doenjang. The South Korean food authority has reported that ingestion of more than 104 colony-forming units (CFUs) of B. cereus per gram of fermented soybean products may cause food poisoning12. It is well known that Korean traditional soybean paste products such as Meju and Doenjang are made by natural fermentation driven by various fungi and bacteria. Meju produced in this manner may be a good growth medium for B. cereus, which is known to cause food poisoning and can affect the production of soybean paste13. Furthermore, it has been reported that Meju fermentation is closely associated with rice straw because rice straw remains directly in contact with soybeans in Meju during the fermentation process (used for hanging soybean bricks in a natural environment), which provides Meju with various types of natural microflora14. In a recent survey, Park et al.15 confirmed the toxin profile of B. cereus and Bacillus thuringiensis (B. thuringiensis) in Korean fermented soybean food. Because spores of B. cereus are highly resistant to various stressors (heat, cold, radiation, desiccation, and disinfectants) and show excellent adhesion to food surfaces, B. cereus contamination is difficult to control in the fermented soybean food industry16. Various methods for inactivating B. cereus have been reported as control strategies. The use of plant-based bactericidal agents has become the most popular method for controlling B. cereus counts in food products. In recent years, biological methods in combination with medicinal plants that reduce microbial contamination levels in foods and feeds have been applied.
One important problem faced in the food sector is that B. thuringiensis is closely related to B. cereus; however, it can be distinguished from pathogenic strains of B. cereus through its ability to produce enterotoxin. The pathogenic spectrum of B. cereus ranges from strains that are used as human probiotics to lethal, highly toxic strains17. B. cereus is thus complex, harboring strains differing considerably in terms of their economic and sanitary importance. It is therefore important to determine the extent to which pathogenic strains can be distinguished from non-pathogenic strains. However, at the chromosomal level, there are currently no specific markers for unambiguously differentiating pathogenic from non-pathogenic strains. The pathogenicity of B. cereus depends on its ability to colonize and persist in the host and subsequently invade tissues. Kamar et al. (2013) reported that food poisoning and clinical strains can be differentiated from non-pathogenic strains based on host colonization phenotypes17. However, in the present work, we focused on determining how to reduce or eliminate B. cereus counts by considering a broad spectrum in which all identified strains might exhibit pathogenic characteristics.
Ginkgo biloba (G. biloba), Allium sativum (A. sativum), and Nelumbo nucifera (N. nucifera) are widely reported to have several biological activities, including antimicrobial, anti-proliferative, hepato-protective, and anti-angiogenic effects18,19,20. Based on these functional and biological activities, we aimed to develop a new strategy by using a combination of these plant extracts (G. biloba, A. sativum, and N. nucifera) at different concentrations (1% and 10% and/or a mixture of these extracts in a ratio of 1:1:1) for the production of soybean-based food products with improved quality. In our preliminary study, different concentrations of plant extracts (0.1%, 1%, 5%, and 10%) were tested for their antimicrobial efficacy against different food-borne pathogenic bacteria, including B. cereus strains that grow during Meju fermentation. Based on their potent anti-microbial efficacy during fermentation, two effective concentrations (1% and 10%) of plant extracts and a mixture of these extracts in a ratio of 1:1:1 were selected for the production of Meju samples.
Although production of commercial Doenjang products has been achieved using herbal products such as garlic and dried lotus leaves, in terms of health benefits, no systematic studies on Doenjang have been conducted on these plant extracts individually or in combination. Therefore, the aim of this study was to develop various types of novel Meju products by single and/or multiple inoculation of ethanolic extracts of various medicinal plants: N. nucifera (lotus) leaves, G. biloba (ginkgo) leaves, and A. sativum (garlic) cloves. Furthermore, we evaluated total phenolic content and α-glucosidase inhibitory effects as well as the anti-bacterial, anti-tyrosinase, and anti-elastase activities of the Meju samples created using single or multiple inoculation of ethanolic extracts derived from medicinal plants to confirm their biological and nutritional properties and quality characteristics. Furthermore, gas chromatography-mass spectrometry (GC-MS) analysis was performed on the Meju samples to evaluate their chemical composition.
Materials and Methods
Chemicals and reagents
Folin-Ciocalteu’s reagent, gallic acid, quercetin, mushroom tyrosinase, 3,4-dihydroxy-L-phenylalanine (DOPA), p-nitrophenyl-α-D-glucopyranoside, yeast α-glucosidase, elastase, aluminum chloride, sodium carbonate, hydrochloric acid, sodium chloride, sodium phosphate, methanol, ethanol, p-iodonitrotetrazolium violet, glucose, Tris base, potassium phosphate monobasic, potassium phosphate dibasic, kojic acid, ursolic acid, N-methyl-N-(trimethylsilyl)-trifluoroacetamide, methoxyamine hydrochloride, and pyridine were purchased from Sigma–Aldrich (St. Louis, MO, USA). Brain heart infusion (BHI), nutrient agar (NA), and nutrient broth (NB) were purchased from Difco (Franklin Lakes, NJ, USA), and mannitol-egg yolk-polymyxin agar was purchased from Oxoid Ltd. (Hampshire, UK). An analytical profile index (API kit) was acquired from (MYP) bioMerieux (Marcy l’Etoile, France), and 96-well microplates were obtained from SPL Life Sciences (Pocheon-si, Gyeonggi-do, Korea). Acarbose was purchased from Tokyo Chemical Industries (Toshima, Tokyo, Japan).
Bacterial strain
B. cereus (KCCM 40935) used in this study was purchased from the Korean Culture Center of Microorganisms (KCCM; Seoul, Korea). Bacteria were cultured in NB for 18 h at 37 °C in a shaking incubator (150 rpm) and stored at −20 °C in glycerol stock form.
Plant materials and preparation of extracts
All selected plant materials, including lotus leaves, ginkgo leaves, and garlic cloves were collected locally in Gyeongsan City, Korea, after which they were washed, cut, and stored at −20 °C for further processing. For preparation of ethanolic extracts, dried plant materials (100 g) were extracted with a 20-fold volume of 70% ethanol for 3 h at 65 °C. The extracts were filtered through Whatman No. 2 filter paper (Advantec; Tokyo, Japan). Filtrates of the ethanolic extracts were concentrated using a vacuum evaporator, freeze-dried, and stored at −20 °C for further use.
Production of Meju fermented with plant extracts
Meju was produced by combining different concentrations of individual ethanolic extracts derived from different medicinal plants and their combinations. The plant extract ratios in Meju are summarized in Table 1. To produce Meju, soybeans (as per the required amount in a Meju product) were steamed in a suitable pot and crushed, after which the ethanolic extract of each plant species (lotus leaves, ginkgo leaves, and garlic cloves) and its mixture (1:1:1 ratio) were mixed at 1% (w/w) and 10% (w/w) concentrations in a square stainless-steel plate. All the mixtures were molded into a brick shape, hung with rice straw, and allowed to ferment naturally for 60 days. Traditional Meju fermented without ethanolic extracts of medicinal plants served as a control. All Meju samples were prepared in triplicate (Figure S1).
