Abstract
Allergic inflammation has crucial roles in allergic diseases such as asthma. It is therefore important to understand why and how the immune system responds to allergens. Here we found that full-length interleukin 33 (IL-33FL), an alarmin cytokine with critical roles in type 2 immunity and asthma, functioned as a protease sensor that detected proteolytic activities associated with various environmental allergens across four kingdoms, including fungi, house dust mites, bacteria and pollens. When exposed to allergen proteases, IL-33FL was rapidly cleaved in its central ‘sensor’ domain, which led to activation of the production of type 2 cytokines in group 2 innate lymphoid cells. Preventing cleavage of IL-33FL reduced allergic airway inflammation. Our findings reveal a molecular mechanism for the rapid induction of allergic type 2 inflammation following allergen exposure, with important implications for allergic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Locksley, R. M. Asthma and allergic inflammation. Cell 140, 777–783 (2010).
Lambrecht, B. N. & Hammad, H. The immunology of asthma. Nat. Immunol. 16, 45–56 (2015).
Palm, N. W., Rosenstein, R. K. & Medzhitov, R. Allergic host defences. Nature 484, 465–472 (2012).
Hammad, H. & Lambrecht, B. N. Barrier epithelial cells and the control of type 2 immunity. Immunity 43, 29–40 (2015).
Trompette, A. et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588 (2009).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Stewart, G. A. & Thompson, P. J. The biochemistry of common aeroallergens. Clin. Exp. Allergy 26, 1020–1044 (1996).
Van Dyken, S. J. et al. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity 40, 414–424 (2014).
Palm, N. W. et al. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 39, 976–985 (2013).
Jarrett, R. et al. Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase. Sci. Transl. Med. 8, 325ra318 (2016).
Sokol, C. L., Barton, G. M., Farr, A. G. & Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 9, 310–318 (2008).
Millien, V. O. et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341, 792–796 (2013).
Rosenstein, R. K., Bezbradica, J. S., Yu, S. & Medzhitov, R. Signaling pathways activated by a protease allergen in basophils. Proc. Natl. Acad. Sci. USA 111, E4963–E4971 (2014).
Gottar, M. et al. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 127, 1425–1437 (2006).
Cheng, Z. et al. Pathogen-secreted proteases activate a novel plant immune pathway. Nature 521, 213–216 (2015).
Cayrol, C. & Girard, J. P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 31C, 31–37 (2014).
Molofsky, A. B., Savage, A. K. & Locksley, R. M. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 42, 1005–1019 (2015).
Liew, F. Y., Girard, J. P. & Turnquist, H. R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 16, 676–689 (2016).
Cayrol, C. & Girard, J. P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 281, 154–168 (2018).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Moffatt, M. F. et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 363, 1211–1221 (2010).
Smith, D. et al. A rare IL33 loss-of-function mutation reduces blood eosinophil counts and protects from asthma. PLoS Genet. 13, e1006659 (2017).
Moussion, C., Ortega, N. & Girard, J. P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 3, e3331 (2008).
Cayrol, C. & Girard, J. P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 106, 9021–9026 (2009).
Luthi, A. U. et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 (2009).
Cohen, E. S. et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat. Commun. 6, 8327 (2015).
Lefrancais, E. et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 109, 1673–1678 (2012).
Lefrancais, E. et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 111, 15502–15507 (2014).
Kouzaki, H., Iijima, K., Kobayashi, T., O’Grady, S. M. & Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 186, 4375–4387 (2011).
Doherty, T. A. et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L577–L588 (2012).
Haenuki, Y. et al. A critical role of IL-33 in experimental allergic rhinitis. J. Allergy Clin. Immunol. 130, 184–194 (2012).
Kamijo, S. et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J. Immunol. 190, 4489–4499 (2013).
Snelgrove, R. J. et al. Alternaria-derived serine protease activity drives IL-33 mediated asthma exacerbations. J. Allergy Clin. Immunol. 134, 583–592 (2014).
Osbourn, M. et al. HpARI protein secreted by a helminth parasite suppresses interleukin-33. Immunity 47, 739–751 (2017).
McSorley, H. J., Blair, N. F., Smith, K. A., McKenzie, A. N. & Maizels, R. M. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 7, 1068–1078 (2014).
