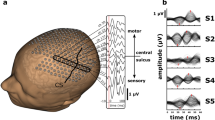
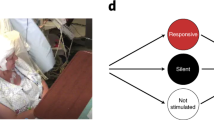
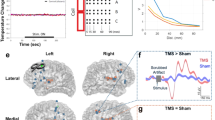
Abstract
Intracranial electroencephalography (iEEG), also known as electrocorticography when using subdural grid electrodes or stereotactic EEG when using depth electrodes, is blossoming in various fields of human neuroscience. In this article, we highlight the potentials of iEEG in exploring functions of the human brain while also considering its limitations. The iEEG signal provides anatomically precise information about the selective engagement of neuronal populations at the millimeter scale and the temporal dynamics of their engagement at the millisecond scale. If several nodes of a given network are monitored simultaneously with implanted electrodes, the iEEG signals can also reveal information about functional interactions within and across networks during different stages of neural computation. As such, human iEEG can complement other methods of neuroscience beyond simply replicating what is already known, or can be known, from noninvasive lines of research in humans or from invasive recordings in nonhuman mammalian brains.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Caton, R. The electric currents of the brain. BMJ 2, 278 (1875).
Beck, A. Die Bestimmung der Localisation der Gehirn- und Ruckenmarkfunctionen vermittelst der electrischen Erscheinungen. Centralbl. Physiol. 4, 473–476 (1890).
Berger, H. Öber das Elektroenkephalogramm des Menschen. Arch. Psychiatr. Nervenkr. 87, 527–570 (1929).
Kwan, P., Schachter, S. C. & Brodie, M. J. Drug-resistant epilepsy. N. Engl. J. Med. 365, 919–926 (2011).
Engel, J. Jr. Surgery for seizures. N. Engl. J. Med. 334, 647–652 (1996).
Semah, F. et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 51, 1256–1262 (1998).
Engel, A. K., Moll, C. K., Fried, I. & Ojemann, G. A. Invasive recordings from the human brain: clinical insights and beyond. Nat. Rev. Neurosci. 6, 35–47 (2005).
Mukamel, R. & Fried, I. Human intracranial recordings and cognitive neuroscience. Annu. Rev. Psychol. 63, 511–537 (2012).
Parvizi, J. Corticocentric myopia: old bias in new cognitive sciences. Trends Cogn. Sci. 13, 354–359 (2009).
Saalmann, Y. B., Pinsk, M. A., Wang, L., Li, X. & Kastner, S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756 (2012).
Wimmer, R. D. et al. Thalamic control of sensory selection in divided attention. Nature 526, 705–709 (2015).
Miller, K. J., Sorensen, L. B., Ojemann, J. G. & den Nijs, M. Power-law scaling in the brain surface electric potential. PLOS Comput. Biol. 5, e1000609 (2009).
Miller, K. J. Broadband spectral change: evidence for a macroscale correlate of population firing rate? J. Neurosci. 30, 6477–6479 (2010).
Winawer, J. et al. Asynchronous broadband signals are the principal source of the BOLD response in human visual cortex. Curr. Biol. 23, 1145–1153 (2013).
Jiruska, P. et al. Update on the mechanisms and roles of high-frequency oscillations in seizures and epileptic disorders. Epilepsia 58, 1330–1339 (2017).
Schevon, C. A. et al. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain 132, 3047–3059 (2009).
Ray, S., Crone, N. E., Niebur, E., Franaszczuk, P. J. & Hsiao, S. S. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J. Neurosci. 28, 11526–11536 (2008).
Ray, S. & Maunsell, J. H. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 9, e1000610 (2011).
Kreiman, G. et al. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron 49, 433–445 (2006).
Liu, J. & Newsome, W. T. Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J. Neurosci. 26, 7779–7790 (2006).
Mukamel, R. et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science 309, 951–954 (2005).
Katzner, S. et al. Local origin of field potentials in visual cortex. Neuron 61, 35–41 (2009).