Physicochemical properties of plant extract-supplemented Meju samples
The physicochemical (moisture, ash, and salt contents) or organoleptic properties (sensory effects) of the plant extract-supplemented Meju samples were determined by the methods of the Association of Official Analytical Chemists21 (AOAC, 2006). For pH measurement of each plant extract-supplemented Meju product, the samples were diluted 10-fold with distilled water and then homogenized, followed by filtration through Whatman No. 2 filter paper (Advantec, Tokyo, Japan). The pH level was measured using a pH meter (Orion 35 star pH Benchtop, Thermo Electron Corporation; Beverly, MA, USA).
Extraction procedure
Meju samples (10 g each) prepared using the ethanolic plant extracts were subjected to reflux extraction with 200 ml of distilled water for 3 h at 70 °C, followed by filtration through Whatman No. 2 filter paper. The residue was again extracted twice with an equal volume of water for 3 h. The filtrate was then freeze-dried. The yields of extracts of Meju samples were in the range of 22.1–25.25%.
Determination of total phenolic content
The total phenolic content of each Meju sample prepared from the ethanolic plant extracts was determined using Folin-Ciocalteu’s reagent with gallic acid as a standard phenolic compound22. Briefly, 20 µl of each diluted Meju sample extract (1 mg/ml) was added to 100 µl of Folin-Ciocalteu’s reagent. After 3 min, 80 ml of a 10% aqueous sodium carbonate solution was added to the mixture. The solution was allowed to stand for 1 h at room temperature (RT), and absorbance of the resulting blue mixture was measured at 765 nm against a blank containing only the extraction solvent (200 µl). Total phenolic content was calculated as gallic acid equivalents (GAE) via the calibration curve constructed using the gallic acid standard solution and expressed in mg GAE/g dry mass.
Determination of total flavonoid content
The total flavonoid content of each Meju sample prepared from ethanolic plant extracts was determined by the colorimetric method23. Briefly, 100 μl of each extract or standard reagent and 400 μl of ethanol were mixed with 500 μl of a 2% AlCl3 solution (diluted in distilled water). After 1 h of incubation at RT, absorbance was measured at 430 nm. Quercetin served as a reference compound to generate a standard curve, and results were expressed in milligrams of quercetin equivalents (mg QE/g dry mass).
Determination of tyrosinase inhibitory activity of Meju samples
Tyrosinase inhibitory activity of Meju samples supplemented with plant extracts was determined by a method described previously24 with some modifications. Briefly, different concentrations (5, 10, 20, or 50 mg/ml) of each Meju sample (70 µl each) were separately added to a reaction mixture containing 40 µl of a 10 mM DOPA solution, 50 µl of 0.175 M sodium phosphate buffer (pH 6.8), and 40 µl of a mushroom tyrosinase solution (110 U/ml). The reaction mixture was allowed to incubate at 37 °C for 2 min, after which the amount of dopachrome produced was measured at 475 nm on a microtiter plate reader. Kojic acid (62.5, 125, 250, 500 and 1000 µg/ml) was used as a standard compound for a positive control. The percentage of tyrosinase inhibition was calculated as follows:
Determination of α-glucosidase inhibitory activity of Meju samples
α-Glucosidase inhibitory activity of Meju samples supplemented with plant extracts was evaluated according to a chromogenic method25, with minor modifications. Briefly, various concentrations (5, 10, 20, and 50 mg/ml) of Meju samples (50 µl) and 100 µl of α-glucosidase (1.0 U/ml) dissolved in 0.1 M phosphate buffer (pH 6.9) were mixed in a 96-well microplate and incubated at 25 °C for 10 min. After pre-incubation, 50 µl of p-nitrophenyl-α-D-glucopyranoside (5 mM) in 0.1 M phosphate buffer (pH 6.9) was added into each well as a substrate solution. The reaction mixture was incubated at 25 °C for 5 min. Absorbance was recorded using a microtiter plate reader at 405 nm before and after incubation with a p-nitrophenyl-α-D-glucopyranoside solution and compared with that of a control containing only 50 µl of the buffer. Acarbose at various concentrations (31.3, 62.5, 125, 250, 500, 1000, and 2000 µg/ml) was used as a standard compound. Each experiment was conducted in triplicate, and the enzyme inhibitory rates of the samples were calculated as follows:
Determination of elastase inhibitory activity
Elastase inhibitory activity of Meju samples supplemented with plant extracts was determined according to a previously reported method26, with minor modifications. For this purpose, 200 μl of 0.05 M Tris-HCl buffer (pH 8.6) and 20 μl of a Meju sample analyzed at various concentrations (5, 10, 20, and 50 mg/ml) were mixed and incubated for 15 min. Then, 10 μl of 2.5 U/ml elastase (optimum reactivity of the enzyme) was added, after which the reaction mixture was incubated for another 15 min; the absorbance was then measured at 410 nm. Ursolic acid at various concentrations (100, 250, 500, and 1000 and 10000 µg/ml) served as a standard compound. The elastase inhibitory rate was calculated as follows:
Determination of B. cereus counts in the Meju samples
To analyze B. cereus cell numbers, 10 g of each Meju sample supplemented with plant extract(s) was homogenized in a blender in 90 ml of a 0.85% sterile NaCl solution and serially diluted to 1:101–1:106 with 0.01 M potassium phosphate buffer (pH 7.2). Enumeration of B. cereus cells was performed by spreading 0.2 ml of each diluted sample onto the surface of mannitol-egg yolk-polymyxin agar plates (a total of five media plates), which were incubated at 30 °C for 24 h27. Pink colonies with transparent zones were counted, and further confirmatory tests were performed by streaking the observed colonies onto the NA medium; the grown colonies were confirmed using the API kit. To determine the enterotoxin-producing capability of each isolate, a loopful of each bacterial colony was inoculated into 10 ml of the BHI medium containing 0.1% glucose with incubation for 16–18 h at 36 °C before performing a Bacillus diarrhea enterotoxin microscopic examination assay (test for protein toxin crystals) to differentiate bacteria from the B. thuringiensis strain28. Confirmatory tests for counting B. cereus cells were carried out by molecular identification at Solgent Co. (Doejon, Korea).
Inhibitory spectrum of the tested plant extracts against B. cereus
Growth and preparation of the bacterial strain
B. cereus was grown in NB at 37 °C for 18–24 h. After proper bacterial growth, the bacterial culture was diluted with peptone water to adjust it to the proper concentration (107 CFU/ml) and was exposed to the extract solution (1% and 10%) of A. sativum, G. biloba, or N. nucifera. Prior to scanning electron microscopy (SEM) analysis, we also performed other inhibitory assays to confirm the inhibitory effects of the plant extracts. The details of the method are described in Supplementary Information Section 1.