Oboki, K. et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. USA 107, 18581–18586 (2010).
Florsheim, E. et al. Integrated innate mechanisms involved in airway allergic inflammation to the serine protease subtilisin. J. Immunol. 194, 4621–4630 (2015).
Halim, T. Y. et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 (2014).
Hara, K. et al. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J. Immunol. 192, 4032–4042 (2014).
Molofsky, A. B. et al. Interleukin-33 and interferon-γ counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 43, 161–174 (2015).
Chen, W. Y., Hong, J., Gannon, J., Kakkar, R. & Lee, R. T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl. Acad. Sci. USA 112, 7249–7254 (2015).
McNeil, P. L., Muthukrishnan, L., Warder, E. & D’Amore, P. A. Growth factors are released by mechanically wounded endothelial cells. J. Cell Biol. 109, 811–822 (1989).
Smithgall, M. D. et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 20, 1019–1030 (2008).
O’Hollaren, M. T. et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N. Engl. J. Med. 324, 359–363 (1991).
Pulimood, T. B., Corden, J. M., Bryden, C., Sharples, L. & Nasser, S. M. Epidemic asthma and the role of the fungal mold Alternaria alternata. J. Allergy Clin. Immunol. 120, 610–617 (2007).
Porter, P. et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2, 504–517 (2009).
Kouzaki, H. et al. Endogenous protease inhibitors in airway epithelial cells contribute to eosinophilic chronic rhinosinusitis. Am. J. Respir. Crit. Care Med. 195, 737–747 (2017).
Fux, M. et al. IL-33 is a mediator rather than a trigger of the acute allergic response in humans. Allergy 69, 216–222 (2014).
Beausejour, A., Grenier, D., Goulet, J. P. & Deslauriers, N. Proteolytic activation of the interleukin-1beta precursor by Candida albicans. Infect. Immun. 66, 676–681 (1998).
LaRock, C. N. et al. IL-1beta is an innate immune sensor of microbial proteolysis. Sci. Immunol. 1 (2016).
Pichery, M. et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 188, 3488–3495 (2012).
Vizcaino, J. A. et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223–226 (2014).
Vizcaino, J. A. et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 (2016).
Acknowledgements
We thank members of the Girard and Burlet-Schiltz laboratories for discussions; the IPBS Anexplo, TRI Imaging and Proteomics core facilities; J. Lee (Mayo Clinic) for antibodies; E. Lefrançais for preliminary observations; J. van Meerwijk and E. Espinosa for comments on the manuscript; F. Viala for iconography; F. Amalric for advice on initial proteomic analyses; and N. Ortega for help with immunohistochemistry and animal ethics. Supported by Agence Nationale de la Recherche (A.D. and M.C.; and ANR-12-BSV3-0005-01 and ANR-16-CE15-0009-01 to J.-P.G.); the French Ministry of Research (P.S.); and Région Midi-Pyrénées, European funds (Fonds Européens de Développement Régional), Toulouse Métropole and the French Ministry of Research with the ‘Investissement d’Avenir Infrastructures Nationales en Biologie et Santé program’ (Proteomics French Infrastructure project ANR-10-INBS-08) (all for the IBiSA Toulouse Proteomics facility).
Author information
Authors and Affiliations
Contributions
C.C and J.-P.G. conceived of the study, supervised the work and planned experiments; C.C performed the majority of biochemical analyses and cellular assays; A.D. isolated ILC2s and performed in vivo experiments with the help of P.S.; S.R. prepared recombinant proteins and samples for mass spectrometry; M.C. and A.S. performed mass spectrometry under the supervision of O.B.-S. and A.G.d.P.; J.-P.G. wrote the manuscript with input from C.C and A.G.d. P.; and all authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 IL-33 is cleaved by both serine and cysteine proteases from environmental allergens
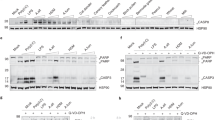
Recombinant human IL-33FL was analyzed by immunoblot after incubation with allergen extracts or proteases for 1 h at 37 °C. Cleavage assay was performed in the presence of cysteine (E64) or serine (AEBSF) protease inhibitors, or EDTA (A. fumigatus, n=2 experiments; H. solani, n=2 experiments; Bromelain, n=2 experiments; Timothy, n=2 experiments; Der p 1, n=2 experiments; Papaïn, n=2 experiments; Subtilisin, n=1 experiment). White arrowheads: IL-33FL; black arrowheads: cleaved IL-33 forms. Cropped images are shown.