Engel, A. K., König, P., Gray, C. M. & Singer, W. Stimulus-dependent neuronal oscillations in cat visual cortex: inter-columnar interaction as determined by cross-correlation analysis. Eur. J. Neurosci. 2, 588–606 (1990).
Park, S. H. et al. Functional subpopulations of neurons in a macaque face patch revealed by single-unit fMRI mapping. Neuron 95, 971–981.e975 (2017).
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001).
Nir, Y. et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr. Biol. 17, 1275–1285 (2007).
Manning, J. R., Jacobs, J., Fried, I. & Kahana, M. J. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J. Neurosci. 29, 13613–13620 (2009).
Niessing, J. et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309, 948–951 (2005).
Flinker, A., Chang, E. F., Barbaro, N. M., Berger, M. S. & Knight, R. T. Sub-centimeter language organization in the human temporal lobe. Brain Lang. 117, 103–109 (2011).
Mesgarani, N., Cheung, C., Johnson, K. & Chang, E. F. Phonetic feature encoding in human superior temporal gyrus. Science 343, 1006–1010 (2014).
Hermes, D. et al. Neurophysiologic correlates of fMRI in human motor cortex. Hum. Brain Mapp. 33, 1689–1699 (2012).
Miller, K. J. et al. Spectral changes in cortical surface potentials during motor movement. J. Neurosci. 27, 2424–2432 (2007).
Foster, B. L., Dastjerdi, M. & Parvizi, J. Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing. Proc. Natl Acad. Sci. USA 109, 15514–15519 (2012).
Buzsaki, G. Rhythms of the Brain. (Oxford University Press, Oxford, 2006).
VanRullen, R. Perceptual cycles. Trends Cogn. Sci. 20, 723–735 (2016).
Canolty, R. T. & Knight, R. T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 14, 506–515 (2010).
Schroeder, C. E. & Lakatos, P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 32, 9–18 (2009).
Voytek, B. et al. Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat. Neurosci. 18, 1318–1324 (2015).
Szczepanski, S. M. et al. Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. PLoS Biol. 12, e1001936 (2014).
Daitch, A. L. et al. Mapping human temporal and parietal neuronal population activity and functional coupling during mathematical cognition. Proc. Natl Acad. Sci. USA 113, E7277–E7286 (2016).
Tang, H. et al. Spatiotemporal dynamics underlying object completion in human ventral visual cortex. Neuron 83, 736–748 (2014).
Raichle, M. E. Neuroscience. The brain’s dark energy. Science 314, 1249–1250 (2006).
Parvizi, J. et al. Electrical stimulation of human fusiform face-selective regions distorts face perception. J. Neurosci. 32, 14915–14920 (2012).
Ball, T., Kern, M., Mutschler, I., Aertsen, A. & Schulze-Bonhage, A. Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage 46, 708–716 (2009).
Chao, Z. C., Nagasaka, Y. & Fujii, N. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Front. Neuroeng. 3, 3 (2010).
Pasley, B. N. et al. Reconstructing speech from human auditory cortex. PLoS Biol. 10, e1001251 (2012).
Rutishauser, U., Ross, I. B., Mamelak, A. N. & Schuman, E. M. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907 (2010).
Jacobs, J., Kahana, M. J., Ekstrom, A. D. & Fried, I. Brain oscillations control timing of single-neuron activity in humans. J. Neurosci. 27, 3839–3844 (2007).
Zanos, S., Zanos, T. P., Marmarelis, V. Z., Ojemann, G. A. & Fetz, E. E. Relationships between spike-free local field potentials and spike timing in human temporal cortex. J. Neurophysiol. 107, 1808–1821 (2012).
Svoboda, E., McKinnon, M. C. & Levine, B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 44, 2189–2208 (2006).
Nir, Y., Dinstein, I., Malach, R. & Heeger, D. J. BOLD and spiking activity. Nat. Neurosci. 11, 523–524 (2008).
Keller, C. J. et al. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J. Neurosci. 33, 6333–6342 (2013).