SEM analysis
SEM was conducted to determine the effects of all tested plant extracts on the morphology of the B. cereus under study at the minimum inhibitory concentration (MIC). Briefly, the bacterial cells (107 CFU/ml) of the control and treatment groups were washed with 0.05 M phosphate buffer (pH 7.0), followed by centrifugation (4000 × g, 10 min), and the cells were first fixed in 2.5% (w/v) glutaraldehyde at room temperature for 2 h. Secondary fixation was conducted in a 1% (v/v) arsenic acid solution at 4 °C overnight. Sample groups were dehydrated by increasing the concentration of ethanol and freeze-dried. Control samples were prepared without the addition of plant extract samples. To examine morphological changes, a published SEM protocol29 was modified. Finally, each bacterial sample was sputter-coated with gold in an ion coater for 2 min, followed by examination under a scanning electron microscope (Hitachi; Hitachi City, Japan).
GC-MS profile of bioactive compounds in Meju samples
Extraction of bioactive compounds from Meju
For GC-MS analysis, different Meju samples were subjected to extraction as suggested by Namgung et al.30. Briefly, Meju samples (5 g each) were extracted using 50 ml of 80% methanol in water (v/v) at 70 °C for 30 min, followed by filtration through Whatman No. 2 filter paper. The residue was again extracted twice with the same volume of 80% methanol for 30 min, followed by filtration. The filtrate was then concentrated under reduced pressure and diluted up to a volume of 10 ml with 80% methanol, followed by centrifugation (10,000 rpm; 10 min). Next, the transparent upper layer of each extract (1 ml) was transferred into a glass vial, and methanol was evaporated under a constant stream of nitrogen at 40 °C, followed by derivatization.
Derivatization of Meju sample extracts
For derivatization, 50 µl of the methoxyamine hydrochloride reagent (50 mg dissolved in 1 ml of pyridine) was added to each methanol-extracted Meju sample in an extraction vial; the components were mixed and kept at 40 °C for 60 min. Then, 100 µl of the N-methyl-N-(trimethylsilyl)-trifluoroacetamide reagent was added into each vial, followed by incubation at 40 °C for 45 min31. Finally, the derivatized samples were filtered using syringe filters (0.45 µM) and kept in GC vials. Bioactive compounds in Meju samples were identified on a GC-MS system (Agilent; Santa Clara, CA, USA).
GC-MS conditions and analysis
Chromatography was carried out on a Hewlett-Packard (Palo Alto, CA, USA) fused silica column DB-5 MS UI (30 m length, 0.25 mm inner diameter, and 0.25 mm thickness). The GC-MS conditions were reported elsewhere31. The relative proportions of the extract constituents were expressed as percentages by peak area normalization. Extract components were identified based on GC retention time relative to computer matching of mass spectra using Wiley and National Institute of Standards and Technology libraries for the GC-MS system.
Statistical analysis
The data were expressed as the mean ± standard deviation of three independent experiments and subjected to one-way analysis of variance and Student’s t test. Data with P values < 0.05 were considered statistically significant. IC50 values (concentration required for 50% inhibition) were calculated using the formula Y = 100 × A1/(X + A1), where A1 = IC50, Y = response, X = inhibitory concentration (linear regression analysis).
Data availability
The authors declare that all the other data supporting the finding of this study are available within the article and from the corresponding author on reasonable request.
Results and Discussion
Production of Meju fermented with plant extracts
All Meju samples were prepared in two different sets to determine the variability of the results, and the microflora in each lot was analyzed. As presented in Figure S1, the presence of natural microflora (fungal growth) in Meju samples fermented for 60 days in a natural environment with and without plant extracts confirmed the fermentation of Meju samples (control as well as plant extract-supplemented samples).
Physico chemical properties of Meju samples
To assess the acceptability of the plant extract-supplemented Meju products, physico chemical properties were analyzed by the standard AOAC methods21. Ash content indicates the amount of mineral salts present in the diet32. The Meju samples supplemented with plant extracts after 60 days of natural fermentation contained ash contents ranging from 11.85 ± 1.02% to 14.37 ± 1.82%. The control Meju sample without plant extracts, however, showed an ash content of 13.82 ± 1.63%. No significant difference in ash content was observed among all the plant extract-supplemented Meju samples. With respect to moisture content, the plant extract-supplemented Meju samples showed a moisture content ranging from 54.46 ± 1.65% to 64.48 ± 1.38%, whereas control samples showed a moisture content of 55.47 ± 0.16%. As the fermentation period increased, the moisture content decreased in all Meju samples. All the plant extract-supplemented Meju samples showed salt contents ranging from 7.51 ± 1.52% to 8.23 ± 3.11%, whereas in the control Meju, the salt content was 10.87 ± 1.44%. The results of pH analysis revealed that there was no significant difference in pH among different samples after different fermentation periods. The pH levels of all the Meju samples prepared with plant extracts were in the range 5.05–6.01. Traditionally, the pH level of fermented Meju samples (without plant extracts) is 6.3. It is a well-established phenomenon that fermented foods with pH levels below 4 are usually safe because most of the pathogens are unable to survive in this pH range33. Based on the parameters obtained, we may obtain official approval of our plant extract-supplemented Meju samples because they have physicochemical and organoleptic properties similar to those of originally produced traditional Meju samples without plant extracts.
Total phenolic and flavonoid contents
There is great interest in natural phenolic and flavonoid antioxidants because of their presence in edible plants, their health benefits, and their possible use as natural food preservatives34. Raw materials such as soybeans and plants are rich in various bioactive phytochemicals (phenolics and flavonoids). Therefore, in the present study, we analyzed the total phenolic and flavonoid contents of Meju samples supplemented with plant extracts and compared the values with those of traditional Meju samples (Table 2). The total phenolic content of Meju samples containing 10% lotus leaf extract (LOM10) showed the highest value, 31.6 ± 0.51 mg GAE/g. On the other hand, the flavonoid contents of Meju samples supplemented with 10% plant extracts (GAM10, LOM10, GIM10, and MIM10) were 7.13 ± 0.07, 23.75 ± 0.62, 7.90 ± 0.14, and 11.69 ± 0.19 mg QE/g, respectively, and were higher than the flavonoid content of the control Meju (7.47 ± 0.28 mg QE/g) prepared without any plant extracts (Table 2). However, some researchers have reported that the flavonoid content of fermented soybean products may vary owing to differences in the raw materials used during fermentation35, 36. Lin et al.37 suggested that the starter culture or microflora can affect the antioxidant activity of soybeans after fermentation. Nam et al.38 reported that flavonoid content can be significantly affected by the soybean cultivar and fermentation period. These results suggest that higher antioxidant activity may be due to the presence of phenolic and flavonoid compounds in Meju samples fermented with various plant extracts. Hence, extracts with a high polyphenolic content likely have strong antioxidant activity.
