Abstract
Major migration events in Holocene Eurasia have been characterized genetically at broad regional scales1,2,3,4. However, insights into the population dynamics in the contact zones are hampered by a lack of ancient genomic data sampled at high spatiotemporal resolution5,6,7. Here, to address this, we analysed shotgun-sequenced genomes from 100 skeletons spanning 7,300 years of the Mesolithic period, Neolithic period and Early Bronze Age in Denmark and integrated these with proxies for diet (13C and 15N content), mobility (87Sr/86Sr ratio) and vegetation cover (pollen). We observe that Danish Mesolithic individuals of the Maglemose, Kongemose and Ertebølle cultures form a distinct genetic cluster related to other Western European hunter-gatherers. Despite shifts in material culture they displayed genetic homogeneity from around 10,500 to 5,900 calibrated years before present, when Neolithic farmers with Anatolian-derived ancestry arrived. Although the Neolithic transition was delayed by more than a millennium relative to Central Europe, it was very abrupt and resulted in a population turnover with limited genetic contribution from local hunter-gatherers. The succeeding Neolithic population, associated with the Funnel Beaker culture, persisted for only about 1,000 years before immigrants with eastern Steppe-derived ancestry arrived. This second and equally rapid population replacement gave rise to the Single Grave culture with an ancestry profile more similar to present-day Danes. In our multiproxy dataset, these major demographic events are manifested as parallel shifts in genotype, phenotype, diet and land use.
Similar content being viewed by others
Main
The Mesolithic and Neolithic periods in southern Scandinavia are marked by a number of pivotal and well-described cultural transitions8. However, the genetic and demographic impacts of these events remain largely uncharacterized. The early postglacial human colonization of the Scandinavian peninsula (Sweden and Norway) is believed to comprise at least two distinct migration waves: a source related to western European hunter-gatherers (WHG) from the south, and an eastern European hunter-gatherer (EHG) source into the far north, before venturing south along the Atlantic coast of Norway9,10. However, insight into the fine-scale structure and mobility of Scandinavian Mesolithic populations is limited, including an almost complete absence of genetic data from southern Scandinavian populations associated with the consecutive Maglemose, Kongemose and Ertebølle cultures in Denmark.
The Neolithic transition represents a watershed event in European prehistory, marked by the spread of domesticated crops and livestock from Southwest Asia, starting around 11,000 bp. Although migrations and population turnovers associated with this transition have been demonstrated at broad geographical and chronological scales1,2,3,4, coarse sampling and a one-sided focus on genetics have hindered insights on social interaction and detailed demographic processes in the contact zones between locals and newcomers5,6,7. Southern Scandinavia occupies an enigmatic position in this discussion. The Neolithic transition was delayed here by a millennium compared to Central Europe, during which hunter-gatherer societies continued to flourish until around 5,900 calibrated years bp (cal. bp), only marginally affected by farmer populations to the south11. The substantial delay could suggest that the transition to farming in Denmark occurred by a different mechanism involving a stronger element of cultural diffusion12 than the migration of people (demic diffusion) observed in the rest of Europe13,14,15.
An extensive archaeological record has indicated that the Funnel Beaker culture (FBC) thrived for the first millennium of the Neolithic in Denmark, before an apparent decline16 was followed by the appearance of the Single Grave culture (SGC). Owing to a lack of genetic data and a robust absolute chronology, the relation between the FBC and the SGC has been extensively debated17,18,19. Population dynamics associated with this second cultural transition in Neolithic Denmark are similarly unresolved, including its possible link to the ‘steppe migrations’ that transformed the gene pools elsewhere in Europe around the same time1,2.
To investigate these defining events at high temporal and spatial resolution, we analyse a detailed and continuous dataset of 100 ancient Danish shotgun-sequenced genomes (0.01× to 7.1× autosomal coverage3), spanning about 7,300 years from the Early Mesolithic Maglemose, the Kongemose and Late Mesolithic Ertebølle epochs, the Early and Middle Neolithic FBC and the SGC, up until the Bronze Age (Fig. 1 and Supplementary Data 1). The archaeological record in Denmark represents a very large assemblage of well-documented Mesolithic and Neolithic human skeletal remains, from a wide range of chronological, topographical and socio-cultural contexts. This is a result of an environment and climate that was amenable to both Mesolithic fisher-hunter-gatherer lifeways20 and the later Neolithic farming practices, combined with taphonomically favourable preservation conditions for skeletal remains, and a long, prolific history of archaeological research. We used a multiproxy approach, combining autosomal imputed genomes3,21 with Y chromosomal and mitochondrial haplogroups, 14C-dating, genetic phenotype predictions, as well as 87Sr/86Sr, δ13C and δ15N isotope data as proxies for mobility and diet. Moreover, to investigate a direct link between demographic and environmental processes, we align the genetic changes observed in the Danish population over time with changes in local vegetation, based on pollen analyses and quantitative vegetation cover reconstruction.
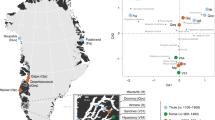
a, Geographic locations and age ranges relating to the 100 sequenced genomes from Denmark. Groupings are designated through a combination of chronology, culture, and ancestry (see Supplementary Notes 1 and 3). b, PCA for 179 ancient Danish individuals (Supplementary Data 3) ranging from the Mesolithic to the Viking Age, including previously published ones1,47,57,76, in the context of broader West Eurasian genetic diversity (n = 983 modern individuals, open grey circles; n = 1,105 ancient individuals, filled grey circles). Ancient individuals from Denmark are coloured according to the period as defined in a and c. c, Unsupervised model-based clustering (ADMIXTURE) for K = 8 ancestry components in Danish individuals, as well as contextual data from selected groups (left) that represent relevant ancestry components. See Extended Data Fig. 1 for individual labels. Black crosses indicate low-coverage genomes represented by pseudo-haploid genotypes. BA, Bronze Age.
The Mesolithic period
It is not known whether shifts in southern Scandinavian Mesolithic material culture occurred in a population continuum or were facilitated by incoming migrants. The Early Mesolithic settlement in Denmark is associated with the Maglemose culture (around 11,000–8,400 cal. bp), characterized archaeologically by small flint projectiles in geometric shapes. Until the recent development of underwater archaeology, this culture was known mainly from inland locations along lakes and rivers22. During the succeeding Kongemose culture (around 8,400–7,400 cal. bp), trapeze-shaped flint points dominate the assemblages of arrowheads23 along with high quality long blades. Most of the larger settlements cluster at good fishing locations along the coasts24, but there are also specialized hunting camps in the interior25. The Late Mesolithic Ertebølle culture (about 7,400–5,900 cal. bp), is characterized by flint points with transverse edges. Pottery was introduced from other hunter-gatherer groups to the east and perhaps the southwest26 and ‘exotic’ shaft-hole axes suggest exchange with farming societies south of the Baltic Sea27. The larger habitation sites, densely scattered along the coasts, probably represent multi-family, year-round occupation24,28 and they have provided important insights into the physical anthropology and spiritual culture of the period.
By analysing genomes from 38 Danish hunter-gatherers and inferring their ancestry, we examine whether cultural transitions observed in the Danish archaeological record are associated with any genetic changes in the population. Model-based clustering (ADMIXTURE), PCA and IBD-sharing analyses show that throughout the Maglemose (n = 4), Kongemose (n = 8) and Ertebølle (n = 27) epochs the region displayed a remarkable genetic homogeneity across a 4,500-year transect (Figs. 1–3 and Extended Data Figs. 1–3), supporting interpretations of demographic continuity favoured by some archaeologists23,24,25. From the earliest known skeleton in Denmark, ‘Koelbjerg Man’ (NEO254, 10,648–10,282 cal. bp29), to the most recent Mesolithic skeleton included here, ‘Rødhals Man’ (NEO645, 5,916–5,795 cal. bp), the individuals derive their ancestry almost exclusively from the same southern European source (Italy_15000BP_9000BP) that predominated in WHG ancestry in Mesolithic Western Europe3.