Foster, B. L., Rangarajan, V., Shirer, W. R. & Parvizi, J. Intrinsic and task-dependent coupling of neuronal population activity in human parietal cortex. Neuron 86, 578–590 (2015).
Desmurget, M. et al. Movement intention after parietal cortex stimulation in humans. Science 324, 811–813 (2009).
Parvizi, J., Rangarajan, V., Shirer, W. R., Desai, N. & Greicius, M. D. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron 80, 1359–1367 (2013).
Fried, I., Wilson, C. L., MacDonald, K. A. & Behnke, E. J. Electric current stimulates laughter. Nature 391, 650 (1998).
Blanke, O., Ortigue, S., Landis, T. & Seeck, M. Stimulating illusory own-body perceptions. Nature 419, 269–270 (2002).
Rangarajan, V. et al. Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. J. Neurosci. 34, 12828–12836 (2014).
Selimbeyoglu, A. & Parvizi, J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Front. Hum. Neurosci. 4, 46 (2010).
Morrell, M. J. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77, 1295–1304 (2011).
Rosin, B. et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72, 370–384 (2011).
Widge, A. S. et al. Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Exp. Neurol. 287, 461–472 (2017).
Pesaran, B., Musallam, S. & Andersen, R. A. Cognitive neural prosthetics. Curr. Biol. 16, R77–R80 (2006).
Cogan, G. B. et al. Sensory-motor transformations for speech occur bilaterally. Nature 507, 94–98 (2014).
Beauchamp, M. S., Sun, P., Baum, S. H., Tolias, A. S. & Yoshor, D. Electrocorticography links human temporoparietal junction to visual perception. Nat. Neurosci. 15, 957–959 (2012).
Tsao, D. Y., Moeller, S. & Freiwald, W. A. Comparing face patch systems in macaques and humans. Proc. Natl Acad. Sci. USA 105, 19514–19519 (2008).
Pinsk, M. A. et al. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J. Neurophysiol. 101, 2581–2600 (2009).
Halgren, E. et al. Laminar profile of spontaneous and evoked theta: Rhythmic modulation of cortical processing during word integration. Neuropsychologia 76, 108–124 (2015).
Cash, S. S. et al. The human K-complex represents an isolated cortical down-state. Science 324, 1084–1087 (2009).
Kiani, R. et al. Natural grouping of neural responses reveals spatially segregated clusters in prearcuate cortex. Neuron 85, 1359–1373 (2015).
Buffalo, E. A., Fries, P., Landman, R., Buschman, T. J. & Desimone, R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc. Natl Acad. Sci. USA 108, 11262–11267 (2011).
Bastos, A. M. et al. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85, 390–401 (2015).
Chang, E. F. Towards large-scale, human-based, mesoscopic neurotechnologies. Neuron 86, 68–78 (2015).
Litt, B. Engineering the next generation of brain scientists. Neuron 86, 16–20 (2015).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2015).
Lilly, J. C., Hughes, J. R., Alvord, E. C. Jr & Galkin, T. W. Brief, noninjurious electric waveform for stimulation of the brain. Science 121, 468–469 (1955).
Foutz, T. J. & McIntyre, C. C. Evaluation of novel stimulus waveforms for deep brain stimulation. J. Neural Eng. 7, 066008 (2010).
Famm, K., Litt, B., Tracey, K. J., Boyden, E. S. & Slaoui, M. Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161 (2013).
Litt, B. Evaluating devices for treating epilepsy. Epilepsia 44 (Suppl. 7), 30–37 (2003).
Schalk, G. & Leuthardt, E. C. Brain-computer interfaces using electrocorticographic signals. IEEE Rev. Biomed. Eng. 4, 140–154 (2011).
Andersen, R. A., Burdick, J. W., Musallam, S., Pesaran, B. & Cham, J. G. Cognitive neural prosthetics. Trends Cogn. Sci. 8, 486–493 (2004).
Bassett, D. S. & Sporns, O. Network neuroscience. Nat. Neurosci. 20, 353–364 (2017).
Power, J. D. et al. Functional network organization of the human brain. Neuron 72, 665–678 (2011).
