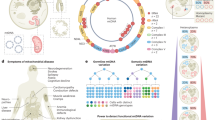
Abstract
Pathogenic mutations in mitochondrial DNA (mtDNA) compromise cellular metabolism, contributing to cellular heterogeneity and disease. Diverse mutations are associated with diverse clinical phenotypes, suggesting distinct organ- and cell-type-specific metabolic vulnerabilities. Here we establish a multi-omics approach to quantify deletions in mtDNA alongside cell state features in single cells derived from six patients across the phenotypic spectrum of single large-scale mtDNA deletions (SLSMDs). By profiling 206,663 cells, we reveal the dynamics of pathogenic mtDNA deletion heteroplasmy consistent with purifying selection and distinct metabolic vulnerabilities across T-cell states in vivo and validate these observations in vitro. By extending analyses to hematopoietic and erythroid progenitors, we reveal mtDNA dynamics and cell-type-specific gene regulatory adaptations, demonstrating the context-dependence of perturbing mitochondrial genomic integrity. Collectively, we report pathogenic mtDNA heteroplasmy dynamics of individual blood and immune cells across lineages, demonstrating the power of single-cell multi-omics for revealing fundamental properties of mitochondrial genetics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data associated with this work is available at GEO accession GSE173936.
Code availability
Software and documentation for mitochondrial DNA variant calling, including deletion calling and heteroplasmy estimation, is available via the mgatk package at http://github.com/caleblareau/mgatk as of version 0.6.3. All custom code to reproduce all analyses supporting this paper is available at https://github.com/caleblareau/pearson_syndrome. Code used in this paper is indexed in Zenodo (https://zenodo.org/record/7853604).
References
Stewart, J. B. & Chinnery, P. F. Extreme heterogeneity of human mitochondrial DNA from organelles to populations. Nat. Rev. Genet. 22, 106–118 (2021).
Stewart, J. B. & Chinnery, P. F. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat. Rev. Genet. 16, 530–542 (2015).
Wallace, D. C. & Chalkia, D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 5, a021220 (2013).
Gorelick G, A. N. et al. Respiratory complex and tissue lineage drive recurrent mutations in tumour mtDNA. Nat Metab. 3, 558–570 (2021).
Smith, A. L. M. et al. Age-associated mitochondrial DNA mutations cause metabolic remodeling that contributes to accelerated intestinal tumorigenesis. Nat. Cancer 1, 976–989 (2020).
Goldstein, A. & Falk, M. J. Mitochondrial DNA Deletion Syndromes (University of Washington, 2023).
Ludwig, L. S. et al. Lineage tracing in humans enabled by mitochondrial mutations and single-cell genomics. Cell 176, 1325–1339 (2019).
Xu, J. et al. Single-cell lineage tracing by endogenous mutations enriched in transposase accessible mitochondrial DNA. eLife 8, e45105 (2019).
Lareau, C. A. et al. Massively parallel single-cell mitochondrial DNA genotyping and chromatin profiling. Nat. Biotechnol. 39, 451–461 (2020).
Walker, M. A. et al. Purifying selection against pathogenic mitochondrial DNA in human T cells. N. Engl. J. Med. 383, 1556–1563 (2020).
Mimitou, E. P. et al. Scalable, multimodal profiling of chromatin accessibility, gene expression and protein levels in single cells. Nat. Biotechnol. 39, 1246–1258 (2021).
Krishnan, K. J. et al. What causes mitochondrial DNA deletions in human cells? Nat. Genet. 40, 275–279 (2008).
Pitceathly, R. D. S., Rahman, S. & Hanna, M. G. Single deletions in mitochondrial DNA–molecular mechanisms and disease phenotypes in clinical practice. Neuromuscul. Disord. 22, 577–586 (2012).
Lujan, S. A. et al. Ultrasensitive deletion detection links mitochondrial DNA replication, disease, and aging. Genome Biol. 21, 248 (2020).
Hjelm, B. E. et al. Splice-Break: exploiting an RNA-seq splice junction algorithm to discover mitochondrial DNA deletion breakpoints and analyses of psychiatric disorders. Nucleic Acids Res. 47, e59 (2019).
Hao, Y. et al. Dictionary learning for integrative, multimodal, and scalable single-cell analysis. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01767-y (2023).
Battaglia, S. et al. Uridine and pyruvate protect T cells’ proliferative capacity from mitochondrial toxic antibiotics: a clinical pilot study. Sci. Rep. 11, 12841 (2021).