Heat map showing relative IBD-sharing rate of 72 imputed ancient individuals from Denmark (n = 67 individuals reported in this Article, n = 5 previously published individuals1,47,57,76) from the Mesolithic to the Bronze Age with selected genetic clusters. Individuals are grouped by their genetic cluster membership. See Supplementary Data 3 for dataset and ancestry category definition.
Evidence of two population turnovers in chronologically sorted multiproxy data from 100 Danish Mesolithic, Neolithic and Early Bronze Age skeletons (Supplement Data 1). The figure shows concomitant changes in (from the top) admixture proportions in non-imputed genome-wide data, Y chromosomal and mitochondrial haplogroups, genetic phenotype predictions (based on imputed data) and 87Sr/86Sr and δ13C and δ15N isotope data as proxies for mobility and diet, respectively. Predicted height values represent differences (in cm) from the average height of the present-day Danish population; probabilities for the hair colours (blond, brown, black and red) and eye colours (blue and brown) are shown, with grey denoting probability of intermediate eye colour (including grey, green and hazel). Lower panel shows the quantitative changes in vegetation cover, based on pollen analyses at Lake Højby in Zealand. Note that the vegetation panel covers a shorter time interval than the other panels. Black vertical lines mark the first presence of Anatolian Neolithic farmer ancestry and Steppe-related ancestry, respectively. Individuals with low genomic coverage, signs of possible contamination and/or low genotype prediction score (GP) are indicated (Methods).
In the IBD-based principal components analysis (PCA), the Danish Mesolithic individuals cluster closely together (Extended Data Fig. 4a), but beyond this tight local genetic connection they share most recent ancestry with the geographically and temporally proximate hunter-gatherer individuals from Western Europe (such as Cheddar Man, Loschbour and Bichon, commonly referred to as WHG; genetic cluster EuropeW_13500BP_8000BP; Fig. 2). A subtle shift of the earliest Danish individuals towards these western individuals probably reflects their closer temporal proximity captured through IBD sharing (Extended Data Fig. 4a). Although pressure-debitage of blades in the Maglemosian culture and pottery in the Ertebølle culture are both argued to have an eastern origin9,10,30,31, our data show no evidence for admixture with more eastern hunter-gatherers during those times. This points to cultural diffusion as the source of these introductions in Denmark. When tested with D-statistics, all Danish Mesolithic individuals form a clade with the earliest individual (NEO254), to the exclusion of Swedish Mesolithic hunter-gatherers (Sweden_10000BP_7500BP; Extended Data Fig. 2a) despite the close proximity to Sweden. However, a weak signal of gene flow with EHGs was shared across the whole Danish Mesolithic transect (Extended Data Fig. 2b), suggesting contact with communities further to the east prior to their expansion into Denmark before or during the earliest Mesolithic.
Genetic phenotype predictions (Supplementary Note 2) indicate a high probability of blue eye pigmentation throughout the Mesolithic, consistent with previous findings1,15,32, showing that this feature was present already in the early Mesolithic but was not fixed in the population. The Mesolithic hunter-gatherers from Denmark all display high probability of brown or black hair and height predictions generally suggest slightly lower and/or less variable stature than in the succeeding Neolithic period. We caution, however, that the relatively large genetic distance to modern individuals included in the genome-wide association studies (GWAS) panel produces scores that are less applicable to Mesolithic individuals than to more recent groups33.
Stable isotope δ13C values in collagen can inform on the proportion of marine versus terrestrially-derived protein, whereas δ15N values reflect the trophic level of the protein sources34. The earliest skeleton (NEO254) shows depleted dietary isotopic values (Fig. 3) representing a lifestyle of inland hunter-gatherers of the Early Mesolithic. This result is mirrored in the second earliest known skeleton from Denmark (Tømmerupgårds Mose34). From later Maglemose (around 9,500 cal. BP) and throughout the Kongemose and Ertebølle epochs, we observe gradually increased δ13C and δ15N values (Extended Data Fig. 5 and Supplementary Figs. 4.1 and 4.2). This implies that marine foods progressed to constitute the major supply of proteins, as suggested previously based on data from more than 30 Mesolithic humans and dogs, from both coastal and inland sites in Denmark34,35. During this period global sea-level rise gradually transformed present-day Denmark into an archipelago, where all human groups had ample access to coastal resources within their annual territories24. The local Mesolithic population adapted their diet and culture over time to the changing landscape and our data show that this occurred in a continuous population, without any detectable influx of migrants over a 4,500-year period. Low variability in 87Sr/86Sr isotope ratios throughout the Mesolithic (Fig. 3 and Supplementary Note 5) could indicate limited long-range mobility and/or deriving dietary sources from more homogeneous environments (for example, marine) than in the succeeding Neolithic periods.
Notably, some of the Danish Mesolithic individuals proved to be closely related3. Close kinship is demonstrated in the case of two individuals (NEO568/NEO569), father and son, interred next to each other in the locus classicus shell midden site of Ertebølle, and in the case of two individuals (NEO732/NEO733), mother and daughter, that were buried together at Dragsholm. The Ertebølle grave was the first discovered human skeleton in Denmark (excavated in the 1890s) that indisputably represented hunter-gatherers. After the excavation of this site, academic reasoning rooted in Biblical narration about early prehistory in Scandinavia lost momentum. The excavation data cannot reveal whether they were buried simultaneously; it can be ascertained only that the boy (infant, less than two years of age) was positioned less than one metre from his father (the ‘Ertebølle Man’). Excavations at Dragsholm in 1973 uncovered a well-preserved double burial containing a grave with two Mesolithic women as well as a male grave with grave goods suggesting an Early Neolithic date for the latter36. A close kin relationship was suggested for the two Dragsholm women on the basis of physical anthropological observations37. It was suggested that they were sisters, but this can now be corrected to a co-burial of a mother and daughter. Our data also show that the male in the adjacent burial (‘Dragsholm Man’, NEO962) was not related to the two women. These cases show that close biological kinship was socially relevant to Late Mesolithic groups in Northern Europe and affected the mortuary treatment of dead members of their society.
Early Neolithic transition
The emergence of the Neolithic FBC in Denmark has occupied a central position in archaeological research and debate throughout the past 175 years8,38,39. The defining element of the Neolithic, a food-producing economy based on domesticates of southwest Asian origin, was indisputably present in Denmark from around 5,900 cal. bp11,38. The neolithization saw a boom of new shapes and types introduced in Danish material culture, including funnel-shaped beakers and polished flint axes. From about 5,800 cal. bp, monumental long barrows of wood and earth were added to the repertoire, and about 200 years later, burials built of soil, surrounded by raised stones and including stone-built chambers, were erected as dominant landmarks in the farmland40. After 5,300 cal. bp, larger and more complex stone-constructed passage graves in large earthen tumuli emerged41. Meanwhile, simple, non-monumental burials continued along with the megalithic tombs all through the FBC epoch42. Habitation deposits, dating to the earliest centuries of the Neolithic, on top of many Mesolithic Ertebølle coastal shell middens may be interpreted as a local continuation of marine gathering and fishing. By contrast, other settlements with regular long houses on easily farmed soils further inland are associated with remains of domestic plants and animals suggesting a very clear distinction from the previous Mesolithic Ertebølle period39,43.
