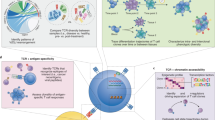
Abstract
RNase H–dependent PCR-enabled T-cell receptor sequencing (rhTCRseq) can be used to determine paired alpha/beta T-cell receptor (TCR) clonotypes in single cells or perform alpha and beta TCR repertoire analysis in bulk RNA samples. With the enhanced specificity of RNase H–dependent PCR (rhPCR), it achieves TCR-specific amplification and addition of dual-index barcodes in a single PCR step. For single cells, the protocol includes sorting of single cells into plates, generation of cDNA libraries, a TCR-specific amplification step, a second PCR on pooled sample to generate a sequencing library, and sequencing. In the bulk method, sorting and cDNA library steps are replaced with a reverse-transcriptase (RT) reaction that adds a unique molecular identifier (UMI) to each cDNA molecule to improve the accuracy of repertoire-frequency measurements. Compared to other methods for TCR sequencing, rhTCRseq has a streamlined workflow and the ability to analyze single cells in 384-well plates. Compared to TCR reconstruction from single-cell transcriptome sequencing data, it improves the success rate for obtaining paired alpha/beta information and ensures recovery of complete complementarity-determining region 3 (CDR3) sequences, a prerequisite for cloning/expression of discovered TCRs. Although it has lower throughput than droplet-based methods, rhTCRseq is well-suited to analysis of small sorted populations, especially when analysis of 96 or 384 single cells is sufficient to identify predominant T-cell clones. For single cells, sorting typically requires 2–4 h and can be performed days, or even months, before library construction and data processing, which takes ~4 d; the bulk RNA protocol takes ~3 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All TCR clonotype data for the results presented in Supplementary Figs. 1, 2, and 4 are provided in Supplementary Tables 3 and 5. All other data are available from the corresponding author upon reasonable request. Example data used for data analysis are publicly available from the GitHub repository at https://github.com/julietforman/rhTCRseq.
Code availability
The code and reference files used for data analysis are publicly available from the GitHub repository at https://github.com/julietforman/rhTCRseq.
References
Dash, P. et al. Paired analysis of TCRα and TCRβ chains at the single-cell level in mice. J. Clin. Invest. 121, 288–295 (2011).
Wang, G. C., Dash, P., McCullers, J. A., Doherty, P. C. & Thomas, P. G. T cell receptor αβ diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci. Transl. Med. 4, 128ra42 (2012).
Han, A., Glanville, J., Hansmann, L. & Davis, M. M. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat. Biotechnol. 32, 684–692 (2014).
Dash, P., Wang, G. C. & Thomas, P. G. Single-cell analysis of T-cell receptor αβ repertoire. Methods Mol. Biol 1343, 181–197 (2015).
Dash, P. et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature 547, 89–93 (2017).
Stubbington, M. J. T. et al. T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods 13, 329–332 (2016).
Redmond, D., Poran, A. & Elemento, O. Single-cell TCRseq: paired recovery of entire T-cell alpha and beta chain transcripts in T-cell receptors from single-cell RNAseq. Genome Med 8, 80 (2016).
Eltahla, A. A. et al. Linking the T cell receptor to the single cell transcriptome in antigen-specific human T cells. Immunol. Cell Biol. 94, 604–611 (2016).
Afik, S. et al. Targeted reconstruction of T cell receptor sequence from single cell RNA-seq links CDR3 length to T cell differentiation state. Nucleic Acids Res. 45, e148 (2017).
Azizi, E. et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308.e36 (2018).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988.e16 (2018).
Howie, B. et al. High-throughput pairing of T cell receptor α and β sequences. Sci. Transl. Med. 7, 301ra131 (2015).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Dobosy, J. R. et al. RNase H-dependent PCR (rhPCR): improved specificity and single nucleotide polymorphism detection using blocked cleavable primers. BMC Biotechnol. 11, 80 (2011).
Trombetta, J. J. et al. Preparation of single-cell RNA-seq libraries for next-generation sequencing. Curr. Protoc. Mol. Biol. 107, 4.22.1–17 (2014).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Kircher, M., Sawyer, S. & Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3 (2012).
MacConaill, L. E. et al. Unique, dual-indexed sequencing adapters with UMIs effectively eliminate index cross-talk and significantly improve sensitivity of massively parallel sequencing. BMC Genomics 19, 30 (2018).
Hug, H. & Schuler, R. Measurement of the number of molecules of a single mRNA species in a complex mRNA preparation. J. Theor. Biol. 221, 615–624 (2003).
Kivioja, T. et al. Counting absolute numbers of molecules using unique molecular identifiers. Nat. Methods 9, 72–74 (2011).