King, M. P. & Attardi, G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 (1989).
Basu, S. et al. Accurate mapping of mitochondrial DNA deletions and duplications using deep sequencing. PLoS Genet. 16, e1009242 (2020).
Goudenège, D. et al. eKLIPse: a sensitive tool for the detection and quantification of mitochondrial DNA deletions from next-generation sequencing data. Genet. Med. 21, 1407–1416 (2019).
Kardos, G. et al. Refractory anemia in childhood: a retrospective analysis of 67 patients with particular reference to monosomy 7. Blood 102, 1997–2003 (2003).
Nishimura, A. et al. Acquisition of monosomy 7 and a RUNX1 mutation in Pearson syndrome. Pediatr. Blood Cancer 68, e28799 (2021).
Granja, J. M. et al. Single-cell multiomic analysis identifies regulatory programs in mixed-phenotype acute leukemia. Nat. Biotechnol. 37, 1458–1465 (2019).
Buenrostro, J. D. et al. Integrated single-cell analysis maps the continuous regulatory landscape of human hematopoietic differentiation. Cell 173, 1535–1548 (2018).
Chung, J.-W. et al. A combination of CD15/CD10, CD64/CD33, CD16/CD13 or CD11b flow cytometric granulocyte panels is sensitive and specific for diagnosis of myelodysplastic syndrome. Ann. Clin. Lab. Sci. 42, 271–280 (2012).
Kussick, S. J. et al. Four-color flow cytometry shows strong concordance with bone marrow morphology and cytogenetics in the evaluation for myelodysplasia. Am. J. Clin. Pathol. 124, 170–181 (2005).
Pekalski, M. L. et al. Neonatal and adult recent thymic emigrants produce IL-8 and express complement receptors CR1 and CR2. JCI Insight 2, e93739 (2017).
Farruggia, P., Di Marco, F. & Dufour, C. Pearson syndrome. Expert Rev. Hematol. 11, 239–246 (2018).
Gagne, K. E. et al. Pearson marrow pancreas syndrome in patients suspected to have Diamond-Blackfan anemia. Blood 124, 437–440 (2014).
Cherry, A. B. C. et al. Induced pluripotent stem cells with a mitochondrial DNA deletion. Stem Cells 31, 1287–1297 (2013).
Ludwig, L. S. et al. Transcriptional states and chromatin accessibility underlying human erythropoiesis. Cell Rep. 27, 3228–3240 (2019).
Bao, X. R. et al. Mitochondrial dysfunction remodels one-carbon metabolism in human cells. eLife 5, e10575 (2016).
Yang, M. & Vousden, K. H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016).
Yang, L. et al. Serine catabolism feeds NADH when respiration is impaired. Cell Metab. 31, 809–821 (2020).
Macaulay, I. C., Ponting, C. P. & Voet, T. Single-cell multiomics: multiple measurements from single cells. Trends Genet. 33, 155–168 (2017).
Stuart, T. & Satija, R. Integrative single-cell analysis. Nat. Rev. Genet. 20, 257–272 (2019).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Kujoth, G. C. et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005).
Vandiver, A. R. et al. Nanopore sequencing identifies a higher frequency and expanded spectrum of mitochondrial DNA deletion mutations in human aging. Aging Cell 22, e13842 (2022).
Lehmann, D. et al. Understanding mitochondrial DNA maintenance disorders at the single muscle fibre level. Nucleic Acids Res. 47, 7430–7443 (2019).
Nido, G. S. et al. Ultradeep mapping of neuronal mitochondrial deletions in Parkinson’s disease. Neurobiol. Aging 63, 120–127 (2018).
Lawless, C., Greaves, L., Reeve, A. K., Turnbull, D. M. & Vincent, A. E. The rise and rise of mitochondrial DNA mutations. Open Biol. 10, 200061 (2020).
Morris, J. et al. Pervasive within-mitochondrion single-nucleotide variant heteroplasmy as revealed by single-mitochondrion sequencing. Cell Rep. 21, 2706–2713 (2017).
Jones, N. et al. Metabolic adaptation of Human CD4+ and CD8+ T cells to T-cell receptor-mediated stimulation. Front. Immunol. 8, 1516 (2017).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8 T cell memory development. Immunity 36, 68–78 (2012).
Hinks, T. S. C. & Zhang, X.-W. MAIT cell activation and functions. Front. Immunol. 11, 1014 (2020).