Regardless of these nuances, at around 5,900 cal. bp, our multiproxy dataset documents a marked and abrupt concomitant shift in genetic, phenotypic, dietary and vegetation parameters (Fig. 3). This is robust evidence for demic diffusion, settling a long-standing debate8,38. As observed elsewhere in Europe13,14,15, the introduction of farming in Denmark was unequivocally associated with the arrival of people with Anatolian farmer-related ancestry. This resulted in a population replacement with limited genetic contribution from the local hunter-gatherers. The earliest example of this typical Neolithic ancestry in our Danish dataset is observed in a bog skeleton of a female from Viksø Mose (NEO601) dated to 5,896–5,718 cal. bp (95%). In the PCA, all Danish Early Neolithic individuals cluster at the ‘late’ end of the European Neolithic farmer cline and consistently show some of the largest amounts of hunter-gatherer ancestry (10–35%) among all European Neolithic farmer genomes included (Figs. 1 and 3 and Extended Data Figs. 1 and 5a and Supplementary Data 4). In IBD clustering analyses, the Danish individuals form part of a genetic cluster (Scandinavia_5600BP_4600BP) together with FBC-associated individuals from Sweden and Poland, and also show close affinity with Polish individuals from the Globular Amphora culture (GAC) (Extended Data Fig. 4b). This could suggest an eastern European proximate origin of the Early Neolithic farmers in Denmark. Using more proximate ancestry modelling, we find that Neolithic FBC-associated individuals across Denmark, Sweden and Poland derived their hunter-gatherer ancestry component predominantly from a source related to WHG (EuropeW_13500BP_8000BP). Ancestry related to Danish Mesolithic hunter-gatherers (Denmark_10500BP_6000BP) is found in smaller proportions (less than around 10%) and only in a subset of the FBC individuals from Denmark (Extended Data Fig. 6). Moreover, this tends to occur in more recent individuals (dated to around 5,400 cal. BP onwards) who are also showing the overall largest amount of total hunter-gatherer ancestry (for example, NEO945 and NEO886; Fig. 3 and Extended Data Figs. 3 and 6a,b). Using DATES44, we found that admixture times for a large proportion of Danish Neolithic individuals predates 5,900 cal. bp when FBC emerged in Denmark, particularly for the earliest individuals (Extended Data Fig. 7). More recent admixture times (post dating the arrival of FBC in Denmark) were mainly observed in individuals dated to after about 5,400 cal. bp, and were associated with overall higher hunter-gatherer proportions. These observations were in marked contrast to FBC-associated individuals from Sweden, where admixture times and hunter-gatherer ancestry did not change over time, and no admixture with local Swedish hunter-gatherers was detected.
Our results demonstrate a population turnover in Denmark at the onset of the neolithisation by incomers who displayed a mix of Anatolian Neolithic farmer ancestry and non-local hunter-gatherer ancestry. Ancestry related to the local Danish hunter-gatherers could be detected only late in the Danish Neolithic gene pool, suggesting gene flow with groups of late surviving hunter-gatherers, as also documented in other European regions (Iron Gates45, Central Europe13 and Spain46). We do not know how the Mesolithic Ertebølle population disappeared. Some may have been isolated in small ‘pockets’ of brief existence and/or adapted to a Neolithic lifestyle. The most recent individual in our Danish dataset with hunter-gatherer ancestry is the aforementioned Dragsholm Man (NEO962), dated to 5,947–5,664 cal. bp (95% confidence interval) and archaeologically assigned to the FBC based on his grave goods37. Our data confirm a typical Neolithic diet matching the cultural affinity but contrasting his hunter-gatherer ancestry. He clearly represents a local person of Mesolithic ancestry who lived in the short Mesolithic-Neolithic transition and adopted the culture and diet of the immigrant farmers. A similar case of late hunter-gatherer ancestry in Denmark was observed when analysing human DNA obtained from a piece of chewed birch pitch from the site of Syltholm on Lolland47, dated to 5,858–5,661 cal. bp (95%). Thus, individuals with hunter-gatherer ancestry persisted for decades and perhaps centuries after the arrival of farming groups in Denmark, although they have left only a minor genomic imprint on the population of the subsequent centuries. Similar ‘relic’ hunter-gatherer ancestry is also found in the Evensås individual (NEO260) from west-coast Sweden, dated to 5913–5731 cal. bp3.
From the onset of the Neolithic in Denmark, diet shifted abruptly to a dominance of terrestrial sources as evidenced by δ13C values around −20‰ and δ15N values around 10‰ (Fig. 3 and Extended Data Fig. 5). In line with archaeological evidence, these isotopic data show that domesticated crops and animals provided the main supply of proteins from this point onwards. Isotope values remained stable at these levels throughout the following periods, although with somewhat greater variation after about 4,500 cal. bp (Fig. 3). Five Neolithic and Early Bronze Age individuals have δ13C and δ15N values that indicate a substantial intake of high trophic marine food. This is especially pronounced for the individual NEO898 (Svinninge Vejle), one of two Danish Neolithic individuals displaying ancestry related to Swedish late hunter-gatherers (see below). A considerably higher variability in individual 87Sr/86Sr values can be seen with the start of the Neolithic. This continues in the later periods (Supplementary Note 5) and is not easily explained by biases in sampling as most of our samples, regardless of ancestry and time period, are concentrated in the more easterly parts of Denmark where bone preservation conditions are generally good (Fig. 1 and Supplementary Fig. 5.3). This pattern could suggest that the Neolithic farmers in Denmark occupied and/or consumed food from more diverse landscapes, or were more mobile than the preceding hunter-gatherers. The Neolithic transition also marks a considerable rise in frequency of major effect alleles associated with light hair pigmentation48, whereas predictions throughout the first millennium of the Neolithic (FBC epoch) mostly indicate a lower stature than present day, echoing previous findings32,49.
Pitted Ware culture (PWC) originated on the Scandinavian peninsula and the Baltic islands east of the Swedish mainland but emerged around 5,100–4,700 cal. bp in the northern and eastern part of Denmark, where it coexisted with the FBC50,51. It is characterized by coarse pottery that is often decorated with pits and subsistence based on a combination of marine species and agricultural products. No burials associated with the PWC have been discovered in Denmark. Of note, however, the genomes of two approximately 5,200-year-old male individuals (NEO33, NEO898) found in Danish wetland deposits proved to be of hunter-gatherer ancestry related to that of PWC individuals from Ajvide on the Baltic island of Gotland (Sweden)52 (Figs. 2, 3 and Extended Data Fig. 4a). Of the two individuals, NEO033 (Vittrup, Northern Jutland) also displays an outlier Sr signature (Fig. 3), perhaps suggesting a non-local origin that matches his unusual ancestry. Overall, our results demonstrate direct contact across the sea between Denmark and the Scandinavian peninsula during this period, which is in line with archaeological findings50,51.
PCA of 2,000 modern Danish genomes from the iPSYCH study62 in the context of ancient western Eurasian individuals. Coloured symbols indicate sample age for ancient Danish individuals, whereas grey symbols indicate 1,145 ancient imputed individuals from across Western Eurasia3. Modern Danish individuals are indicated by black filled circles and are shown on the right. Inset, a magnified view of the cluster with modern Danes. The colour scale in the inset represents the age range of the ancient samples within the magnified region only.