Tyrosinase inhibitory activity
Tyrosinase is a major enzyme of the melanin synthesis pathway in melanocytes. Because melanin causes dark spots and freckles on the skin39, 40, inhibition of tyrosinase could be an important strategy for blocking melanogenesis40. Commercial tyrosinase inhibitors such as kojic acid, arbutin, ascorbic acid derivatives, retinoic acid, and azelaic acid are used as ingredients in cosmetics to prevent hyperpigmentation owing to their skin-whitening efficacy39. In the present study, Meju samples supplemented with various plant extracts individually or in combination at a concentration of 20 mg/ml showed tyrosinase inhibitory effects ranging from 36.84 ± 3.61% to 52.34 ± 3.99%, whereas the inhibitory effect of the control Meju sample (with no added plant extracts) (20 mg/ml) was 41.25 ± 4.53% (Fig. 1). Kojic acid at various concentrations (62.5, 125, 250, 500 and 1000 µg/ml) showed tyrosinase inhibitory activities of 36.35 ± 0.73%, 45.67 ± 0.64%, 67.28 ± 0.19%, 79.41 ± 3.80%, and 86.34 ± 1.94%, respectively. The concentrations of tested Meju samples supplemented with plant extracts individually or in combination and of the control Meju sample required for 50% inhibition were 32.80–296.74 mg/ml and 62.35 mg/ml, respectively, whereas a concentration of 0.141 mg/ml was required for kojic acid (Table 2).
The majority of natural tyrosinase inhibitors derived from functional food products (food/formulations) consist of phenolic or flavonoid components39. In addition, the tyrosinase inhibitory activity may depend on the hydroxyl groups of phenolic compounds, which form hydrogen bonds with the enzyme’s active site, thereby causing steric hindrance, conformational changes, and ultimately suppression of enzymatic activity41. Thus, the phenolics present in our Meju samples may play a major role in inhibiting tyrosinase activity. Polyphenols may also be used as depigmentation agents because of their structural similarity to tyrosine, a substrate of tyrosinase41. In addition, antioxidants inhibit pigmentation by various mechanisms, including scavenging of ROS and reactive nitrogen species as well as reduction of o-quinones or other intermediates of melanin biosynthesis, thus delaying oxidative polymerization42. Therefore, polyphenolic compounds are partially responsible for the efficacy of substances used as whitening agents in skin care products. Similarly, dose-dependent tyrosinase inhibition is observed in fermented soybean broth owing to increased concentrations of total phenolics43. Chai et al.44 examined the tyrosinase inhibitory activities of various fermented-soybean-based food products and reported that almost all samples at a particular concentration exerted similar inhibitory activities, except for a Meju sample showing very high activity: from 5 mg/ml to 20 mg/ml. Our results suggest that Meju samples supplemented with plant extracts are rich in phenolic and flavonoid compounds and may be applicable to the treatment of melanin-related pigmentation as skin-whitening agents.
Elastase inhibitory activity
Elastin is a major component of skin connective tissue and plays an important role in maintaining skin elasticity39. Elastin also participates in the formation of a network with collagenous fibers under the epidermis45. Elastase hydrolyzes peripheral and structural proteins in dermal connective tissue and has a strong ability to degrade elastin45. Because decomposition of elastin results from the activation of elastase caused by UV irradiation or ROS formation, inhibition of elastase activity could be a therapeutic target for protection against elastin-induced skin aging46. The active ingredient responsible for this inhibitory activity is believed to be phenolics and flavonoids present in plant extracts47.
The elastase inhibitory activities of Meju samples supplemented with plant extracts individually or in combination and the activity of a Meju sample without plant extracts are shown in Fig. 2. In this assay, Meju samples supplemented with extracts individually and in combination yielded optimal results at 20 mg/ml. Notably, all the tested Meju samples (20 mg/ml) supplemented with plant extracts of garlic cloves, lotus leaves, and ginkgo leaves individually or in combination (1:1:1) exerted elastase inhibitory activities of 9.90 ± 1.92%, 8.74 ± 1.07%, 3.01 ± 1.35%, and 5.52 ± 1.01%, respectively, although their values were lower than the activity of the control Meju sample (11.18 ± 1.51%; Fig. 2). In this assay, the IC50 values of all the experimental and control Meju samples were 199.70–1364.86 mg/ml and 171.86 mg/ml, respectively. On the other hand, the IC50 value of the positive control (ursolic acid) for elastase inhibition was 0.85 mg/ml (Table 2). Although the Meju samples supplemented with plant extracts did not show significant elastase inhibitory activities for possible skin improvement, they showed positive results regarding melanin-reducing (tyrosinase inhibitory) activity.
In vitro studies on both purified elastases and cultured fibroblasts showed that soybean extracts can affect the extracellular matrix and inhibit enzymatic activities of several elastases48. Based on these studies, it can be inferred that all our Meju samples fermented with plant extracts as well as the control Meju sample may have beneficial effects on human skin and certain dermatological disorders because of the enhanced production of bioactive compounds (phenolics and flavonoids) during fermentation. The increasing use of phenolic and flavonoid compounds in the cosmetic industry could be attributed to the compounds’ health benefits, including antioxidant properties and skin-improving effects49. These findings suggest that the consumption of Meju supplemented with plant extracts may have positive effects and be useful for the treatment of skin pigmentation.
α-Glucosidase inhibitory activity
The application of Meju samples fermented with herbal extracts for treating diabetes remains largely unexplored; this situation has prompted some scientists to develop novel agents with inhibitory effects on intestinal glucosidases. The in vitro α-glucosidase inhibitory activities of Meju samples are summarized in Table 2 and Fig. 3. The α-glucosidase inhibitory activities of all the tested Meju samples fermented with extracts of garlic cloves, lotus leaves, and ginkgo leaves individually or in combination (1:1:1) were determined using p-nitrophenyl-α-D-glucopyranoside as a substrate and compared with the activity of the control Meju sample (without addition of plant extracts; Table 2). Acarbose served as a standard compound. In this assay, the percentages of α-glucosidase inhibition shown by Meju samples fermented with individual plant extracts were similar to that of Meju samples fermented with a combination of extracts (1:1:1), indicating a synergistic or additive interaction between the plant extracts and Meju samples. The control Meju sample showed a lower α-glucosidase inhibitory activity than did the other Meju samples. The IC50 values of all the tested Meju samples fermented with ethanolic extracts derived from garlic cloves, lotus leaves, and ginkgo leaves individually or in combination (1:1:1) ranged from 2.86 mg/ml to 21.82 mg/ml (Table 2). The control Meju sample showed a higher IC50 value (10.48 mg/ml) than did the Meju sample supplemented with lotus leaf extract (LOM1 and LOM10: 5.83 mg/ml and 4.87 mg/ml, respectively; Table 2). On the other hand, the IC50 value of the standard compound (acarbose) for α-glucosidase inhibition was 0.70 mg/ml (Table 2). In addition, all the tested Meju samples (20 mg/ml) supplemented with a 1% plant extract of garlic cloves, lotus leaves, and ginkgo leaves individually or in combination exerted considerable α-glucosidase inhibitory activities: 68.66% ± 4.47%, 72.12% ± 9.71%, 77.94 ± 2.04%, and 63.76 ± 4.60%, respectively. In contrast, this inhibitory activity was slightly lower for the control Meju sample (60.07 ± 6.53%; Fig. 3). The inhibitory activities of Meju samples fermented with plant extracts against α-glucosidase may be due to the increased glycoside content of soybeans caused by either fermentation or the addition of plant extracts. Glycosides consist of sugars that may be structurally similar to the carbohydrate substrate of α-glucosidase50. Meju samples fermented with plant extracts were found to have lower IC50 values than the control Meju sample because their active chemical compounds did not undergo fractionation and may synergistically inhibit α-glucosidase51. Despite several studies on Doenjang samples31, 35, 43, 44, 51 with Meju as a fermentation starter, there has been no investigation of enzyme-based α-glucosidase inhibition in normal Meju samples. In addition, there are various scientific reports on the health benefits of garlic cloves, lotus leaves, and ginkgo leaves, including anti-obesity and anti-diabetic effects52, 53, suggesting that Meju samples fermented with these plant extracts could be used to develop functional foods for the prevention of diabetes with enhanced biological activities.