Lisci, M. et al. Mitochondrial translation is required for sustained killing by cytotoxic T cells. Science 374, eabe9977 (2021).
Korge, P., Calmettes, G. & Weiss, J. N. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta 1847, 514–525 (2015).
Sharma, R. et al. Circulating markers of NADH-reductive stress correlate with mitochondrial disease severity. J. Clin. Invest. 131, e136055 (2021).
Enns, G. M. et al. Degree of glutathione deficiency and redox imbalance depend on subtype of mitochondrial disease and clinical status. PLoS ONE 9, e100001 (2014).
De Franceschi, L. et al. Oxidative stress modulates heme synthesis and induces peroxiredoxin-2 as a novel cytoprotective response in β-thalassemic erythropoiesis. Haematologica 96, 1595–1604 (2011).
Sankaran, V. G. & Weiss, M. J. Anemia: progress in molecular mechanisms and therapies. Nat. Med. 21, 221–230 (2015).
Rosales, X. Q. et al. The North American mitochondrial disease registry. J. Transl. Genet Genom. 4, 81–90 (2020).
Hu, J. et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood 121, 3246–3253 (2013).
Giani, F. C. et al. Targeted application of human genetic variation can improve red blood cell production from stem cells. Cell Stem Cell 18, 73–78 (2016).
Al’Khafaji, A. M. et al. High-throughput RNA isoform sequencing using programmable cDNA concatenation. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01815-7 (2023).
Li, H. et al. The sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Lott, M. T. et al. mtDNA variation and analysis using mitomap and mitomaster. Curr. Protoc. Bioinforma. 44, 1.23.1–1.23.6 (2013).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Stuart, T., Srivastava, A., Madad, S., Lareau, C. A. & Satija, R. Single-cell chromatin state analysis with Signac. Nat. Methods 18, 1333–1341 (2021).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 8, 281–291 (2019).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Soneson, C. & Robinson, M. D. Bias, robustness and scalability in single-cell differential expression analysis. Nat. Methods 15, 255–261 (2018).
Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z. & Zhang, B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47, W199–W205 (2019).
GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Bray, N., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal RNA-seq quantification with kallisto. Nat. Biotechnol. 34, 525–527 (2016).
Müller, S., Cho, A., Liu, S. J., Lim, D. A. & Diaz, A. CONICS integrates scRNA-seq with DNA sequencing to map gene expression to tumor sub-clones. Bioinformatics 34, 3217–3219 (2018).
Granja, J. M. et al. ArchR is a scalable software package for integrative single-cell chromatin accessibility analysis. Nat. Genet. 53, 403–411 (2021).
Satpathy, A. T. et al. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat. Biotechnol. 37, 925–936 (2019).
Acknowledgements
We are deeply grateful to the patients and families who made this work possible. Patient samples and data from adult donors with KSS and CPEO were provided by the North American Mitochondrial Disease Consortium (NAMDC), which is funded by a grant from NIH (U54NS078059). We thank A. Shimamura (Boston Children’s Hospital) and the Boston Children’s Hospital Bone Marrow Failure and Myelodysplastic Syndrome program for their support in this study (supported by NIH grant RC2DK122533). We thank the MDC/BIH Genomics Platform, Berlin (FacilityID = 1565, The CoreMarketplace: MDC&BIH Technology Platform Genomics) for technical support relating to flow cytometry and sequencing efforts and Claudia Quedenau for conducting the MAS-ISO-seq experiments. We acknowledge support from the Broad Institute and the Whitehead Institute Flow Cytometry core facilities. This work was supported by NIH K99 HG012579 (to C.A.L.), RC2 DK122533-01 (to M.D.F.), R01 DK103794 (to V.G.S.), R33 HL120791 (to V.G.S.), R01 DK107716 (to S.A.), R33 HL154133 (to S.A.) and UM1 HG012076 (to C.A.L., A.T.S. and L.S.L.). This research was supported by a Stanford Science Fellowship (to C.A.L.), BroadIgnite Award (to L.S.L.), an Emmy Noether fellowship by the German Research Foundation (D.F.G.; LU 2336/2-1 to L.S.L.), the Hector Fellow Academy (to Y.H.H., P.K., L.N. and L.S.L.), a Lloyd J. Old STAR Award from the Cancer Research Institute (A.T.S.), an ASH Scholar Award from the American Society of Hematology (to A.T.S.), a gift from the Lodish Family to Boston Children’s Hospital (to V.G.S.), the New York Stem Cell Foundation (NYSCF to V.G.S.), the Howard Hughes Medical Institute and Klarman Cell Observatory (to A.R.), the Champ Foundation (to S.A.) and the Associazione Luigi Comini Onlus (to S.A.).