Later Neolithic and Bronze Age
Europe was transformed by large-scale migrations from the Pontic–Caspian Steppe around 5,000–4,800 cal. bp. This introduced steppe-related ancestry to most parts of the continent within a 1,000-year span and gave rise to the Corded Ware culture (CWC) complex1,2. In Denmark, this coincided with the transition from the FBC to the SGC, the regional manifestation of the CWC complex. The transition to single graves in round tumuli has been characterized archaeologically by two expansion phases: a primary and rapid occupation of central, western and northern Jutland (west Denmark) starting around 4,800 cal. bp and a later and slower expansion across the Eastern Danish Islands starting around 4,600 cal. bp53,54. In the eastern parts of the country, SGC traits are less visible, whereas FBC traditions such as burial in megalithic grave chambers persisted55. This cultural shift represents another classical archaeological enigma, with explanations favouring immigration versus cultural acculturation competing for generations19,56.
Insights from a few low-coverage genomes1,57 have indeed shown a link to the Steppe expansions, but by mapping out ancestry components in the 100 ancient genomes we now uncover the full impact of this event and demonstrate a second near-complete population turnover in Denmark within just 1,000 years. This genetic shift was evident from PCA and ADMIXTURE analyses, in which Danish individuals dating to the SGC and Late Neolithic and Bronze Age (LNBA) cluster with other European LNBA individuals and show large proportions of ancestry components associated with Yamnaya groups from the Steppe (Figs. 1 and 3 and Extended Data Fig. 1). We estimate around 60–85% of ancestry related to Steppe groups (Steppe_5000BP_4300BP), with the remainder contributed from individuals with farmer-related ancestry associated with Eastern European GAC (Poland_5000BP_4700BP; 10–23%) and to a lesser extent from local Neolithic Scandinavian farmers (Scandinavia_5600BP_4600BP; 3–18%) (Extended Data Fig. 6a,b). Although the emergence of SGC introduced a major new ancestry component in the Danish gene pool, it was not accompanied by apparent shifts in dietary isotopic ratios, or Sr isotope ratios (Fig. 3). Our complex trait predictions, however, indicate an increase in height (Fig. 3 and Supplementary Note 2), which is consistent with ancient Steppe individuals being predicted taller than average European Neolithic individuals before the steppe migrations32,49,58.
Because of poor preservation conditions in most of western Denmark, we do not have skeletons from the earliest phase of the SGC (around 4,800 cal. bp) so we cannot unequivocally demonstrate that these people carried steppe-related ancestry. SGC burial customs were implemented in different ways in the southern and the GAC-related northern parts of the peninsula, respectively18 and considering recent genetic results in other regions59, it is plausible that differing demographic processes unfolded within Denmark. However, we know that steppe ancestry was present 200 years later in SGC-associated skeletons from the Gjerrild grave57. The age of the Gjerrild skeletons (from around 4,600 cal. bp) matches the earliest example of steppe-related ancestry in our current study, identified in a skeleton from a megalithic tomb at Næs (NEO792). We estimated around 85% of Steppe-related ancestry in this individual, the highest amount among all Danish LNBA individuals (Extended Data Fig. 6a). Notably, NEO792 is also contemporaneous with the two most recent individuals in our dataset showing Anatolian farmer-related ancestry without any steppe-related ancestry (NEO580, Klokkehøj and NEO943, Stenderup Hage) testifying to a short period of ancestry co-existence before the FBC disappeared—similar to the disappearance of the Mesolithic Ertebølle people of hunter-gatherer ancestry a thousand years earlier. Using Bayesian modelling we estimate the duration between the first appearance of Anatolian farmer-related ancestry to the first appearance of Steppe-related ancestry in Denmark to be between 876 and 1,100 years (95% prob. interval, Supplementary Note 3) implying that the former type of ancestry was dominant for less than 50 generations.
The following Late Neolithic ‘Dagger’ epoch (around 4,300–3,700 cal. bp) in Denmark has been described as a time of integration of culturally and genetically distinct groups54. Bronze became dominant in the local production of weapons while elegantly surface-flaked daggers in flint were still the dominant male burial gift. Unlike the SGC epoch, this period is richly represented by human skeletal material. Although broad population genomic signatures suggest genetic stability in the LNBA (Figs. 1 and 3), patterns of pairwise IBD-sharing and Y chromosome haplogroup distributions in a temporal transect of 38 LNBA Danish and southern Swedish individuals indicate at least three distinct ancestry phases during this approximately 1,000-year time span (Extended Data Figs. 4c and 8).
LNBA phase I: an early stage between around 4,600 and 4,300 cal. bp, in which Scandinavians cluster with early CWC individuals from Eastern Europe, rich in Steppe-related ancestry and males with an R1a Y chromosomal haplotype (Extended Data Fig. 8a,b). Archaeologically, these individuals are associated with the later stages of the Danish SGC and the Swedish Battle Axe Culture.
LNBA phase II: an intermediate stage largely coinciding with the Dagger epoch (around 4,300–3,700 cal. bp), in which Danish individuals cluster with central and western European LNBA individuals dominated by males with distinct sub-lineages of R1b-L513 (Extended Data Fig. 8c,d). Among them are individuals from Borreby (NEO735, 737) and Madesø (NEO752).
LNBA phase III: a final stage from around 4,000 cal. bp onwards, in which a distinct cluster of Scandinavian individuals dominated by males with I1 Y-haplogroups appears (Extended Data Fig. 8e). Y chromosome haplogroup I1 is one of the dominant haplogroups in present-day Scandinavians, and we here document its earliest occurrence in an approximately 4,000-year-old individual from Falköping in southern Sweden (NEO220). The rapid increase in frequency of this haplogroup and associated genome-wide ancestry coincides with increase in human mobility seen in Swedish Sr isotope data, suggesting an influx of people from eastern or northeastern regions of Scandinavia, and the emergence of stone cist burials in Southern Sweden60, which were also introduced in eastern Denmark during that period54,61.
Using genomes from LNBA phase III (Scandinavia_4000BP_3000BP) in supervised ancestry modelling, we find that they form the predominant ancestry source for later Iron and Viking Age Scandinavians (Extended Data Fig. 6d) and other ancient European groups with a documented Scandinavian or Germanic association (for example, Anglo-Saxons and Goths; Extended Data Fig. 6e). When projecting 2,000 modern Danish genomes62 on a PCA of ancient Eurasians, the modern individuals occupy an intermediate space on a cline between the LNBA and Viking Age individuals (Fig. 4). This result shows that the foundation for the present-day gene pool was already in place in LNBA groups 3,000 years ago, but the genetic structure of the Danish population was continually reshaped during succeeding millenia.
Environmental impact
The two documented major population turnovers were accompanied by substantial changes in land use, as apparent from the high-resolution pollen diagram from Lake Højby in Northwest Zealand (Fig. 3) reconstructed using the landscape-reconstruction algorithm (LRA; Supplementary Note 6). We uncovered a direct synchronic link between shifts in a populations’ ancestry profile and land use. During the Mesolithic, the landscape was dominated by primary forest trees (Tilia, Ulmus, Quercus, Fraxinus, Alnus and so on). At the onset of the Neolithic, the primary forest diminished, cleared by FBC farmers. A new type of forest with more secondary and early successional trees (Betula and then Corylus) appeared, whereas the proportion between forest and open land remained almost unaltered. From about 5,650 cal. bp deforestation intensified, resulting in an open grassland-dominated landscape. This open phase was short-lived, and the secondary forest expanded again from around 5,500 to 5,000 cal. bp, until another episode of forest clearance occurred during the last part of the FBC epoch. We conclude that the agricultural practice during the FBC was characterized by repeated clearing of the forest followed by regrowth. After about 4,600 cal bp, this strategy changed with the emergence of the SGC and the arrival of Steppe-related ancestry in Denmark. In Western Denmark (Jutland), the arrival of the SGC was characterized by permanent large-scale opening of the landscape to create pastureland63,64 and we observe here a similar increase in grassland and cropland at Højby Sø in Eastern Denmark around 4,600 cal. bp (Fig. 3). Notably, this was accompanied by an increase in primary forest cover, especially Tilia and Ulmus, probably reflecting a development of a more permanent division of the landscape into open grazing areas and primary forests.
