Abstract
Bispecific T cell engagers (BiTEs) kill B cells by engaging T cells. BiTEs are highly effective in acute lymphoblastic leukemia. Here we treated six patients with multidrug-resistant rheumatoid arthritis (RA) with the CD19xCD3 BiTE blinatumomab under compassionate use. Low doses of blinatumomab led to B cell depletion and concomitant decrease of T cells, documenting their engager function. Treatment was safe, with brief increase in body temperature and acute phase proteins during first infusion but no signs of clinically relevant cytokine-release syndrome. Blinatumomab led to a rapid decline in RA clinical disease activity in all patients, improved synovitis in ultrasound and FAPI-PET-CT and reduced autoantibodies. High-dimensional flow cytometry analysis of B cells documented an immune reset with depletion of activated memory B cells, which were replaced by nonclass-switched IgD-positive naïve B cells. Together, these data suggest the feasibility and potential for BiTEs to treat RA. This approach warrants further exploration on other B-cell-mediated autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All numerical data in this paper can be obtained from the corresponding author within 4 weeks upon request via email to georg.schett@uk-erlangen.de. Patient data can be shared only in pseudonymized form. Otherwise, there are no restrictions to data access. All data graphs in the figures (Figs. 1b–f, 2a,e–g, 3a–e,i and 4a–i and Extended Data Figs. 1, 2a–g and 3a–m) show raw data and depict individual values. Source data are provided with this paper.
Code availability
No custom code was created for this analysis.
References
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Gravallese, E. M. & Firestein, G. S. Rheumatoid arthritis—common origins, divergent mechanisms. N. Engl. J. Med. 388, 529–542 (2023).
Malmström, V., Catrina, A. I. & Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat. Rev. Immunol. 17, 60–75 (2016).
Humby, F. et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 6, 59–75 (2009).
Edwards, J. C. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 (2004).
Buch, M. H. Defining refractory rheumatoid arthritis. Ann. Rheum. Dis. 77, 966–969 (2018).
Rivellese, F. et al. Rituximab versus tocilizumab in rheumatoid arthritis: synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat. Med. 28, 1256–1268 (2022).
Kremer, J. M. et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N. Engl. J. Med. 349, 1907–1915 (2003).
Goebeler, M. E. & Bargou, R. C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 17, 418–434 (2020).
Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 (2017).
Gruen, M., Bommert, K. & Bargou, R. C. T-cell-mediated lysis of B cells induced by a CD19×CD3 bispecific single-chain antibody is perforin dependent and death receptor independent. Cancer Immunol. Immunother. 53, 625–632 (2004).
Loffler, A. et al. A recombinant bispecific single-chain antibody, CD19×CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 95, 2098–2103 (2000).
Hoffmann, P. et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J. Cancer 115, 98–104 (2005).
Offner, S., Hofmeister, R., Romaniuk, A., Kufer, P. & Baeuerle, P. A. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol. Immunol. 43, 763–771 (2006).
Bargou, R. et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 (2008).
Topp, M. S. et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 29, 2493–2498 (2011).
Vos, K. et al. Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis Rheum. 56, 772–778 (2007).
Schett, G., Tanaka, Y. & Isaacs, J. D. Why remission is not enough: underlying disease mechanisms in RA that prevent cure. Nat. Rev. Rheumatol. 17, 135–144 (2021).
Genovese, M. C. et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 353, 1114–1123 (2005).
Jois, R. N., Masding, A., Somerville, M., Gaffney, K. & Scott, D. G. Rituximab therapy in patients with resistant rheumatoid arthritis: real-life experience. Rheumatol. (Oxf.) 46, 980–982 (2007).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022).
Bergmann, C. et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann. Rheum. Dis. 82, 1117–1120 (2023).
Muller, F. et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet 401, 815–818 (2023).
Muller, F. et al. CD19 CAR T-cell therapy in autoimmune disease - a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Hutchings, M. et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 398, 1157–1169 (2021).
Moreau, P. et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 387, 495–505 (2022).
Lesokhin, A. M. et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat. Med. 29, 2259–2267 (2023).
Taubmann, J. et al. Chimeric antigen receptor T cell treatment: unraveling the role of B cells in systemic lupus erythematosus. Arthritis Rheumatol. 76, 497–504 (2024).
Teachey, D. T. et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121, 5154–5157 (2013).
Aletaha, D. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588 (2010).
Wells, G. et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann. Rheum. Dis. 68, 954–960 (2009).
D’Agostino, M. A. et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open 3, e000428 (2017).