Reduction in B. cereus counts in Meju samples fermented with plant extracts
In this assay, all the selected plant extracts derived from garlic cloves, lotus leaves, and ginkgo leaves individually or in combination (1:1:1) were used to prepare Meju during fermentation to examine their ability to reduce B. cereus counts in samples of the soybean-based Meju starter culture. Results showed that B. cereus counts in Meju samples fermented with individual plant extracts of garlic cloves, lotus leaves, and ginkgo leaves or their combination were drastically reduced compared with those in the traditional Meju sample fermented without any plant extract. Meju samples fermented with individual plant extracts showed B. cereus counts ranging from 0 CFU/g to (1.0 ± 0.1) × 102 CFU/g. On the other hand, the control Meju sample showed B. cereus counts ranging from (1.7 ± 0.01) × 103 CFU/g to (1.8 ± 0.07) × 104 CFU/g (Table 3). Meju samples fermented with a higher concentration (10%) of plant extracts inhibited the growth of B. cereus better than samples fermented with a low concentration (1%) of plant extracts (Table 3). To confirm that the bacterium found in the Meju samples (1% plant extract-supplemented Meju and control Meju) was B. cereus, colonies on MYP agar were identified using the API kit, and all the analyzed colonies tested positive for B. cereus with 99.9% ID and T-index values ranging from 0.37 to 0.62. These data confirmed how closely the profile corresponds to the taxon in question relative to all other taxa in the database, and how closely the profile corresponds to the most typical set of reactions for each taxon, respectively (Table 3). Moreover, among all the Meju samples fermented with plant extracts, the Meju samples that were fermented with the 1% and 10% ginkgo leaf extract showed a remarkable reduction in B. cereus counts (Table 3). Confirmatory tests for counted B. cereus cells were also performed by molecular identification. Results showed that all the colonies suspected of containing B. cereus tested positive for B. cereus with 100% similarity (data not shown).
According to the abovementioned results, the addition of 10% ethanolic extracts of selected plants individually or in combination may be a feasible approach to controlling the growth of food-borne B. cereus in starter culture Meju samples. Lim and Lee13 observed a growth reduction of B. cereus in Meju samples prepared from licorice extracts at different concentrations. Although various methods for inactivating B. cereus have been developed, including ethanol, sodium chloride, and microwave methods13, 54, the application of plant extracts of natural origin to Meju samples appears to be the most effective method for controlling the growth of hazardous pathogens such as B. cereus. In addition, Lim and Lee13 produced licorice extracts to enhance Meju samples with respect to sensory attributes and consumer acceptability. In the present work, fermented soybean paste samples were approved by performing sensory examinations and other consumer acceptability tests, which were not elaborated when licorice extracts were incorporated into Meju samples. Furthermore, the Korean population is well aware of the use of a few of these plant extracts (garlic cloves and lotus leaves) in different types of fermented soybean products. These findings suggest that selected plant extracts derived from garlic cloves, lotus leaves, and ginkgo leaves individually or in combination can be utilized in conjunction with other conventional food safety measures to inhibit the growth of B. cereus in Meju samples.
Confirmation of inhibitory effects of plant extracts against B. cereus
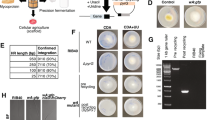
Physical and morphological alterations may induce deterioration of the cell wall surface of bacterial pathogens upon treatment with a suitable antimicrobial agent. Hence, SEM analysis was carried out to visualize the effects of these plant extracts individually and in combination on the morphology of B. cereus cells relative to a control group (Fig. 4). Ethanolic extracts of lotus leaves and ginkgo leaves revealed possible inhibitory effects, as confirmed by severe morphological alteration of the cell wall of B. cereus, leading to cell disruption and lysis (Fig. 4B and C). Furthermore, B. cereus cells treated with plant extracts showed severely damaged cell morphology, including disruption of the cell membrane, abnormal cell breaking, and swelling. In contrast, control cells of the pathogen tested (in the absence of any plant extract) showed regular and smooth surfaces (Fig. 4A). In our preliminary study, the in vitro inhibitory activities of ethanolic extracts of garlic cloves, lotus leaves, ginkgo leaves added individually and in combination (1:1:1) against B. cereus were qualitatively and quantitatively assessed by the presence of inhibition zones and MIC values (Supplementary Section 1). Results showed that extracts derived from ginkgo leaves, lotus leaves, and combinations thereof had possible inhibitory effects on B. cereus (data not shown). Several researchers have also demonstrated the anti-bacterial effects of garlic cloves, lotus leaves, and ginkgo leaves against a variety of food-borne pathogenic bacterial strains, including B. cereus 55, 56.
Scanning electron microscopy of Bacillus cereus treated with single or combined ethanolic extracts of lotus leaves, ginkgo leaves, and garlic cloves. (A) Without any treatment (showing regular and smooth surface of cells); Bacillus cereus cells treated with ethanolic extracts of lotus leaves (B), ginkgo leaves (C), garlic cloves (D), and combination of extracts in a ratio of 1:1:1 (all showing disruption, swelling, surface collapse, and cell lysis); (E) A. sativum (lesser cell lysis).
The literature suggests that the active ingredients of plant extracts such as phenolics and flavonoids may bind to the cell surface and penetrate target sites such as the plasma membrane containing membrane-bound enzymes, disrupting the cell wall structure57. Thus, among all the tested plant extracts and combinations thereof, the ethanolic extract of ginkgo leaves showed the strongest bactericidal activity, as indicated by the significant reduction in microbial counts and complete inhibition of B. cereus growth. Based on these results, selected plant extracts can be considered promising anti-microbials for improving food safety by controlling B. cereus counts in food products.