Drivers of change
We have demonstrated examples of both cultural and demic diffusion during the Mesolithic and Neolithic periods in Denmark. Shifts in the Mesolithic material culture appeared without any detectable levels of changes in ancestry, whereas the two cultural shifts in the Neolithic period were clearly driven by new people coming in. Accordingly, groupings of artefacts and monuments into archaeological cultures do not always represent genetically distinct populations and the underlying mechanisms responsible for prehistoric cultural shifts must be examined on a case-by-case basis.
It remains a mystery why the Neolithic farming expansion came to a 1,000-year standstill before entering Southern Scandinavia. It may be that it was complicated by a high Mesolithic hunter-gatherer population density owing to a very productive marine and coastal environment20,65. Further, the Danish Ertebølle population may have been acquainted with armed conflict11,66 enabling territorial defence against intruders. Alternatively, it has been argued that changing climatic conditions around 6,000 cal. bp became a driver since it enhanced the potential for farming further north67, but other studies have not confirmed this68. The second population turnover in the late Neolithic resulted in a short period of three competing cultural complexes in Denmark, namely the FBC, the PWC and the SGC. The latter introduced the steppe-related ancestry which has prevailed to this day. There is archaeological evidence that this was a violent time, both in Denmark69 and elsewhere70,71. Additionally, ancient DNA evidence has demonstrated that plague was widespread during this period72,73. In tandem with other indicators of population declines74, and widespread reforestation after 5,000 cal. bp75, it suggests that the local populations of Central and Northern Europe may have been severely impacted prior to the arrival of newcomers with Steppe-related ancestry. This could explain the rapid population turnover and limited admixture with locals we observe.
While the two major shifts in Danish Mesolithic and Neolithic material culture may have had different drivers and causes, the outcomes were ultimately the same: new people arrived and rapidly took over the territory. With this arrival, the local landscape was modified to fit the lifestyle and culture of the immigrants. This is the hallmark of the Anthropocene, observed here in high resolution in prehistoric Denmark.
Methods
Ancient genomic analyses
The 100 ancient Danish genomes analysed here contribute to the 317 shotgun-sequenced genomes in Allentoft et al.3. All details concerning sampling, DNA extraction, library preparation, sequencing, basic bioinformatics, authentication and dataset construction are found in ref. 3 together with all site descriptions and sample metadata. A condensed list of metainformation on the 100 Danish individuals is released here (Supplementary Data 1) together with a text summarizing the study sites and skeletons (Supplementary Note 1). In brief, laboratory work was carried out in dedicated ancient DNA cleanlab facilities (University of Copenhagen) using optimized ancient DNA methods1,77. Double-stranded blunt-end libraries were sequenced (80 bp and 100 bp single-end reads) on Illumina HiSeq 2500 and 4000 platforms. Initial shallow shotgun screening was used to identify samples with sufficient DNA preservation for deeper genomic sequencing. Of the 100 Danish samples that qualified for this, 65 were from tooth cementum, 29 were petrous bones, and 6 were obtained from other bones (Supplementary Data 1). Sequence reads were bioinformatically mapped to the human reference genome (build 37), filtered and merged to sample level followed by estimates of genomic overage, post-mortem DNA damage, contamination, and genetic sex ID (see3). For these 100 samples we observed C-to-T deamination fractions ranging from 12.2% to 66.7%, with an average of 34.9% across all samples (Supplementary Data 1), consistent with highly degraded ancient DNA. We genetically identified 67 males, 32 females and one undetermined in our dataset (Supplementary Data 1).
We utilized a new computational method optimized for low-coverage data21, to impute genotypes based on genotype likelihoods of ancient individuals with the samtools/bcftools pipeline, and using the 1000 Genomes phased data78 as a reference panel. To generate the main dataset in3 this was jointly applied to 1,664 shotgun-sequenced ancient genomes, including our 100 ancient Danish genomes, and resulted in a dataset of 8.5 million common SNPs (>1% minor allele frequency and imputation info score > 0.5) for imputed diploid ancient genomes. After removing genomes with low coverage (<0.1X), low imputation quality (average genotype probability <0.98), contamination estimates >5%, or close relatives (first or second degree, lowest coverage relative removed), 67 of the 100 Danish genomes were retained as imputed in downstream analyses. The remaining 33 genomes were analysed as pseudo-haploid genotypes.
For population genetic analyses, we combined ancient samples with two different modern reference panels:
-
‘1000 G’ dataset: whole-genome sequencing data of 2,504 individuals from 26 world-wide populations from the 1000 Genomes project, with genotypes at 7,321,965 autosomal SNPs.
-
‘HO’ dataset: SNP array data of 2,180 modern individuals from 213 world-wide populations, with genotypes at 535,880 autosomal SNPs.
Analyses were based on the 1000 G dataset unless otherwise noted. Individuals not passing imputation quality control cutoffs mentioned above were included in PCA and ADMIXTURE analyses as pseudo-haploid genotypes. Four Danish individuals showed possible signs of DNA contamination (Fig. 3 and Supplementary Data 1) and were excluded from most analyses. To take full advantage of the extensive multiproxy data they were, however, included in Fig. 3. Individual metadata for all genetic analyses related to the ancient Danish individuals as well as selected subset of relevant West Eurasian individuals, are reported in Supplementary Data 3.
For PCA combining ancient and modern Western Eurasians (Fig. 1b), we used the data and framework from3 to capture West Eurasian genetic diversity based on n = 983 modern genomes and n = 1,105 ancient genomes (HO dataset). Data from a total of 179 ancient Danish genomes are shown in Fig. 1b of which 83 are previously published1,47,57,76 (Supplementary Data 3)—the latter being primarily from the Bronze Age and Viking periods. To perform PCA projection for low-coverage individuals, we used smartpca with options ‘lsqproject: YES’ and ‘autoshrink: YES’.
The ADMIXTURE results presented in this study represent subsets of individuals from the full ADMIXTURE runs in3 where 1,593 ancient individuals were analysed (n = 1,492 imputed, n = 101 pseudo-haploid, n = 71 excluded as close relatives or with a contamination estimate >5%; HO dataset). Figure 1c represents 176 ancient Danish genomes after excluding three close relatives (Supplementary Data 1 and 4).
D-statistics were obtained using pseudo-haploid genotypes at transversion SNPs in the 1000 G dataset, grouping the non-Danish individuals into populations using their membership in the genetic clusters inferred from IBD sharing (Supplementary Data IV). We computed D-statistics from genotypes in PLINK format using the qpdstat function implemented in the ADMIXTOOLS 2 R package79.
Analysis of IBD sharing and mixture models were carried out as described3, using the same set of inferred genetic clusters (see Supplementary Data 4). In brief, we used IBDseq80 to detect IBD segments, a carried out genetic clustering of the individuals using hierarchical community detection on a network of pairwise IBD-sharing similarities. IBD-based PCA was carried out in R using the eigen function on a covariance matrix of pairwise IBD sharing between the respective ancient individuals. We estimated ancestry proportion in supervised modelling of target individuals as mixtures of different sets of putative source groups via non-negative least squares on relative IBD-sharing rate vectors.