Supplementary Figure 2 Mass spectrometry analysis of cleavage sites for different protease allergens in human IL-33FL
(a) Illustration of the peptide mapping process on the IL-33 sequence, leading to peptide sequences listed in table S1. Peptides used to map the N-ter or C-ter domains after trypsin cleavage (underlined in red) or endoGlu-C cleavage (underlined in blue) are indicated, as well as the main trypsin and endoGlu-C cleavage sites in the central domain generating MS detectable neo N-terminal peptides from the allergen-processed forms of IL-33 (start shown with grey arrows). (b) Extracted chromatograms corresponding to the MS signal of the doubly charged peptide ion 109–121 HDSSITGISPITE 2+ (monoisotopic m/z=678.83), detected only after processing of IL-33FL with some allergens (Full-length control, n=5 replicates; Timothy, n=1 replicate; Papaïn, n=2 replicates; Ragweed, n=1 replicate; A. Oryzae, n=2 replicates; A. alternata, n=3 replicates; A. fumigatus, n=1 replicate; H. solani, n=1 replicate; Der p 1, n=2 replicates; Subtilisin, n=1 replicate). Blue, red and green curves correspond respectively to the first, second, and third isotopes of the peptide ion. (c) Example of MS/MS sequencing spectra supporting the identification of some of neo-N terminal peptides reported in Extended Data Table 1: sequence 95–103 AFGISGVQK (Mascot ion score of 37, peptide ion m/z=453.7557, processing with Timothy and trypsin cleavage); sequence 103–121 KYTRALHDSSITGISPITE (Mascot ion score of 19, peptide ion m/z=697.0364, processing with A. alternata and endoGlu-C cleavage); sequence 106–121 RALHDSSITGISPITE (Mascot ion score of 18, peptide ion m/z=566.3003, processing with ragweed and endoGlu-C cleavage); sequence 108–121 LHDSSITGISPITE (Mascot ion score of 92, peptide ion m/z=735.3787, processing with A. oryzae and endoGlu-C cleavage); sequence 109–121 HDSSITGISPITE (Mascot ion score of 80, peptide ion m/z=678.8368, processing with Subtilisin and endoGlu-C cleavage).
Supplementary Figure 3 Release of endogenous IL-33 following exposure to A. alternata
(a) Oxidative stress plays a critical role in IL-33 release following exposure of human primary cells to A. alternata. Intact monolayers of IL-33-producing endothelial cells were exposed to different stimuli in the presence or absence of antioxidant glutathione (GSH, 10 mM) and release of endogenous IL-33 in cell supernatants was analyzed by ELISA. Stimuli included A. alternata extract (20 μg/ml, 20 min), allergen-derived PLA2 (125 μg/ml, 3h) and an inducer of oxidative stress (tert-butyl hydroperoxide, tBHP, 2 mM, 3h). Pre-treatment of the cells with GSH inhibited IL-33 release induced by the different stimuli. Treatment with tBHP was sufficient to induce IL-33 release. Data are shown as mean +/- standard deviation (s.d.) of cell cultures replicates (n=3) and are representative of 3 independent experiments. A one-way ANOVA analysis followed by a multiple comparison test (Tukey) (A. alternata) or an unpaired two-tailed student’s t-test (PLA2, tBHP) were performed to determine statistical significance. *P<0.0001. (b) Endogenous IL-33 is not detected in cell supernatants after exposure of the cells to A. alternata (20 μg/ml, 20 or 60 min, 37 °C) extracts in the presence of GSH (20 mM). IL-33 forms in cells supernatants were analyzed by pull down (PD) assay with ST2-Fc followed by immunoblot analysis with anti-IL-33 mAbs (cropped images is shown). Recombinant IL-33FL (FL), IL-3395-270 (95) and IL-33112-270 (112) proteins were co-migrated on the gels. White arrowheads: IL-33FL; black arrowheads: cleaved IL-33 forms. #, non-specific bands. Data are representative of two independent experiments. (c-f) Endogenous murine IL-33 is rapidly released and cleaved in vivo following i.n. exposure to A. alternata. Levels of IL-33 in BAL fluids from wild type C57BL/6J mice at different time points after i.n. treatment with A. alternata (c, 1 h, 3 injections, 12.5 μg; d, 15 min, 1 injection, 100 μg) are shown. Data are pooled from multiple experiments (c, PBS, n = 8, A. alternata, n = 32; d, n = 16) and are shown as mean +/- s.e.m. Two-tailed Mann-Whitney test (c), *p<0.0001. Cleaved endogenous IL-33 was detected in BAL fluids shortly after i.n. exposure of wild type C57BL/6J mice to A. alternata (e,f, 1 injection, 100 μg, 15 min), but it was not observed when A. alternata extract was treated with AEBSF, an inhibitor of IL-33 release (f). IL-33 forms in BAL fluids were analyzed by pull down (PD) assay with ST2-Fc and immunoblot (IB, cropped images are shown). White arrowhead: IL-33FL; black arrowhead: cleaved IL-33 form. #, non-specific bands. Recombinant mIL-33FL, mIL-33102-266 and mIL-33103-266 murine proteins were used as controls (e,f). Data are representative of three independent experiments.