In our previous study, we evaluated the fungal microflora in Meju samples fermented with single and/or multiple plant extracts, including lotus leaves, ginkgo leaves, and garlic cloves, at different concentrations (1%, 10%, and a mixture of these extracts in a ratio of 1:1:1). Meju samples fermented with individual and/or multiple plant extracts showed the presence of various non-hazardous fungal strains, including Aspergillus species, Mucor species, Paecilomyces species, and Penicillium chrysogenum. In contrast, the control Meju samples fermented without plant extracts revealed the presence of other fungal microflora such as Aspergillus ruber 58, which is known to produce aflatoxin B1 and ochratoxin A59. The findings of the present study confirm that the fermentation of Meju with plant extracts may strongly affect the fungal microflora by eliminating hazardous fungal pathogens through the suppressive effects of the extracts against toxin-producing fungal pathogens, improving the quality of Meju.
In addition, we analyzed functional properties of selected plant extracts. The literature and our experimental data show that these plant extracts and their bioactive compounds have various known pharmacological effects18, 19. As a reference, there are many other plant components that are directly related to several other functional activities, including anti-microbial activities18. Additionally, similar studies have demonstrated the direct and indirect inhibitory effects of several plant extracts and their active ingredients on certain biological activities60, 61.
GC-MS profile of bioactive compounds in Meju samples
GC-MS is an analytical method that combines the features of gas-liquid chromatography and mass spectrometry to identify different substances in a variety of samples. GC-MS analysis of plant extract-supplemented Meju samples and a control Meju sample resulted in the identification of 113 compounds representing 98.44–99.98% of the total extract. All tested Meju samples yielded compounds largely composed of alcohols, sugars, amides, furanones, phenolics, terpenoids, steroids, alkaloids, and flavanones, as well as hydrazine and imidazole derivatives and other phytochemicals. It was noted in this study that the percentage of compounds increased in all the tested Meju samples as the concentration of plant extracts increased from 1% to 10% (Table S1). A detailed chemical profile of various plant extract-supplemented Meju samples and the control Meju sample (with no added plant extracts) is presented in Table S1.
Docosenamide (Table S1), an amide compound, was present in all the tested Meju samples. After comparing with control Meju samples, the 1% plant extract-supplemented samples showed relative areas ranging from 0.12% to 0.14%, whereas 10% plant extract-supplemented Meju samples yielded a higher range (0.27–0.89%) of the relative area. Ghazali et al.62 reported the presence of docosenamide in the root extract of Ixora coccinea, which showed anti-microbial effects. Kitaoka et al.63 isolated and purified laminaribiose from Euglena gracilis, a disaccharide important in the field of agriculture as an antiseptic. Laminaribiose (Table S1) was also present in Meju samples supplemented with garlic cloves, lotus leaves, or ginkgo leaves as well as in mixed Meju samples, but it was absent in the control Meju sample. It was found that in the 10% plant extract-supplemented Meju samples, the relative peak area was slightly larger (0.19–0.35%) than that for the 1% plant extract-supplemented Meju samples (0.07–0.18%). Another key compound that was present in all the tested Meju samples is erythritol, a four-carbon sugar alcohol (polyol). Generally recognized as safe, erythritol is used in the food industry as a low-calorie sweetener64. The alkaloid N-methylasimilobine, which was present only in 1% and 10% lotus leaf extract-supplemented Meju samples and in 1% and 10% mixed extract-supplemented Meju samples, has been reported to possess acetylcholinesterase inhibitory activity65. In our previous study18, we reported the presence of N-methylasimilobine in the methanol extract of lotus leaves, indicating the potential therapeutic usefulness of this extract.
In addition, control and plant extract-supplemented Meju samples contained an aromatic acid component, azaazoniaboratine (Table S1), which has been found to exert an anti-cancer effect66. On the other hand, furanone and its various derivatives possessing antioxidant and anti-inflammatory activities67 were also present in the Meju samples analyzed, indicating the medicinal utility of the Meju samples under study. Among furan derivatives, the furan ring is a constituent of several important natural products, including fructofuranose, psicofuranose, sorbofuranose, allofuranose, and many other natural terpenoids.
Other volatile organic acids such as lactic acid, acetic acid, propanoic acid, citric acid, acrylic acid, palmitic acid, and butenedioic acid were also present in all the tested Meju samples and can provide an important contribution to the flavor characteristics of various foods while imparting antioxidant and other pharmacologically important activities. These results are in strong agreement with the findings of Tang et al.68. Propionic acid is a naturally occurring carboxylic acid that inhibits the growth of molds and some bacteria when added to food at concentrations between 0.1% and 1%69; the acid was also detected in our Meju samples. Moreover, anthraquinone, which has been reported to possess strong anti-microbial properties70, was found in abundance in garlic and ginkgo leaf extract-supplemented Meju samples and in mixed-extract-supplemented Meju samples, suggesting that anthraquinone alone or in combination with other bioactive compounds of Meju samples may be responsible for the observed anti-microbial effect. In this study, GC-MS analysis of different Meju samples detected the presence of common bioactive compounds and specific compounds because different plant extracts were used for Meju production.
Conclusions
The purpose of this study was to develop a new system for producing novel types of traditional Korean Meju products by incorporating various plant extracts with significant biological effects during fermentation. GC-MS analysis of various plant extract-supplemented Meju samples identified various bioactive compounds such as organic acids, amino acids, fatty acids, terpenes, phenolics, sterols, alkaloids, flavonoids, and sugars, as well as furans and their derivatives. Moreover, the percentage of various bioactive compounds found in plant extract-supplemented Meju samples gradually increased with the concentration of plant extracts during Meju fermentation. Fermented soy products such as Meju prepared from selected plant extracts derived from garlic cloves, lotus leaves, and ginkgo leaves individually or in combination at different concentrations (1% and 10%) exerted strong inhibitory effects against the growth of B. cereus. Moreover, the fermented Meju samples incorporated with ginkgo leaf extract (10%) showed considerable tyrosinase and α-glucosidase inhibitory effect compared with traditional Meju samples fermented without plant extracts. These findings reinforce the notion that the various biological and functional properties observed for Meju samples incorporated with ginkgo leaf extract may be attributed to various biologically active polyphenolic compounds, which may act either individually or synergistically. Based on the abovementioned findings, it can be concluded that these plant extracts can serve as natural additives during the production of functional Meju products with acceptable attributes.
References
Kwon, D. Y., Daily, J. W., Kim, H. J. & Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 30, 1–13 (2010).
Yoo, S. K., Kang, S. M. & Noh, Y. S. Quality properties on soybean pastes made with microorganisms isolated from traditional soybean pastes. Korean J. Food Sci. Technol. 2, 1266–1270 (2000).
Jeong, S. C., Hyun, K. W., Kim, J. H. & Lee, J. S. Isolation of a halotolerant yeast and the production of extracellular protease. Korean J. Biotechnol. Bioeng. 16, 158–162 (2001).
Shukla, S., Lee, J. S., Park, H. K., Kim, J. K. & Kim, M. Effect of novel starter culture on reduction of biogenic amines, quality improvement, and sensory properties of Doenjang, a traditional Korean soybean fermented sauce variety. J. Food Sci. 80, 794–803 (2015).
Anagnostopoulou, M. A., Kefalas, P., Papageorgiou, V. P., Assimepoulou, A. N. & Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 94, 19–25 (2006).