Admixture time inference for FBC-associated individuals was carried out using the linkage-disequilibrium-based method DATES44 (HO dataset). We estimated admixture time separately for each target individual from Denmark and Sweden, using hunter-gatherer individuals (n = 58) and early farmer individuals (n = 49) as the two source groups.
For the PCAs presented in Fig. 4 including modern Danish samples we projected 2,000 imputed samples81 of individuals born in 1981–2005 from the iPSYCH2012 case-cohort study62 onto the PCA space spanned by the 1,145 non-low coverage or related european and western Asian ancient imputed samples3. Otherwise, the analysis is identical to the one described above. The modern individuals were selected from a subset of the random population subcohort component of iPSYCH2012 having all four grandparents born in Denmark, and being of Danish or European ancestry as determined in a separate already existing PCA of main modern-day ancestry groups81. This was done using Eigensoft 7.2.1 on the intersect of imputed SNPs from the ancient and modern samples, filtered by minor allele frequency 0.05, pruned using PLINK v1.90b6.2182 based on source samples (parameters: –indep-pairwise 1000 50 0.25) leaving 146,895 variants.
The genetic predictions of eye and hair colour were done based on the HIrisPlex system83. We used imputed effect allele dosages of 18 out of 24 main effect HIrisPlex variants, available for the ancient samples, to derive probabilities for brown, blue and grey/intermediate eye colour and blond, brown, black and red hair colour, following HIrisPlex formulas (see further details in Supplementary Note 2). We predicted relative ‘genetic height’ using allelic effect estimates from 310 common autosomal SNPs with robustly genome-wide significant allelic effects (P < 10−15) in a recent GWAS of height in the UK Biobank84. Per-sample height polygenic score (PGS) was calculated for ancient individuals as well as 3,467 Danish ancestry male conscripts from the random population subcohort of the iPSYCH2012 case-cohort study62 by summing allelic effect multiplied with the effect allele imputed dosage81 across the 310 loci. For further details see Supplementary Note 2. Only a fraction of the 100 Danish skeletons were suitable for stature estimation by actual measurement, which is why these values are not reported here.
Radiocarbon dates and Bayesian modelling of ancestry chronology
For the 100 sample ages in this study we use midpoint estimates of the calibrated and reservoir corrected probability distribution of the radiocarbon age (Supplementary Data 1; further 14C dates, associated isotopic measurements, calibrations and reservoir corrections are accessible in ref. 3). Focusing on estimating the interval between the two major population turnovers, we established a precise chronology using 81 radiocarbon dates from 64 Danish sites relevant to this particular interval (Supplementary Note 3). A Bayesian approach applied to the radiocarbon dates unifies radiocarbon results, ancestry information, and the high precision curve into one calibration process, thereby gaining greater precision. All models and data calibrations were performed using OxCal v4.485,86,87,88 and the calibration dataset from Reimer et al.89. We used a trapezoidal phase prior90,91 for the calculation of the transitional time interval to determine duration between the first appearance of Anatolian farmer-related ancestry to the first appearance of Steppe-related ancestry in Denmark. We corrected the reservoir effect on bones with significantly increased isotope values (δ13C, −18.00 and δ15N, +12.00) directly in the models using previously defined reservoir ages as input and calculated the diet reconstruction estimates for the individual in 14C years based on the collagen isotope values (Supplementary Note 3 and Supplementary Figs. 3.1 and 3.2); for a similar method see refs. 92,93. For combining radiocarbon dates related to the same individual we used the R_Combine() function.
Stable isotope proxies for diet and mobility
Bulk collagen isotope values of carbon (δ13C) and nitrogen (δ15N) represent protein sources consumed over several years before death, depending on the skeletal part and the age at death of the individual94,95. Generally, δ13C values inform on the proportion of marine versus terrestrial protein, whereas δ15N values reflect the trophic level from which the proteins were acquired96,97. See Supplementary Note 4 for further discussion. Stable isotope values were measured in collagen from all 100 skeletons and the full assemblage of isotopic measurements is available in Supplementary Data 2, and further discussed in Supplementary Note 4. Most of the δ13C and δ15N measurements were conducted at the 14C Centre, University of Belfast according to standard protocols98, based on a modified Longin method including ultra-filtration98,99. Measured uncertainty was within the generally accepted range of ±0.2‰ (1 s.d.) and all samples were within the acceptable atomic C:N range of 2.9–3.6, showing low likelihood of diagenesis100,101.
Strontium isotope analyses can provide a proxy for individual mobility102,103,104. The 87Sr/86Sr ratio in specific skeletal elements may reflect the local geological signature obtained through diet by the individual during early childhood and it will usually remain unchanged during life and after death105. Ongoing controversies exist over the exact use of geographically-defined baseline values106,107, which is why we restrict our observations and interpretations of Sr variation to patterns that are only relative to our own data. Measurements of 87Sr/86Sr ratios in teeth and petrous bones were conducted at the Geochronology and Isotope Geochemistry Laboratory (Department of Geological Sciences, University of North Carolina- Chapel Hill) and data are found in Supplementary Data 2. For further details see Supplementary Note 5.
Vegetation modelling
Using a high-resolution pollen diagram from Lake Højby, Northwest Zealand108, we reconstruct the changes in vegetation cover during the period 5,000–2,400 cal. bc using the landscape-reconstruction algorithm (LRA109,110). Although the LRA has previously been applied at low temporal resolution regional scale (fer example, in refs. 111,112.), and to Iron Age (and later) pollen diagrams113,114, to our knowledge, this is the first time that this quantitative method is applied at local scale to a pollen record spanning the Mesolithic and Neolithic periods in Denmark. In total 60 pollen samples between 6,900 and 4,400 cal. bp were included and the temporal resolution between samples is approximately 40 years. Regional vegetation was estimated with the model REVEALS109 based on pollen data from six other lakes on Zealand (see Supplementary Fig. 6.1). From this, regional pollen rain is calculated and local scale vegetation around Højby Sø calculated using the LOVE model110. Average pollen productivity estimates for Europe115 for 25 wind pollinated species were applied. The reconstructed cover for plant species were then combined into four land cover categories, crops (only cereals), grassland (all other herbs), secondary forest (Betula and Corylus) and primary forest (all other trees). The vegetation reconstruction from Højby Sø is used to illustrate the vegetation development at the Mesolithic/Neolithic transition in eastern Denmark. For more details see Supplementary Note 6.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Sequencing data analysed in this study is released in the accompanying study ‘Population genomics of post-glacial western Eurasia’3. These are publicly available on the European Nucleotide Archive under accession PRJEB64656, together with sequence alignment map files, aligned using human build GRCh37. The full analysis dataset including both imputed and pseudo-haploid genotypes for all ancient individuals used in this study is available at https://doi.org/10.17894/ucph.d71a6a5a-8107-4fd9-9440-bdafdfe81455. Aggregated IBD-sharing data as well as hi-resolution versions of supplementary figures are available at Zenodo under accession https://doi.org/10.5281/zenodo.8196989. Maps were created in R using public domain Natural Earth map data.
References
Allentoft, M. E. et al. Population genomics of Bronze Age Eurasia. Nature 522, 167–172 (2015).
Haak, W. et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522, 207–211 (2015).
Allentoft, M. E. et al. Population genomics of post-glacial western Eurasia. Nature https://doi.org/10.1038/s41586-023-06865-0 (2024).