Author information
Authors and Affiliations
Contributions
C.A.L., A.R., V.G.S., S.A. and L.S.L. conceived and designed the project. C.A.L. developed the mgatk-del software and led all analyses. L.S.L. led, designed and performed experiments with F.A.B., Y.H.H., P.K., L.N. and Y.Y., and assistance from S.M.D., W.L. and C.M. E.P.M. and P.S. contributed to reagents and advised on the ASAP-seq experiments. L.O., J.S. and C.E. contributed to healthy pediatric control samples and clinical perspectives. C.A.L., K.G. and J.M.V. developed the mtDNA simulation framework. F.B., Y.H.H., P.K., L.N., S.D.P., J.C.G., E.F., R.M., P.M., SuP, A.K., S.A. and L.S.L. contributed to data interpretation. S.M.D., S.D.P., M.D.F., A.K., S.A.V., A.T.S., A.R., V.G.S., S.A. and L.S.L. supervised various aspects of this work. C.A.L. and L.S.L. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The Broad Institute has filed for a patent relating to the use of the technology described in this paper where C.A.L., L.S.L., C.M., A.R. and V.G.S. are named inventors (US provisional patent application 62/683,502). C.A.L. and L.S.L. are consultants to Cartography Biosciences. ATS is a founder of Immunai and Cartography Biosciences and receives research funding from Allogene Therapeutics and Merck Research Laboratories. A.R. is a founder and equity holder of Celsius Therapeutics, an equity holder in Immunitas Therapeutics, and until August 31, 2020, was an SAB member of Syros Pharmaceuticals, Neogene Therapeutics, Asimov and ThermoFisher Scientific. Since August 1, 2020, A.R. has been an employee of Genentech. V.G.S. serves as an advisor to and/or has equity in Branch Biosciences, Novartis, Forma, Cellarity and Ensoma. S.A.V. is an advisor to Immunai and has provided consulting services to Koch Disruptive Technologies and ADC Therapeutics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Na Cai, Dan Mishmar, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Deletion and heteroplasmy estimation using mgatk-del.
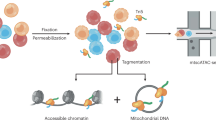
(a) Schematic of mgatk-del pipeline, which utilizes the outputs of CellRanger-ATAC. Two critical steps of base-resolution deletion calling (‘find’) and estimation of single-cell heteroplasmy (‘quantify’) are illustrated. (b) Output of mgatk-del ‘find’ for Pearson syndrome deletion 1 (PS1). The red vertical lines represent the called deletion breakpoints where the regions were joined (blue arc) via a secondary alignment (‘SA’ tag in.bam file). (c) Schematic of the simulation framework. Synthetic cells with known heteroplasmy were generated via mixtures of reference and PS mtDNA for all previously reported deletions. (d) Summary of results from a 50% mix showing the heteroplasmy as estimated from the ratio of clipped to unclipped reads. Parameters ‘inner-param’ and ‘outer-param’ define the number of bases that are discarded on the read when estimating the overall heteroplasmy per cell. (e) Results of exhaustive simulation for three mtDNA deletions used in the cell mixing experiment. The minimum value of the root mean squared error (RMSE) of the estimated and true heteroplasmy is noted with an asterisk over the grid search. (f) Difference in mean estimated heteroplasmy (RMSE) in optimal and default parameters across a variety of settings indicating the stability. (g) Decomposition of variance using a permuted model. Black shows the observed variance whereas green shows the spread under a permuted (null) model. The percent of the variance explained by this null model is shown. (h) Single-cell correlation of clipped (Fig. 1d) versus coverage-based (Fig. 1e) heteroplasmy estimates for valid deletions per indicated deletion/donor. The Pearson correlation for the three deletions is indicated. (i) % of cells with non-zero heteroplasmy for different deletions at different coverages using indicated methods. The left panel assesses sensitivity where the true proportion of cells with the deletion is 100%. The right panel assesses specificity where the true proportion of cells with the deletion is 0%. For both plots, detection of the deletion requires ≥1% heteroplasmy. (j) The mean absolute error in heteroplasmy at 50x coverage is indicated by the value shown on the graph for two methods of heteroplasmy estimation, as in (i).