Supplementary Figure 4 Treatment of Alternaria alternata extracts with serine protease inhibitor A1AT results in extracellular accumulation of uncleaved IL-33FL and reduced allergic airway inflammation
(a) Monolayers of IL-33-producing primary human endothelial cells were exposed to A. alternata extracts (20 μg/ml, 20 min, 37 °C) pre-incubated or not with alpha-1 anti-trypsin (A1AT, 10 μg/ml or 20 μg/ml), and release of endogenous IL-33 in cell supernatants was analyzed by ELISA. NT, not treated. Data are shown as mean +/- standard deviation (s.d.) of cell cultures replicates (n=3) and are representative of three independent experiments. A one-way ANOVA analysis followed by a multiple comparison test (Tukey) was performed to determine statistical significance. *P<0.0001, ns, P=0.7255 (A1AT, 10), P=0.1631 (A1AT, 20). (b) Uncleaved IL-33FL was found to accumulate in cell supernatants after exposure of IL-33-producing cells to A. alternata extracts (20 μg/ml, 20 min, 37 °C) pre-incubated with A1AT (10 μg/ml or 20 μg/ml). IL-33 forms in cells supernatants were analyzed by pull down (PD) assay with ST2-Fc followed by immunoblot analysis with anti-IL-33 mAbs (cropped image is shown). Recombinant IL-33FL (FL), IL-3395-270 (95) and IL-33112-270 (112) proteins were co-migrated on the gels. NT, not treated. #, non-specific bands. Data are representative of three independent experiments with similar results. (c) Treatment with A1AT reduces A. alternata-induced allergic airway inflammation in vivo. Representative flow cytometry plots and frequencies of SiglecF+ CD11c– eosinophils in BAL fluids (c) after i.n. exposure of wild type C57BL/6J mice to PBS or A. alternata (1 injection, 12.5 μg) treated with A1AT (12.5 μg, A1AT) or not (control), are shown. Data are pooled from 3 experiments (n = 6) and are shown as mean +/- s.e.m. A one-way ANOVA analysis followed by a multiple comparison test (Tukey) was performed to determine statistical significance. *p<0.0001.
Supplementary Figure 5 Species-specific differences in IL-33 regulation by allergen proteases
(a) Murine IL-33 is sensitive to degradation by A. alternata allergen proteases. Murine IL-33FL recombinant protein was incubated with increasing doses of A. alternata for 1 h at 37 °C. Cleavage of IL-33FL was analyzed by immunoblot (IB, cropped image is shown). (b-e) Human IL-33FL is processed into stable mature forms by allergen proteases whereas murine IL-33FL is cleaved but rapidly degraded after cleavage. White arrowheads: IL-33FL; black arrowheads: cleaved IL-33 forms. Identical amounts of human IL-33FL (2.5 ng) and murine IL-33FL (2.5 ng) recombinant proteins, produced in rabbit reticulocyte lysates, were incubated for the indicated time (min) at 37 °C with A. alternata (500 ng, b), Asp fumigatus (250 ng, c), papain (125 ng, d) or subtilisin (8 ng, e). Proteins forms were analyzed by immunoblots (IB) with anti-human or anti-mouse IL-33 antibodies (anti-hIL-33 or anti-mIL-33, cropped images are shown). Data are representative of two independent experiments with similar results (a-e).
