Abstract
There is compelling evidence that the human cerebellum is engaged in a wide array of motor and cognitive tasks. A fundamental question centers on whether the cerebellum is organized into distinct functional subregions. To address this question, we employed a rich task battery designed to tap into a broad range of cognitive processes. During four functional MRI sessions, participants performed a battery of 26 diverse tasks comprising 47 unique conditions. Using the data from this multi-domain task battery, we derived a comprehensive functional parcellation of the cerebellar cortex and evaluated it by predicting functional boundaries in a novel set of tasks. The new parcellation successfully identified distinct functional subregions, providing significant improvements over existing parcellations derived from task-free data. Lobular boundaries, commonly used to summarize functional data, did not coincide with functional subdivisions. The new parcellation provides a functional atlas to guide future neuroimaging studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The activation maps and functional parcellations are available from http://www.diedrichsenlab.org/imaging/mdtb.htm. The raw behavioral and imaging data for the cerebellum are also available on the data sharing repository https://openneuro.org/.
Code availability
The experimental code is available at https://github.com/maedbhk/MDTB-Cerebellum.
References
Ivry, R. B. & Baldo, J. V. Is the cerebellum involved in learning and cognition? Curr. Opin. Neurobiol. 2, 212–216 (1992).
Kelly, R. M. & Strick, P. L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 23, 8432–8444 (2003).
Allen, G., Buxton, R. B., Wong, E. C. & Courchesne, E. Attentional activation of the cerebellum independent of motor involvement. Science 275, 1940–1943 (1997).
Larsell, O. The development of the cerebellum in man in relation to its comparative anatomy. J. Comp. Neurol. 87, 85–129 (1947).
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C. & Yeo, B. T. T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322–2345 (2011).
Ji, J. L. et al. Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage 185, 35–57 (2019).
Guell, X., Schmahmann, J. D., Gabrieli, J. & Ghosh, S. S. Functional gradients of the cerebellum. eLife 7, e36652 (2018).
Stoodley, C. J., Valera, E. M. & Schmahmann, J. D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59, 1560–1570 (2012).
Stoodley, C. J. & Schmahmann, J. D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44, 489–501 (2009).
Wiestler, T., McGonigle, D. J. & Diedrichsen, J. Integration of sensory and motor representations of single fingers in the human cerebellum. J. Neurophysiol. 105, 3042–3053 (2011).
Ohtsuka, K. & Noda, H. Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey. J. Neurophysiol. 74, 1828–1840 (1995).
Nitschke, M. F. et al. Activation of cerebellar hemispheres in spatial memorization of saccadic eye movements: an fMRI study. Hum. Brain Mapp. 22, 155–164 (2004).
Horak, F. B. & Diener, H. C. Cerebellar control of postural scaling and central set in stance. J. Neurophysiol. 72, 479–493 (1994).
Diedrichsen, J. & Zotow, E. Surface-based display of volume-averaged cerebellar imaging data. PLoS ONE 10, e0133402 (2015).
Nguyen, V. T. et al. Distinct cerebellar contributions to cognitive-perceptual dynamics during natural viewing. Cereb. Cortex 27, 5652–5662 (2017).
Barch, D. M. et al. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 80, 169–189 (2013).
Diedrichsen, J. & Kriegeskorte, N. Representational models: a common framework for understanding encoding, pattern-component, and representational-similarity analysis. PLoS Comput. Biol. 13, e1005508 (2017).
Poldrack, R. A. et al. The Cognitive Atlas: toward a knowledge foundation for cognitive neuroscience. Front. Neuroinform. 5, 17 (2011).
Marek, S. et al. Spatial and temporal organization of the individual human cerebellum. Neuron 100, 977–993.e7 (2018).
Diedrichsen, J., Balsters, J. H., Flavell, J., Cussans, E. & Ramnani, N. A probabilistic MR atlas of the human cerebellum. Neuroimage 46, 39–46 (2009).
Airey, D. C., Lu, L. & Williams, R. W. Genetic control of the mouse cerebellum: identification of quantitative trait loci modulating size and architecture. J. Neurosci. 21, 5099–5109 (2001).