Daamen, W. F., Veerkamp, J. H., Hest, J. C. M. & Kuppevelt, T. H. Elastin as a biomaterial for tissue engineering. Biomaterials 28, 4378–4398 (2007).
Nar, H., Werle, K., Bauer, M. M. T., Dollinger, H. & Jung, B. Crystal structure of human macrophage elastase (MMP-12) in complex with a hydroxamic acid inhibitor. J. Mol. Biol. 312, 743–751 (2001).
Kim, Y. H. et al. Inhibitory effects of natural plants of Jeju island on elastase and MMP-1 expression. J. Cosm. Sci. 58, 19–33 (2007).
Briganti, S., Camera, E. & Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 16, 101–110 (2003).
Oskoueian, A., Haghighi, R. S., Ebrahimi, M. & Oskoueian, E. Bioactive compounds, antioxidant, tyrosinase inhibition, xanthine oxidase inhibition, anticholinesterase and anti-inflammatory activities of Prunus mahaleb L. seed. J. Med. Plant Res. 6, 225–233 (2011).
Sukito, A. & Tachibana, S. Potent α-glucosidase inhibitors isolated from Ginkgo biloba leaves. Pak. J. Biol. Sci. 17, 1170–1178 (2014).
Eom, J. S. & Choi, H. S. Inhibition of Bacillus cereus growth and toxin production by Bacillus amyloliquefaciens RD7-7 in fermented soybean products. J. Microbiol. Biotechnol. 26, 44–55 (2016).
Lim, S. I. & Lee, B. Y. Effect of licorice (Glycyrrhiza glabra) ethanol extracts on growth of Bacillus cereus at the Meju producing stage. J. Korean Soc. Appl. Biol. Chem. 53, 184–191 (2010).
Kim, D. H., Kim, S. H., Kwon, S. W., Lee, J. K. & Hong, S. B. Fungal diversity of rice straw for meju fermentation. J. Microbiol. Biotechnol. 23, 1654–1663 (2013).
Park, K. M., Kim, H. J., Jeong, M. C. & Koo, M. Occurrence of toxigenic Bacillus cereus and Bacillus thuringiensis in Doenjang, a Korean fermented soybean paste. J. Food Prot. 79, 605–612 (2016).
Checinska, A., Paszczynski, A. & Burbank, M. Bacillus and other spore-forming genera: variations in responses and mechanisms for survival. Annu. Rev. Food Sci. Technol. 6, 351–369 (2015).
Kamar, R. et al. Pathogenic potential of Bacillus cereus strains as revealed by phenotypic analysis. J. Clin. Microbiol. 51, 320–330 (2013).
Lee, J. S., Shukla, S., Kim, J. A. & Kim, M. Anti-angiogenic effect of Nelumbo nucifera leaf extracts in human umbilical vein endothelial cells with antioxidant potential. PlosOne 10, e0118552 (2015).
Chen, J., Zhang, T., Jiang, B., Mu, W. & Miao, M. Characterization and antioxidant activity of Ginkgo biloba exocarp polysaccharides. Carbohyd. Polym. 87, 40–45 (2012).
Mikaili, P., Maadirad, S., Moloudizargari, M., Aghajanshakeri, S. & Sarahroodi, S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J. Basic Med. Sci. 16, 1031–1048 (2013).
AOAC - Association of Official Analytical Chemists. Official methods of analysis of the association of official analytical chemists. AOAC, Washington, D.C. (2006).
Singleton, V. L., Orthofer, R. & Lamuela-Raventos, R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Ed: Packer L, Oxidants and antioxidants, Part A, Methods in Enzymology. Academic Press, New York, NY, USA 299, 152–178 (1999).
Sakanaka, S., Tachibana, Y. & Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon tea (kakinoha-cha). Food Chem. 89, 569–575 (2005).
Fawole, O. A., Makunga, N. P. & Opara, U. L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Comp. Alt. Med. 12, 202–211 (2012).
Yuan, T., Wan, C., Liu, K. & Seeram, N. P. New maplexin FI and phenolic glycosides from red maple (Acer rubrum) bark. Tetrahedron 68, 959–964 (2012).
Kim, J. H., Byun, J. C., Bandl, A. K. R., Hyun, C. G. & Lee, N. H. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J. Med. Plant Res. 3, 914–920 (2009).
KFDA - Korea Food and Drug Administration. Food-borne pathogen test methods, Seoul, Korea Available from: http://www.kfda.go.kr (2010).
Sandra, A. et al. Bacillus cereus and Bacillus thuringiensis in ready-to-eat cooked rice in Malaysia. Int. Food Res. J. 19, 829–836 (2012).
Kockro, R. A. et al. Use of scanning electron microscopy to investigate the prophylactic efficacy of rifampin-impregnated CSF shunts catheters. J. Med. Microbiol. 49, 441–450 (2000).
Namgung, H. J. et al. Metabolite profiling of Doenjang, fermented soybean paste, during fermentation. J. Sci. Food Agric. 90, 1926–1935 (2009).
Huang, D., Charchoghlyan, H., Lee, J. S. & Kim, M. Bioactive compounds and antioxidant activities of the Korean lotus leaf (Nelumbo nucifera) condiment: volatile and nonvolatile metabolite profiling during fermentation. Int. J. Food Sci. Technol. 50, 1988–1995 (2015).
Ikujenlola, A. V., Oguntuase, S. O. & Omosuli, S. V. Physico-chemical properties of complementary food from malted quality protein maize (Zea mays L.) and defatted fluted pumpkin flour (Telfairia occidentalis Hook, F). Food Public Health. 3, 323–328 (2013).
Padonou, S. W. et al. Development of starter culture for improved processing of Lafun, an African fermented cassava food product. J. Appl. Microbiol. 109, 1402–1410 (2010).
Aneta, W., Jan, O. S. & Renata, C. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105, 940–949 (2007).
Kim, S. H. & Lee, K. A. Evaluation of taste compounds in water-soluble extract of a Doenjang (soybean paste). Food Chem. 83, 339–342 (2003).
Jang, C. H. et al. Metabolism of isoflavone derivatives during manufacturing of traditional Meju and Doenjang. Food Sci. Biotechnol. 17, 442–445 (2008).
Lin, C. H., Wei, Y. T. & Chou, C. C. Enhanced antioxidant activity of soybean koji prepared with various filamentous fungi. Food Microbiol. 23, 628–633 (2006).
Nam, D. H. et al. Simultaneous enhancement of free isoflavone content and antioxidant potential of soybean fermentation with Aspergillus oryzae. J. Food Sci. 76, 194–200 (2011).
Hong, Y. H., Jung, E. Y., Noh, D. O. & Suh, H. J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: physical stability, collagenase, elastase, and tyrosinase activities. Integ. Med. Res. 3, 25–33 (2014).
Park, S. H. et al. Terrein: a new melanogenesis inhibitor and its mechanism. Cell. Mol. Life Sci. 61, 2878–2885 (2004).