Extended Data Fig. 2 Supporting information for PS PBMC mtscATAC-seq analyses.
(a) Result of mgatk-del hyperparameter optimization via a simulation framework. The minimum value of the root mean squared error (RMSE) of the estimated and true heteroplasmy is noted with an asterisk over the grid search. (b) Summary of % of cells with 0% heteroplasmy across all hematopoietic cells for the three PS donors. (c) Violin plots of each respective mtDNA deletion for all three patients in selected T cell populations. Black indicates the observed data. Gray represents heteroplasmy under a null model of one mean and the variation attributed to differences in coverage per cell. CD8.TEM and MAIT cells have a bimodal distribution (indicating purifying selection) whereas CD8+ naive cells have a distribution that is more consistent with a single mode of heteroplasmy. The percentage of cells with 0% heteroplasmy under observed and null settings are noted for each population below the violins. (d) UMAP visualization of MELAS bridge reference projection across three donors previously reported. (e) Summary of % of cells with 0% heteroplasmy across all hematopoietic cells for the three MELAS donors10 with refined cell type annotations from the bridge reference projection.
Extended Data Fig. 3 Supporting analyses for primary PS T cell cultures.
(a) Representative flow cytometry plot of CD4+ and CD8+ T cells. Cells from the four donors (columns) were assessed for CD45RA and CD45RO expression. The box indicates the proportion of CD45RAhi/CD45RO− cells summarized in Fig. 3d. (b) Summary of flow cytometry data from independent T cell culture comparing cells from a healthy pediatric donor to an adult donor. T cells were derived from both bone marrow mononuclear cells (BMMNCs) and peripheral blood mononuclear cells (PBMCs) for the pediatric donor. (c) Embedding of day 14T cells colored by antibody derived tags (ADTs) for CCR4 and IL2RA. (d) Dynamics of heteroplasmy for 76 heteroplasmic single nucleotide variants (SNVs) during T cell culture. m.12631T > C and m.4225A > G are highlighted. (e) Single-cell heteroplasmy of m.12631T > C and m.4225A > G, variants expanding during T cell culture, annotated in the day 14 embedding. Blue arrow indicates the subpopulation positive for the m.4225A > G variant. (f) Single-cell heteroplasmy of the PS deletion annotated on day 21 derived T cells profiled via mtscATAC-seq. (g) Single-cell heteroplasmy of m.12631T > C and m.4225A > G, variants expanding during T cell culture, annotated in the day 21 cell embedding. Blue arrow indicates the subpopulation positive for the m.4225A > G variant. (h) Day 21 embedding clustering, annotations, and per-cluster heteroplasmy quantified via a cumulative distribution function. (i) Independent validation of in vitro CD8+ T cell relative expansion (left) and overall T cell proliferation (right) defects from PT1 PBMCs. (j) Summary of cell clusters, heteroplasmy, and marker gene scores for PT1 T cells following culture. (k) Day 14 PT1 per-cluster heteroplasmy quantified via a cumulative distribution function for PT1 T cells after culture.
Extended Data Fig. 4 Supporting information for del7q calling and CD34+ mtscATAC-seq analyses.
(a) Flow cytometry gating strategy for the sorting of live CD66b−CD34+ bone marrow mononuclear cells. (b) Summary of 7q fragment abundances in healthy CD34+ and PBMC mtscATAC-seq samples9; compare to Fig. 4b with the same cutoff. (c) Result of Gaussian mixture model applied to indicated samples. The red trace indicates the first mixture component estimated (lower mean) whereas the blue trace represents the second component with a higher mean. The healthy PBMC sample does not contain a chromosome alteration. (d) Graphical density of cells from mixture model (y-axis) and from crude fragment abundance (x-axis; see Fig. 4b). The dotted line indicates the cutoff for wild type and del7q annotations. (e) Histograms of mtDNA deletion heteroplasmy proportions (%) stratified on del7q status. (f) Projection of a healthy control CD34+ mtscATAC-seq sample onto the reference embedding as shown in Fig. 5d. (g) Stacked bar plots of cell type proportions for projected cell types from PT3 with PS/MDS stratified by del7q status (MDS for positive and wild type for negative) and a healthy control donor. (h) Annotation of del7q status in PBMCs, which is primarily identified in myeloid, NK, and B-cell populations; see Fig. 2d for cluster annotations.