Apps, R. & Hawkes, R. Cerebellar cortical organization: a one-map hypothesis. Nat. Rev. Neurosci. 10, 670–681 (2009).
Sugihara, I. & Shinoda, Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J. Neurosci. 24, 8771–8785 (2004).
Leclerc, N., Doré, L., Parent, A. & Hawkes, R. The compartmentalization of the monkey and rat cerebellar cortex: zebrin I and cytochrome oxidase. Brain Res. 506, 70–78 (1990).
Lauritzen, M. Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. J. Cereb. Blood Flow Metab. 21, 1367–1383 (2001).
Hawkes, R., Gallagher, E. & Ozol, K. Blebs in the mouse cerebellar granular layer as a sign of structural inhomogeneity. 1. Anterior lobe vermis. Acta Anat. (Basel) 158, 205–214 (1997).
Tavor, I. et al. Task-free MRI predicts individual differences in brain activity during task performance. Science 352, 216–220 (2016).
Martin, T. A., Keating, J. G., Goodkin, H. P., Bastian, A. J. & Thach, W. T. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119, 1183–1198 (1996).
Andreasen, N. C. & Pierson, R. The role of the cerebellum in schizophrenia. Biol. Psychiatry 64, 81–88 (2008).
Moberget, T. et al. Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol. Psychiatry 23, 1512–1520 (2018).
Cornelissen, F. W. & Peters, E. M. The Eyelink Toolbox: eye tracking with MATLAB and the Psychophysics Toolbox. Behav. Res. Methods Instrum. Comput. 34, 613–617 (2002).
Ashburner, J. et al. SPM12 Manual www.fil.ion.ucl.ac.uk/spm/doc/spm12_manual.pdf (2019).
Van Essen, D. C. Cortical cartography and Caret software. Neuroimage 62, 757–764 (2012).
Diedrichsen, J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 33, 127–138 (2006).
Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113 (2007).
Sereno, M. I., Diedrichsen, J., Tachrout, M., Silva, G. & De Zeeuw, C. Reconstruction and unfolding of the human cerebellar cortex from high-resolution post-mortem MRI. Society for Neuroscience, abstr. 733 (2014).
Oosterhof, N. N., Wiestler, T., Downing, P. E. & Diedrichsen, J. A comparison of volume-based and surface-based multi-voxel pattern analysis. Neuroimage 56, 593–600 (2011).
Ding, C. H., Li, T. & Jordan, M. I. Convex and semi-nonnegative matrix factorizations. IEEE Trans. Pattern Anal. Mach. Intell. 32, 45–55 (2010).
Walther, A. et al. Reliability of dissimilarity measures for multi-voxel pattern analysis. Neuroimage 137, 188–200 (2016).
Acknowledgements
This work was supported by the James S. McDonnell Foundation (Scholar award to J.D.), the Canadian Institutes of Health Research (no. PJT 159520 to J.D.), a Platform Support Grant from Brain Canada and the Canada First Research Excellence Fund (BrainsCAN to Western University), the National Science Foundation (no. OAC-1649658 to R.A.P.) and the National Institute of Health (nos. NS092079 and NS105839 to R.B.I.). Data from the Human Connectome Project were analyzed by the Washington University–University of Minnesota Consortium (principal investigators: D. Van Essen and K. Ugurbil; no. 1U54MH091657) funded by the 16 National Institutes of Health (NIH) institutes and centers that support the NIH Blueprint for Neuroscience Research and by the McDonnell Center for Systems Neuroscience at Washington University. Thanks to J. Walters for assistance in task annotation.
Author information
Authors and Affiliations
Contributions
R.B.I. and J.D. originally conceived of the project. M.K., J.D. and R.B.I. designed the study. M.K. and C.R.H.-C. collected the data. M.K. and J.D. performed the analyses. R.A.P., M.K., J.D. and R.B.I. annotated the cognitive tasks. M.K., J.D. and R.B.I. wrote the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Neuroscience thanks Fenna Krienen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Integrated supplementary information
Supplementary Figure 1 Unthresholded, group-averaged activation maps for the 47 unique task conditions displayed on a surface-based representation of the cerebellar cortex14.