Kim, Y. J. Anti-melanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 30, 1052–1055 (2007).
Seo, S. Y., Sharma, V. K. & Sharma, N. Mushroom tyrosinase: recent prospects. J. Agric. Food Chem. 51, 2837–2853 (2003).
Yang, J. H., Mau, J. L., Ko, P. T. & Huang, L. C. Antioxidant properties of fermented soybean broth. Food Chem. 71, 249–254 (2000).
Chai, C. et al. Determination of bioactive compounds in fermented soybean products using GC/MS and further investigation of correlation of their bioactivities. J. Chromatograph. B 880, 42–49 (2012).
Thring, T. S., Hili, P. & Naughton, D. P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Comp. Alt. Med. 9, e27 (2009).
Meyer, W., Neurand, K. & Radke, B. Elastic fiber arrangement in the skin of the pig. Arch. Dermatol. Res. 270, 391–401 (1981).
Wittenauer, J., Mackle, S., Submann, D., Schweiggert-Weisz, U. & Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 101, 179–187 (2015).
Zhao, R. et al. Extracts from Glycine max (soybean) induce elastin synthesis and inhibit elastase activity. Exp. Dermatol. 18, 883–886 (2009).
Arct, J., Oborska, A., Mojski, M., Binkowska, A. & Swidzikowska, B. Common cosmetic hydrophilic ingredients as penetration modifiers of flavonoids. Int. J. Cosm. Sci. 24, 357–366 (2002).
Sugiwati, S., Setiasi, S. & Afifah, E. Antihyperglycemic activity of the mahkota dewa [Phaleria macrocarpa (Scheff.) Boerl.] leaf extracts as an alpha-glucosidase inhibitor. Makara Kesehatan 13, 74–78 (2009).
Shukla, S. et al. Total phenolic content, antioxidant, tyrosinase and α-glucosidase inhibitory activities of water soluble extracts of novel starter culture Doenjang, a Korean fermented soybean sauce variety. Food Control 59, 854–861 (2016).
Lee, M. S., Kim, I. H., Kim, C. T. & Kim, Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J. Nutr. 141, 1947–1953 (2011).
Banin, R. M. et al. Beneficial effects of Ginkgo biloba extract on insulin signaling cascade, dyslipidemia, and body adiposity of diet-induced obese rats. Braz. J. Med. Biol. Res. 47, 780–788 (2014).
Jang, J. H. et al. Growth inhibition effects of ethanol and sodium chloride on Bacillus cereus. Korean J. Food Sci. Technol. 35, 998–1002 (2003).
Sati, S. C. & Joshi, S. Antibacterial activities of Ginkgo biloba L. leaf extracts. Scientific. World J. 11, 2237–2242 (2011).
Mohsenipour, Z. & Hassanshahian, M. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundisahpur J. Microbiol. 8, e18971 (2015).
Zhu, S. Y., Yang, Y., Yu, H. D., Ying, Y. & Zou, G. L. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 96, 151–158 (2005).
Shukla, S. et al. Evaluation of fungal microflora for aflatoxin producing possibility in novel quality Meju fermented with single and/or multiple additions of Nelumbo nucifera, Ginkgo biloba, and Allium sativum extracts. J. Food Safety e12368 (2017).
Lee, J. H., Kim, M. H. & Im, S. S. Antioxidative materials in domestic Meju and Doenjang. J. Korean Soc. Food Sci. Nutri. 20, 148–155 (1991).
Carrasco, D. et al. Use of plant extracts as an effective manner to control Clostridium perfringens induced necrotic enteritis in poultry. BioMed Res. Int. 2016, 1–15 (2016).
Hess, H. D. et al. Supplementation of a tropical grass diet with forage legumes and Sapindus saponaria fruits: effects on in vitro ruminal nitrogen turnover and methanogenesis. Aust. J. Agric. Res. 54, 703–713 (2003).
Ghazali, N., Abdullah, N. A., Bakar, A. & Mohamad, N. K. GC-MS analysis of some bioactive components in the root extracts of Ixora coccinea Linn. Int. J. Pharma Bio. Sci. 5, 197–203 (2014).
Kitaoka, M., Sasaki, T. & Taniguchi, H. Purification and properties of laminaribiose phosphorylase (EC 2.4 1.31) from Euglena gracilis Z. Arch. Biochem. Biophys. 304, 508–514 (1993).
Kawanabe, J., Hirasawa, M., Takeuchi, T., Oda, T. & Ikeda, T. Noncariogenicity of erythritol as a substrate. Caries Res. 26, 358–362 (1992).
Yang, Z. et al. An aporphine alkaloid from Nelumbo nucifera as an acetylcholinesterase inhibitor and the primary investigation for structure-activity correlations. Nat. Prod. Res. 26, 387–392 (2012).
Diana, S., Halina, C., Maria, H. B. & Jacek, N. The anticancer activity of propolis. Folia Histochem. Cytobiol. 50, 25–37 (2012).
Weber, V. et al. Novel 4,5-diaryl-3-hydroxy-2(5H)-furanones as anti-oxidants and anti-inflammatory agents. Bioorg. Medi. Chem. 10, 1647–1658 (2002).
Tang, X. et al. The cardioprotective effects of citric acid and L-malic acid on myocardial ischemia/reperfusion injury. Evidence-Based Comp. Alt. Med. 95, 1–11 (2013).
Nguyen, N. H. T. et al. Propionate increases neuronal histone acetylation, but is metabolized oxidatively by glia. Relevance for propionic acidemia. J. Neurochem. 101, 806–814 (2007).
Iuri Bezerra, B. et al. Phytochemical and antifungal activity of anthraquinones and root and leaf extracts of Coccoloba mollis on phytopathogens. Braz. Arch. Biol. Technol. 54, 535–541 (2011).
Acknowledgements
This research was supported by the National Research Foundation (Grant No. NRF-2014R1A1A3050670) in 2014.
Author information
Authors and Affiliations
Contributions
M.K. and S.S. conceived and designed the experiments. S.S., J.P., J.H.P., and J.S.L. performed the experiments. S.S., and M.K. analyzed the data. S.S. wrote the paper. All authors reviewed the manuscript. M.K. approved the final version of the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shukla, S., Park, J., Park, J.H. et al. Development of novel Meju starter culture using plant extracts with reduced Bacillus cereus counts and enhanced functional properties. Sci Rep 7, 11409 (2017). https://doi.org/10.1038/s41598-017-09551-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09551-0
This article is cited by
-
Lotus leaf extract inhibits ER− breast cancer cell migration and metastasis
Nutrition & Metabolism (2021)
-
Alkaloid profiling and antimicrobial activities of Papaver glaucum and P. decaisnei
BMC Research Notes (2021)
-
Effect of Rhizopus nigricans (Rhizopus stolonifera)-based novel starter culture on quality and safety attributes of doenjang, a traditional Korean soybean fermented food product
Scientific Reports (2020)
-
Garlic augments the functional and nutritional behavior of Doenjang, a traditional Korean fermented soybean paste
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.