Supplementary Figure 6 Plant, bacterial and fungal allergen proteases induce IL-33-dependent airway eosinophilia
(a-c) Representative flow cytometry plots and frequencies of SiglecF+ CD11c– eosinophils in BAL fluids from wild type (WT) and Il-33-/- C57BL/6J mice treated i.n. with plant protease papain (3 injections, 50 μg; a), bacterial protease subtilisin (3 injections, 5 μg; b) or A. fumigatus extracts (3 injections, 50 μg; c) are shown. Data are pooled from 2–3 experiments (a, WT PBS, n=9, WT papain, n=9, Il33-/- PBS, n=7, Il33-/- papain, n=9; b, WT PBS, n=6, WT subtilisin, n=6, Il33-/- PBS, n=4, Il33-/- subtilisin, n=5; c, WT PBS, n=6, WT A. fumigatus, n=6, Il33-/- PBS, n=6, Il33-/- A. fumigatus, n=6) and are shown as mean +/- s.e.m. A one-way ANOVA analysis followed by a multiple comparison test (Tukey) was performed to determine statistical significance. ****P<0.0001 (a, WT papain vs WT PBS; a, WT papain vs Il33-/- papain), ***P=0.0003 (b, WT subtilisin vs WT PBS), ***P=0.0005 (c, WT A. fumigatus vs WT PBS), **P=0.0079 (b, WT subtilisin vs Il33-/- subtilisin), **P=0.0037 (c, WT A. fumigatus vs Il33-/- A. fumigatus), *P=0.0243 (a, Il33-/- papain vs PBS), ns P=0.5645 (b, Il33-/- subtilisin vs PBS), ns P=0.7708 (c, Il33-/- A. fumigatus vs PBS).
Supplementary Figure 7 Recombinant IL-33FL restores allergen protease-induced BAL eosinophilia in Il33–/– mice
(a-c) Representative flow cytometry plots and frequencies of SiglecF+ CD11c– eosinophils in BAL fluids from Il33-/- mice treated i.n. with A. alternaria extracts (3 injections, 12.5 μg; a), A. fumigatus extracts (3 injections, 50 μg; b) or papain (3 injections, 50 μg; c), and/or recombinant human IL-33FL are shown. Allergens and IL-33FL were not co-incubated prior to administration. Data are pooled from multiple experiments (a, A. alternata, n=9, IL-33FL, n=10, A. alternata + IL-33FL, n=11; b, A. fumigatus, n=9, IL-33FL, n=10, A. fumigatus + IL-33FL, n=11; c, papain, n=4, IL-33FL, n=6, papain + IL-33FL, n=6) and are shown as mean +/- s.e.m. A one-way ANOVA analysis followed by a multiple comparison test (Tukey) was performed to determine statistical significance. **P<0.0001 (a-c).
Supplementary Figure 8 Full scans of immunoblots
Images from all immunoblot experiments reported in the main figures and supplementary figures are shown. The red boxes correspond to the cropped images shown in the individual figure panels.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1-8
Rights and permissions
About this article
Cite this article
Cayrol, C., Duval, A., Schmitt, P. et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol 19, 375–385 (2018). https://doi.org/10.1038/s41590-018-0067-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0067-5
This article is cited by
-
Matrix-bound nanovesicle-associated IL-33 supports functional recovery after skeletal muscle injury by initiating a pro-regenerative macrophage phenotypic transition
npj Regenerative Medicine (2024)
-
Serum Level of Interleukin-33 in Infantile Atopic Dermatitis
Indian Journal of Pediatrics (2024)
-
Group 2 innate lymphoid cells and their surrounding environment
Inflammation and Regeneration (2023)
-
Discovery of highly immunogenic spleen-resident FCGR3+CD103+ cDC1s differentiated by IL-33-primed ST2+ basophils
Cellular & Molecular Immunology (2023)
-
Unpacking the complexity of nuclear IL-33 (nIL-33): a crucial regulator of transcription and signal transduction
Journal of Cell Communication and Signaling (2023)