All activations are calculated relative to the mean activation across all conditions. Red-to-yellow colors indicate increases in activation and blue colors indicate decreases in activation. Activity is normalized by the root-mean-square-error of the time-series fit for each voxel.
Supplementary Figure 2 Unthresholded, individual activation maps for 4 representative tasks and motor feature maps for 11 representative participants.
All activations are calculated relative to the mean activation across all conditions. Red-to-yellow colors indicate increases in activation and blue colors indicate decreases in activation. Activity is normalized by the root-mean-square-error of the time-series fit for each voxel.
Supplementary Figure 3 Stability of task performance.
Percent accuracy, averaged across two scanning sessions, each composed of eight runs. Average across all tasks is shown in black. Poorest performance was on the spatial map task (red line) and best performance was on the IAPS emotion task (green line). Error-bars indicate between-subject (N=24) standard error.
Supplementary Figure 4 Representational task space for 47 unique task conditions.
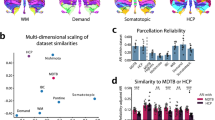
(a) Group-averaged representational dissimilarity matrix (RDM) data for the unique 47 task conditions. Shared tasks are averaged across the four scanning sessions. Dark blue represents low dissimilarity between pairwise task-evoked activity patterns while high distances (bright yellow) represent high dissimilarity between pairwise task-evoked activity patterns. Thresholded values are shown below the diagonal (dark blue cells indicating pairwise comparisons between task conditions were not significant (p<.001, e.g., pleasant and unpleasant scenes). (b) A multi-dimensional scaling plot (MDS, using first three PCs for display purposes), showing the relative similarity of the task-evoked activity patterns after correction for activity related to basic motor output. Hierarchical clustering was applied to the tasks, with colors in both the RDM and MDS indicating cluster membership.
Supplementary Figure 5 Comparison of task-based and task-free parcellations.
7, 10, and 17 region parcellations derived from task-free HCP (a-c) and MDTB (d-f) data. (g) Average Rand coefficient between task-free parcellations, computed locally (1cm sphere) around each cerebellar voxel. (h) Average Rand coefficient between MDTB parcellations. (i) Average difference of Rand coefficients for the MDTB and task-free parcellations.
Supplementary Figure 6 Cross-validated evaluation of MDTB parcellation on a subset of 7 tasks, selected to be most dissimilar to task conditions included in the data set.
For comparison purposes, task-free parcellations are evaluated on the same tasks. (a) MDTB parcellation trained on Set A and evaluated on 7 tasks from Set B (Mental Rotation Easy, Mental Rotation Medium, Mental Rotation Hard, Spatial Map Medium, Spatial Map Hard, Animated Movie, and Nature Movie). (b) MDTB parcellation trained on Set B and evaluated on 7 tasks from Set A (Sad Faces, Interval Timing, Go, Theory of Mind, Word Reading, Motor Imagery, Math). Error-bars indicate between-subject standard error (N=24).
Supplementary Figure 7 Pearson correlation between the task-profiles of the 10 regions of the MDTB parcellation.
The values in the correlation matrix are scaled between 0 (blue) and 1 (yellow). The bar on the right denotes the colors of each of the 10 regions (see Fig 5).
Supplementary information
Rights and permissions
About this article
Cite this article
King, M., Hernandez-Castillo, C.R., Poldrack, R.A. et al. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci 22, 1371–1378 (2019). https://doi.org/10.1038/s41593-019-0436-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0436-x
This article is cited by
-
Cerebellar microstructural abnormalities in patients with somatic symptom disorders
BMC Psychiatry (2025)
-
Atypical early neural responses to native and non-native language in infants at high likelihood for developing autism
Molecular Autism (2025)
-
Errors of attention adaptively warp spatial cognition
Nature Human Behaviour (2025)
-
Neuroimaging-based variability in subtyping biomarkers for psychiatric heterogeneity
Molecular Psychiatry (2025)
-
Alterations in cerebral perfusion and corresponding brain functional networks in systemic lupus erythematosus with cognitive impairment
Scientific Reports (2025)