Posth, C. et al. Palaeogenomics of Upper Palaeolithic to Neolithic European hunter-gatherers. Nature 615, 117–126 (2023).
Johannsen, N. N., Larson, G., Meltzer, D. J. & Vander Linden, M. A composite window into human history. Science 356, 1118–1120 (2017).
Furholt, M. Mobility and social change: understanding the European Neolithic period after the archaeogenetic revolution. J. Archaeol. Res. 29, 481–535 (2021).
Kristiansen, K. Archaeology and the Genetic Revolution in European Prehistory (Elements in the Archaeology of Europe) (Cambridge Univ. Press, 2022).
Fischer, A. & Kristiansen, K. The Neolithisation of Denmark. 150 Years of Debate (J. R. Collis, 2002).
Günther, T. et al. Population genomics of Mesolithic Scandinavia: investigating early postglacial migration routes and high-latitude adaptation. PLoS Biol. 16, e2003703 (2018).
Kashuba, N. et al. Ancient DNA from mastics solidifies connection between material culture and genetics of mesolithic hunter–gatherers in Scandinavia. Commun. Biol. 2, 185 (2019).
Fischer, A. in The Neolithisation of Denmark—150 years of debate (eds Fischer, A. & Kristiansen, K.) 343–393 (J. R. Collis, 2002).
Price, T. D. Europe’s First Farmers (Cambridge Univ. Press, 2000).
Lipson, M. et al. Parallel palaeogenomic transects reveal complex genetic history of early European farmers. Nature 551, 368–372 (2017).
Mathieson, I. et al. The genomic history of southeastern Europe. Nature 555, 197–203 (2018).
Brace, S. et al. Ancient genomes indicate population replacement in Early Neolithic Britain. Nat. Ecol. Evol. 3, 765–771 (2019).
Midgley, M. TRB Culture: The First Farmers of the North European Plain (Edinburgh Univ. Press, 1992).
Iversen, R. in Tracing the Indo-Europeans: New evidence from Archaeology and Historical Linguistics (eds Olsen, B. A., Olander, T. & Kristiansen, K.) 73–95 (Oxbow, 2019).
Nielsen, S. K. & Johannsen, N. N. Mortuary palisades, single graves, and cultural admixture: the establishment of Corded Ware culture on the Jutland Peninsula. Praehistorische Zeitschrift https://doi.org/10.1515/pz-2023-2022 (2023).
Kristiansen, K. Prehistoric migrations—the case of the Single Grave and Corded Ware Cultures. J. Dan. Archaeol. 8, 211–225 (1991).
Lewis, J. P. et al. Marine resource abundance drove pre-agricultural population increase in Stone Age Scandinavia. Nat. Commun. 11, 2006 (2020).
Sousa da Mota, B. et al. Imputation of ancient human genomes. Nat. Commun. 14, 3660 (2023).
Schilling, H. in Mesolithic on the Move (eds Larsson, L. et al.) 351–358 (2003).
Petersen, P. V. Chronological and regional variation in the Late Mesolithic of Eastern Denmark. J. Dan. Archaeol. 3, 7–18 (1984).
Fischer, A. in The Danish Storebælt Since the Ice Age (eds Pedersen, L., Fischer, A. & Aaby, B.) 63–77 (Sea and Forest, 1997).
Sørensen, S. A. The Kongemose Culture (Univ. Press of Southern Denmark, 2017).
Dolbunova, E. et al. The transmission of pottery technology among prehistoric European hunter-gatherers. Nat. Hum. Behav. 7, 171–183 (2023).
Klassen, L. Jade und Kupfer: Untersuchungen zum Neolithisierungsprozess im westlichen Ostseeraum unter besonderer Berücksichtigung der Kulturentwicklung Europas 5500–3500 bc vol. 47 (Aarhus Universitetsforlag, 2004).
Price, T. D. Seeking the First Farmers in Western Sjælland, Denmark: The Archaeology of the Transition to Agriculture in Northern Europe (Oxbow Books, 2022).
Hansen, J. et al. The Maglemosian skeleton from Koelbjerg revisited: Identifying sex and provenance. Dan. J. Archaeol. 6, 55–66 (2017).
Sørensen, M. in Ecology of Early Settlement in Northern Europe: Conditions for Subsistence and Survival. The Early Settlement of Northern Europe Vol. 1 (eds. Persson, P., Riede, F. & Skar, B.) 277–301 (Equinox, 2018).
Piezonka, H. et al. Stone Age pottery chronology in the Northeast European Forest Zone: new AMS and EA-IRMS results on foodcrusts. Radiocarbon 58, 267–289 (2016).
Mathieson, I. et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528, 499–503 (2015).
Irving-Pease, E. K. et al. The selection landscape and genetic legacy of ancient Eurasians. Nature https://doi.org/10.1038/s41586-023-06705-1 (2024).
Fischer, A. et al. Coast-inland mobility and diet in the Danish Mesolithic and Neolithic: evidence from stable isotope values of humans and dogs. J. Archaeol. Sci. 34, 2125–2150 (2007).
Fischer, A. et al. The composition of Mesolithic food—evidence from a submerged settlement on the Argus Bank, Denmark. Acta Archaeol. 78, 163–178 (2007).
Brinch Petersen, E. Gravene ved Dragsholm. Fra jægere til bønder for 6000 år siden. Nationalmuseets Arbejdsmark 1974, 112–120 (1974).
Price, T. D. et al. New information on the Stone Age graves at Dragsholm, Denmark. Acta Archaeol. 78, 193–219 (2007).
Sørensen, L. From Hunter to Farmer in Northern Europe. Migration and adaptation during the Neolithic and Bronze Age. Acta Archaeologica Vol. 85 (Wiley–Blackwell, 2014).
Nielsen, P. O. & Nielsen, F. O. S. First Farmers on the Island of Bornholm (The Royal Society of Northern Antiquaries and Univ. Press of Southern Denmark, 2020).
Sjögren, K.-G. & Fischer, A. The chronology of Danish dolmens. Results from 14C dates on human bones. J. Neolit. Archaeol. 25, https://doi.org/10.12766/jna.2023.1 (2023).
Dehn, T. & Hansen, S. I. Birch bark in Danish passage graves. J. Dan. Archaeol. 14, 23–44 (2006).
Ebbesen, K. Simple, tidligneolitiske grave. Aarbøger for nordisk Oldkyndighed og Historie 1992, 47–102 (1994).
Gron, K. J. & Sørensen, L. Cultural and economic negotiation: a new perspective on the Neolithic Transition of Southern Scandinavia. Antiquity 92, 958–974 (2018).
Chintalapati, M., Patterson, N. & Moorjani, P. The spatiotemporal patterns of major human admixture events during the European Holocene. eLife 11, e77625 (2022).
González-Fortes, G. et al. Paleogenomic evidence for multi-generational mixing between Neolithic farmers and Mesolithic hunter-gatherers in the Lower Danube Basin. Curr. Biol. 27, 1801–1810.e10 (2017).
Villalba-Mouco, V. et al. Survival of Late Pleistocene hunter-gatherer ancestry in the Iberian Peninsula. Curr. Biol. 29, 1169–1177.e7 (2019).
Jensen, T. Z. T. et al. A 5700 year-old human genome and oral microbiome from chewed birch pitch. Nat. Commun. 10, 5520 (2019).
Olalde, I. et al. Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature 507, 225–228 (2014).
Cox, S. L., Ruff, C. B., Maier, R. M. & Mathieson, I. Genetic contributions to variation in human stature in prehistoric Europe. Proc. Natl Acad. Sci. USA 116, 21484–21492 (2019).