Britanova, O. V. et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J. Immunol. 192, 2689–2698 (2014).
Shugay, M. et al. Towards error-free profiling of immune repertoires. Nat. Methods 11, 653–655 (2014).
Egorov, E. S. et al. Quantitative profiling of immune repertoires for minor lymphocyte counts using unique molecular identifiers. J. Immunol. 194, 6155–6163 (2015).
Turchaninova, M. A. et al. High-quality full-length immunoglobulin profiling with unique molecular barcoding. Nat. Protoc. 11, 1599–1616 (2016).
Ma, K.-Y. et al. Immune repertoire sequencing using molecular identifiers enables accurate clonality discovery and clone size quantification. Front. Immunol. 9, 33 (2018).
Heather, J. M., Ismail, M., Oakes, T. & Chain, B. High-throughput sequencing of the T-cell receptor repertoire: pitfalls and opportunities. Brief. Bioinformatics 19, 554–565 (2018).
Hu, Z. et al. A cloning and expression system to probe T-cell receptor specificity and assess functional avidity to neoantigens. Blood 132, 1911–1921 (2018).
Robert, L. et al. Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology 3, e29244 (2014).
Cha, E. et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 6, 238ra70 (2014).
Postow, M. A. et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J. Immunother. Cancer 3, 23 (2015).
Heather, J. M. et al. Dynamic perturbations of the T-cell receptor repertoire in chronic HIV infection and following antiretroviral therapy. Front. Immunol. 6, 644 (2015).
Li, B. et al. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nat. Genet. 48, 725–732 (2016).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Inoue, H. et al. Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma. Oncoimmunology 5, e1204507 (2016).
Riaz, N. et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171, 934–949.e16 (2017).
Roh, W. et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci. Transl. Med. 9, eaah3560 (2017); erratum 9, eaan3788 (2017).
Beausang, J. F. et al. T cell receptor sequencing of early-stage breast cancer tumors identifies altered clonal structure of the T cell repertoire. Proc. Natl. Acad. Sci. USA 114, E10409–E10417 (2017).
van Dongen, J. J. M. et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17, 2257–2317 (2003).
Boyd, S. D. et al. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci. Transl. Med. 1, 12ra23 (2009).
Robins, H. S. et al. Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci. Transl. Med. 2, 47ra64 (2010).
Mariani, S. et al. Comprehensive assessment of the TCRBV repertoire in small T-cell samples by means of an improved and convenient multiplex PCR method. Exp. Hematol. 37, 728–738 (2009).
Wang, C. et al. High throughput sequencing reveals a complex pattern of dynamic interrelationships among human T cell subsets. Proc. Natl. Acad. Sci. USA 107, 1518–1523 (2010).
Sherwood, A. M. et al. Deep sequencing of the human TCRγ and TCRβ repertoires suggests that TCRβ rearranges after αβ and γδ T cell commitment. Sci. Transl. Med. 3, 90ra61 (2011).
Quail, M. A. et al. A large genome center’s improvements to the Illumina sequencing system. Nat. Methods 5, 1005–1010 (2008).
Knierim, E., Lucke, B., Schwarz, J. M., Schuelke, M. & Seelow, D. Systematic comparison of three methods for fragmentation of long-range PCR products for next generation sequencing. PLoS ONE 6, e28240 (2011).
Head, S. R. et al. Library construction for next-generation sequencing: overviews and challenges. BioTechniques 56, 61–64 (2014). 66, 68.
Bolotin, D. A. et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods 12, 380–381 (2015).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009).
Rodrigues, O. R. & Monard, S. A rapid method to verify single-cell deposition setup for cell sorters. Cytometry A 89, 594–600 (2016).
Acknowledgements
We thank W. Zhang for preparation of total RNA from PBMC samples. S.L., J.F., D.B.K., S.A.S., and K.J.L. were supported by NIH/NCI U24 CA224331. G.O. received generous support from the Mathers Family Foundation. J.F. was a participant in the Broad Cancer Genomics Scholars program at the Broad Institute. D.B.K. was supported by NIH/NCI R21 CA216772-01A1 and NCI-SPORE-2P50CA101942-11A1. S.A.S. was supported by NIH R50CA211482. C.J.W. was supported by NCI 1R01 CA155010-01A1 and Leukemia Lymphoma Translational Research Program Award 6460-15, and was a Scholar of the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Contributions
S.L. and K.J.L. devised the rhTCRseq scheme. S.L. developed and performed the rhTCRseq experimentation. J.S., R.A., J.F., R.J., L.R.O., and S.A.S. devised and wrote the computational pipeline and performed the data analysis. K.D. and Y.B. developed the rhPCR protocols and provided rhPCR primers. G.O. and D.B.K. provided single cells sorted into plates for the development of the rhTCRseq protocol. G.O. performed the single-cell preparation and sorting for Supplementary Fig. 3. C.J.W. and K.J.L. directed the overall study design. S.L., J.F., C.J.W., and K.J.L. wrote the manuscript with comments and editing by all authors.