Kuwert, T., Schmidkonz, C., Prante, O., Schett, G. & Ramming, A. FAPI PET opens a new window to understanding immune-mediated inflammatory diseases. J. Nucl. Med. 63, 1136–1137 (2022).
Alivernini, S. et al. Inclusion of synovial tissue-derived characteristics in a nomogram for the prediction of treatment response in treatment-naive rheumatoid arthritis patients. Arthritis Rheumatol. 73, 1601–1613 (2021).
Krenn, V. et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 49, 358–364 (2006).
Acknowledgements
We thank J. Verhagen and A. Wegner for performing tissue imaging, H. Bang (Orgentec) for performing antimodified peptide autoantibody measurements and T. Winkler for helpful discussions. This study was supported by the Deutsche Forschungsgemeinschaft (DFG) through the Leibniz Award (G.S.), GR 5979/2-1 (R.G.-B.), the research group FOR2886 and the CRC1181, CRC1483, CRC/TRR305 and CRC/TRR221, the Else Kröner-Fresenius-Stiftung through 2022_EKEA.72 (to R.G.-B.) and the IZKF Erlangen (grant N10, to R.G.-B.). Further funding has been obtained from the Bundesministerium für Bildung und Forschung through the MASCARA project and the European Union (ERC Synergy grant 4D Nanoscope). We also acknowledge funding from Staedtler Stiftung.
Author information
Authors and Affiliations
Contributions
L.B., M.H., G.S. and R.G.-B. conceived the study and contributed to the study design. L.B., M.H., G.S. and R.G.-B. performed clinical and experimental data generation and statistical analysis. T.R., M.G.R., F.F., C.T., A. Wirsching, J.W., A. Wilhelm, J.-P.A., M.P., M.E., S.A., A.Z., G.K., S.U., A.B. and M.-A.D’A. contributed to clinical data generation, data analysis, interpretation and writing. All authors contributed to data interpretation and edited the paper.
Corresponding author
Ethics declarations
Competing interests
There are no conflicts of interest related to this study. The study received no commercial funding. M.-A.D.’A. has received speaker honoraria from Abbvie, Novartis and Janssen, which were not related to this study. G.S. has received speaker honoraria from Janssen and Novartis, which were not related to this study. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Lihua Budde, David Fox and Deepak Rao for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
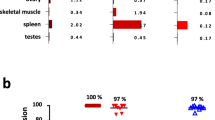
Extended Data Fig. 1 Acute phase cytokines by individual patient.
Serum cytokines were analyzed by Luminex assay (N = 6 for time points T0, T2, T3, T5 and N = 4 for time points T1, T4).
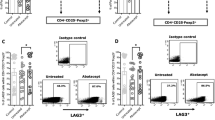
Extended Data Fig. 2 T cell subsets after blinatumomab treatment in RA.
Effects of blinatumomab on (a) CD4+ and CD8 + T cells, (b) the CD4/CD8 ratio, (c) the proportion of naïve, effector memory (EM) and central memory (CM) CD4 + T cells, (d) the proportion of naïve, effector memory (EM) and central memory (CM) CD8 + T cells, (e) terminally differentiated effector memory CD4+ cells re-expressing CD45RA (TEMRA) (f) Foxp3+ regulatory T cells, (g) terminally differentiated effector memory CD8+ cells re-expressing CD45RA (TEMRA), (h) RORgt+ Th17 cells. N = 5 individual patients. Dark line depicts the median.
Extended Data Fig. 3 24 week follow-up after blinatumomab therapy during abatacept maintenance.
(a) tender joint count, (b) swollen joint count, (c) patient global disease activity (visual analogue scale; 0–100) and (d) disease activity score 28 (DAS28). Hatched lines show cut-off for remission (2.6). (e) rheumatoid factor, (f) anti-cyclic citrullinated peptide (CCP)-2 autoantibodies, (g) anti-citrullinated, (h) anti-carbamylated, (i) anti-acetylated vimentin autoantibodies and (j) non-modified vimentin autoantibodies (control) (k) immunoglobulin G, (l) immunoglobulin A and (m) immunoglobulin M levels. N = 6 individual patients. Dark line depicts the median.
Supplementary information
Supplementary Data 1
Source data for Extended Data Fig. 1.
Supplementary Data 2
Source data for Extended Data Fig. 2.
Supplementary Data 3
Source data for Extended Data Fig. 3.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bucci, L., Hagen, M., Rothe, T. et al. Bispecific T cell engager therapy for refractory rheumatoid arthritis. Nat Med 30, 1593–1601 (2024). https://doi.org/10.1038/s41591-024-02964-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-02964-1