Kriegeskorte, N. et al. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron 60, 1126–1141 (2008).
Shum, J. et al. A brain area for visual numerals. J. Neurosci. 33, 6709–6715 (2013).
Penfield, W. & Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443 (1937).
Milner, B., Squire, L. R. & Kandel, E. R. Cognitive neuroscience and the study of memory. Neuron 20, 445–468 (1998).
Gazzaniga, M. S. Review of the split brain. J. Neurol. 209, 75–79 (1975).
Elger, C. E., Helmstaedter, C. & Kurthen, M. Chronic epilepsy and cognition. Lancet Neurol. 3, 663–672 (2004).
Motamedi, G. & Meador, K. Epilepsy and cognition. Epilepsy Behav. 4 (Suppl. 2), S25–S38 (2003).
Meador, K. J. Networks, cognition, and epilepsy. Neurology 77, 930–931 (2011).
Holmes, M. D. & Tucker, D. M. Identifying the epileptic network. Front. Neurol. 4, 84 (2013).
Holmes, G. L. & Lenck-Santini, P. P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 8, 504–515 (2006).
Aldenkamp, A. P. & Arends, J. Effects of epileptiform EEG discharges on cognitive function: is the concept of “transient cognitive impairment” still valid? Epilepsy Behav. 5 (Suppl. 1), S25–S34 (2004).
Binnie, C. D. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2, 725–730 (2003).
Jones-Gotman, M. Localization of lesions by neuropsychological testing. Epilepsia 32 (Suppl. 5), S41–S52 (1991).
Glennon, J. M. et al. Interictal epileptiform discharges have an independent association with cognitive impairment in children with lesional epilepsy. Epilepsia 57, 1436–1442 (2016).
Loring, D. W., Kapur, R., Meador, K. J. & Morrell, M. J. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia 56, 1836–1844 (2015).
Groppe, D. M. et al. iELVis: An open source MATLAB toolbox for localizing and visualizing human intracranial electrode data. J. Neurosci. Methods 281, 40–48 (2017).
Hermes, D., Miller, K. J., Noordmans, H. J., Vansteensel, M. J. & Ramsey, N. F. Automated electrocorticographic electrode localization on individually rendered brain surfaces. J. Neurosci. Methods 185, 293–298 (2010).
Acknowledgements
We thank the following colleagues for their time discussing the promises and limitations of iEEG with us: G. Buszáki, S. Dehaene, J. DiCarlo, E. Halgren, R. Kiani, M. Kahana, R. Knight, C. Koch, N. Logotethis, R. Malach, M.-B. Moser, B. Newsome, A. Nieder, U. Rutishauser and A. Wagner. We gratefully acknowledge funding support from the US National Institute of Health (1R01MH109954-01 to J.P.; 2RO1MH064043-12, 5RO1EY017699-09, Silvio O. Conte Center 21560-685 to S.K. and J.P.), the US National Science Foundation (BCS1358907 to JP; BCS-1328270 to S.K.) and the James S. McDonnell Foundation to S.K.
Author information
Authors and Affiliations
Contributions
J.P. and S.K. designed the form and content of the manuscript; J.P. wrote the first draft of the manuscript and J.P. and S.K. edited several revisions of it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parvizi, J., Kastner, S. Promises and limitations of human intracranial electroencephalography. Nat Neurosci 21, 474–483 (2018). https://doi.org/10.1038/s41593-018-0108-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0108-2
This article is cited by
-
Non-invasive canine electroencephalography (EEG): a systematic review
BMC Veterinary Research (2025)
-
The case for hemispheric lateralization of the human amygdala in fear processing
Molecular Psychiatry (2025)
-
Revisiting the role of computational neuroimaging in the era of integrative neuroscience
Neuropsychopharmacology (2025)
-
Simultaneous intracranial recordings of interacting brains reveal neurocognitive dynamics of human cooperation
Nature Neuroscience (2025)
-
Cortical processing of discrete prosodic patterns in continuous speech
Nature Communications (2025)