Corresponding author
Ethics declarations
Competing interests
J.S. is currently an employee of Moderna Therapeutics. K.D. is an employee of IDT; Y.B. was an employee of IDT during the time of this work; K.J.L. was a paid consultant of IDT during a portion of this work; D.B.K. has previously advised Neon Therapeutics and owns equity in Aduro Biotech, Agenus Inc., Ampliphi BioSciences Corp., Biomarin Pharmaceutical Inc., Bristol-Myers Squibb Company, Celldex Therapeutics Inc., Editas Medicine Inc., Exelixis Inc., Gilead Sciences Inc., IMV Inc., Lexicon Pharmaceuticals Inc., Sangamo Therapeutics, and Stemline Therapeutics Inc.; C.J.W. is a founder of Neon Therapeutics and a member of its scientific advisory board. C.J.W. is subject to a conflict of interest management plan for the reported studies because of her competing financial interests in Neon Therapeutics. Under this plan, C.J.W. may not access identifiable human subjects data nor otherwise participate directly in the IRB-approved protocol reported herein. C.J.W.’s contributions to the overall program strategy and data analyses occurred on a de-identified basis. The remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related link
Key reference using this protocol
Keskin, D. B. et al. Nature 565, 234–239 (2019): https://doi.org/10.1038/s41586-018-0792-9.
Integrated supplementary information
Supplementary Figure 1 TCR repertoire analysis for five individuals.
RNA extracted from PBMCs of five healthy adult volunteers (anonymous donors designated N1-N5) was analyzed using the bulk RNA rhTCRseq protocol. The heat maps show the frequency distributions for TRAV (a) and TRBV (b) alleles for 320-ng samples. The allele designations are along the top of each heat map and the frequency of each allele is indicated by the color code.
Supplementary Figure 2 Reproducibility of the bulk RNA rhTCRseq protocol.
RNA extracted from PBMCs of anonymous donors N1-N5 was analyzed in eight replicates of 10 ng RNA and eight replicates of 40 ng per donor. Replicate results were combined to generate 80- and 320-ng samples. The plots show the clonotype-specific correlations for alpha (a) and beta (b) comparing the 80 ng and 320 ng results for sample N2. Correlation coefficients for the other four samples are reported in Supplementary Table 4. Correlation analysis was limited to comparing higher frequency clonotypes. For each individual, the frequency in the 80-ng results that corresponds to five UMI counts was used as the threshold frequency. Clonotypes in the 80-ng or the 320-ng data above this threshold were used for the correlation analysis. For clonotypes that are above the threshold in one dataset and below the threshold in the other, the actual frequency values below the threshold were used in the comparison.
Supplementary Figure 3 Gating strategy for isolating tumor-specific T cells.
The gating strategy for sorting T cells stimulated with autologous melanoma cells consisted of the following steps: (i) gating on single cells with lymphocyte physical parameter, (ii) gating on viable (Zombie Aqua negative) CD3+ CD4+ events, and (iii) gating on IL2-, TNFα-, and IFNγ-positive cells using unstimulated control sample to establish background signal. Single cells were sorted into plates using a 70-µm nozzle.
Supplementary Figure 4 Distribution of clones identified by single-cell TCR sequencing of tumor-stimulated T cells.
Histogram of number of cells per clone for 49 TCR clones identified using the rhTCRseq protocol for single cells.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4, Supplementary Note, and Supplementary Table 4
Supplementary Table 1
TCR primers
Supplementary Table 2
Barcode primers
Supplementary Table 3
Bulk RNA clonotypes
Supplementary Table 5
Single-cell clonotypes
Rights and permissions
About this article
Cite this article
Li, S., Sun, J., Allesøe, R. et al. RNase H–dependent PCR-enabled T-cell receptor sequencing for highly specific and efficient targeted sequencing of T-cell receptor mRNA for single-cell and repertoire analysis. Nat Protoc 14, 2571–2594 (2019). https://doi.org/10.1038/s41596-019-0195-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0195-x
This article is cited by
-
High-sensitive spatially resolved T cell receptor sequencing with SPTCR-seq
Nature Communications (2023)
-
The technological landscape and applications of single-cell multi-omics
Nature Reviews Molecular Cell Biology (2023)
-
Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy
Nature Reviews Clinical Oncology (2021)
-
Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma
Nature Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.