Extended Data Fig. 5 Supporting analyses for PT3 bone marrow mononuclear cell ASAP-seq dataset.
(a) Projection of select protein-derived antibody tag abundances for indicated proteins. Select arrows indicate populations positive for the respective marker. UMAP coordinates same as Fig. 6c. (b) Projection of protein surface markers CD56 (NK cells and MDS-associated cells) and CD335 (only NK cells) with arrows indicating the two cell populations. (c) UMAP of ASAP-seq processed bone marrow mononuclear cells from a PS (top) and a healthy control11 (bottom) with hematopoietic stem and progenitor cells (‘progenitors’) indicated in the red boxes. (d) Projection of protein tags within the boxed progenitor populations as in (c), contrasting the presence of only CD71+ cells among CD34+/c-Kit+ cells in PS as compared to the healthy control. (e) Volcano plot showing differential gene activity scores for CD8 recent thymic emigrants (RTEs) compared to other CD8 naive T cells. Annotated genes in red represent known marker genes for RTEs. (f) Zoom (top) and mtDNA deletion heteroplasmy (bottom) in differentiated CD8 T and NK cells from the BMMNC populations. (g) Volcano plot illustrating the association between protein levels and mtDNA deletion heteroplasmy in single cells. P-values were computed from the default two-sided Seurat Wilcoxon test with Bonferroni p-value adjustment. (h) Projection of cell state surface markers (CD3, CD8) and top antibody tags (CD16, CD195) as determined in (g). (i,j) Reclustering and UMAP depiction of PT3 PBMC mtscATAC-seq data identify (i) low heteroplasmy and (j) recent thymic emigrants (RTEs). Cell type annotations as indicated. (k) Landscape of 69 heteroplasmic somatic mtDNA mutations identified in BMMNC. Statistical test: two-sided Fisher’s exact test. (l) Substitution rate of mgatk identified heteroplasmic mutations (y-axis) in each class of mononucleotide and trinucleotide change resolved by the heavy (H) and light (L) strands of the mitochondrial genome. (m) Scatter plot of 69 somatic mtDNA variants identified in panel (l) stratified based on cells annotated as del7q (x-axis) and wild type for chr7 (y-axis). (n) Projection of wild type (diploid chr7)-enriched somatic mtDNA mutations m.14476G > A (50%) and m.12242A > G (25%).
Extended Data Fig. 6 Supporting information for in vitro erythroid differentiation experiments.
(a) Reference embedding of bone marrow mononuclear cell CITE-seq reference dataset (left) with gene module scores for selected pathways annotated on the UMAP embedding. (b) Flow cytometry gating scheme used for sorting of in vitro differentiated healthy control and PS cells, related to Fig. 7. (c) Flow cytometry plots showing the distribution of CD71 and CD235a surface marker expression of in vitro differentiated healthy control and PS cells at indicated days of culture. (d) MayGrunwald Giemsa stained cytospins of in vitro differentiated healthy control and PS cells at day 8 of culture at 63x magnification. (e) UMAP of scRNA-seq data colored by predicted cell cycle state. Cluster annotations as in Fig. 7e–g. (f) Comparison of differential gene expression between PT3 donor cells with MDS (x-axis) and without MDS (y-axis) related to healthy control. The Pearson correlation between all genes is annotated (0.82). Genes from relevant pathways or genomic annotations are highlighted in specific colors. (g-i) Projection of gene expression of selected differentially expressed genes between PS and healthy control erythroblasts, including (g) PHGDH, (h) CPOX, and (i) HEBP2. Gene expression coloring is scaled for all plots between the first and 99th quantile per gene.
Supplementary information
Supplementary Information
Supplemental Methods and Supplementary Figs. 1–4.
Supplementary Tables
Supplementary Table 1—Coordinates of mtDNA deletions investigated in this study. Supplementary Table 2—Summary of deletion heteroplasmy per donor and cell type in PBMCs. Supplementary Table 3—List of TotalSeq antibodies, clones and barcodes for ASAP-seq and DOGMA-seq.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lareau, C.A., Dubois, S.M., Buquicchio, F.A. et al. Single-cell multi-omics of mitochondrial DNA disorders reveals dynamics of purifying selection across human immune cells. Nat Genet 55, 1198–1209 (2023). https://doi.org/10.1038/s41588-023-01433-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01433-8