Klassen, L. (ed.) The Pitted Ware Culture on Djursland: Supra-regional Significance and Contacts in the Middle Neolithic of Southern Scandinavia (Aarhus Univ. Press, 2020).
Iversen, R., Philippsen, B. & Persson, P. Reconsidering the Pitted Ware chronology. Praehistorische Zeitschrift 96, 44–88 (2021).
Coutinho, A. et al. The Neolithic Pitted Ware culture foragers were culturally but not genetically influenced by the Battle Axe culture herders. Am. J. Phys. Anthropol. 172, 638–649 (2020).
Glob, P. V. Studier over den Jyske Enkeltgravskultur (Gyldendal, 1945).
Müller, J. & Vandkilde, H. in Contrasts of the Nordic Bronze Age. Essays in Honour of Christopher Prescott (eds. Austvoll, K.I., Hem Eriksen, M., Fredriksen, P.D., Melheim, A.L., Prøsch-Danielsen, L., Skogstrand, L.) 29–48 (Brepols, 2020).
Iversen, R. The Transformation of Neolithic Societies. An Eastern Danish Perspective on the 3rd Millennium BC Vol. 88 (Jutland Archaeological Society, 2015).
Damm, C. The Danish Single Grave Culture—ethnic migration or social construction? J. Dan. Archaeol. 10, 199–204 (1991).
Egfjord, A. F.-H. et al. Genomic steppe ancestry in skeletons from the Neolithic Single Grave Culture in Denmark. PLoS ONE 16, e0244872 (2021).
Grasgruber, P., Sebera, M., Hrazdíra, E., Cacek, J. & Kalina, T. Major correlates of male height: a study of 105 countries. Econ. Hum. Biol. 21, 172–195 (2016).
Papac, L. et al. Dynamic changes in genomic and social structures in third millennium bce central Europe. Sci. Adv. 7, eabi6941 (2021).
Blank, M. Mobility, Subsistence and Mortuary practices. An Interdisciplinary Study of Neolithic and Early Bronze Age Megalithic Populations of Southwestern Sweden. PhD thesis, Univ. of Gothenburg (2021).
Winther Johannsen, J. Late Neolithic expansion. Dan. J. Archaeol. 12, https://doi.org/10.7146/dja.v12i1.132093 (2023).
Pedersen, C. B. et al. The iPSYCH2012 case–cohort sample: new directions for unravelling genetic and environmental architectures of severe mental disorders. Mol. Psychiatry 23, 6–14 (2017).
Odgaard, B. V. The Holocene vegetation history of northern West Jutland, Denmark. Nord. J. Bot. 14, 546–546 (1994).
Haak, W. et al. in The Indo-European Puzzle Revisited: Integrating Archaeology, Genetics, and Linguistics (eds Kristiansen, K., Kroonen, G. & Willerslev, E.) 63–80 (2023).
Fischer, A., Gotfredsen, A. B., Meadows, J., Pedersen, L. & Stafford, M. The Rødhals kitchen midden—marine adaptations at the end of the Mesolithic world. J. Archaeol. Sci. 39, 103102 (2021).
Bennike, P. in The Danish Storebælt Since the Ice Age (eds Pedersen, L., Fischer, A. & Aaby, B.) 99–105 (A/S Storebælt Fixed Link, 1997).
Warden, L. et al. Climate induced human demographic and cultural change in northern Europe during the mid-Holocene. Sci Rep. 7, 15251 (2017).
Krossa, V. R. et al. Regional climate change and the onset of farming in northern Germany and southern Scandinavia. Holocene 27, 1589–1599 (2017).
Iversen, R. Arrowheads as indicators of interpersonal violence and group identity among the Neolithic Pitted Ware hunters of southwestern Scandinavia. J. Anthropol. Archaeol. 44, 69–86 (2016).
Lidke, G. Violence in the Single Grave Culture of northern Germany? in Sticks, Stones, and Broken Bones: Neolithic Violence in a European Perspective (eds Schulting, R. J. & Fibiger, L.) 139-150 https://doi.org/10.1093/acprof:osobl/9780199573066.003.0008 (Oxford, 2012).
Schroeder, H. et al. Unraveling ancestry, kinship, and violence in a Late Neolithic mass grave. Proc. Natl Acad. Sci. USA 116, 10705–10710 (2019).
Rasmussen, S. et al. Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago. Cell 163, 571–582 (2015).
Rascovan, N. et al. Emergence and spread of basal lineages of Yersinia pestis during the Neolithic decline. Cell 176, 295–305.e10 (2019).
Hinz, M. et al. in Neolithic Diversities: Perspectives from a Conference in Lund, Sweden 43–51 (Lund Univ., 2015).
Feeser, I., Dörfler, W., Kneisel, J., Hinz, M. & Dreibrodt, S. Human impact and population dynamics in the Neolithic and Bronze Age: multi-proxy evidence from north-western Central Europe. Holocene 29, 1596–1606 (2019).
Margaryan, A. et al. Population genomics of the Viking world. Nature 585, 390–396 (2020).
Damgaard, P. B. et al. Improving access to endogenous DNA in ancient bones and teeth. Sci. Rep. 5, 11184 (2015).
1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Maier, R., Flegontov, P., Flegontova, O., Işıldak, U., Changmai, P. & Reich, D. On the limits of fitting complex models of population history to f-statistics. Elife 12, e85492 (2023).
Browning, B. L. & Browning, S. R. Detecting identity by descent and estimating genotype error rates in sequence data. Am. J. Hum. Genet. 93, 840–851 (2013).
Appadurai, V. et al. Accuracy of haplotype estimation and whole genome imputation affects complex trait analyses in complex biobanks. Commun. Biol. 6, 101 (2023).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Walsh, S. et al. The HIrisPlex system for simultaneous prediction of hair and eye colour from DNA. Forensic Sci. Int. Genet. 7, 98–115 (2013).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Bronk Ramsey, C. Development of the radiocarbon calibration program OxCal. Radiocarbon 43, 355–363 (2001).
Bronk Ramsey, C. Deposition models for chronological records. Q. Sci. Rev. 27, 42–60 (2008).
Bronk Ramsey, C. Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 (2009).
Bronk Ramsey, C. Dealing with outliers and offsets. Radiocarbon 51, 1023–1045 (2009).
Reimer, P., Austin, W. & Bard, E. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kbp). Radiocarbon 62, 725–757 (2020).
Karlsberg, A. J. Flexible Bayesian Methods for Archaeological Dating. PhD thesis, Univ. Sheffield (2006).
Lee, S. & Ramsey, C. Development and application of the trapezoidal model for archaeological chronologies. Radiocarbon 54, 107–122 (2012).
Meadows, J. et al. Dietary freshwater reservoir effects and the radiocarbon ages of prehistoric human bones from Zvejnieki, Latvia. J. Archaeol. Sci. 6, 678–689 (2016).
Rose, H. A., Meadows, J. & Bjerregaard, M. High-resolution dating of a medieval multiple grave. Radiocarbon 60, 1547–1559 (2018).
Hedges, R. E. M., Clement, J. G., David, C., Thomas, L. & O’Connell, T. C. Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements. Am. J. Phys. Anthropol. 133, 808–816 (2007).
Jørkov, M. L. S., Heinemeier, J. & Lynnerup, N. The petrous bone-a new sampling site for identifying early dietary patterns in stable isotopic studies. Am. J. Phys. Anthropol. 138, 199–209 (2009).
Schoeninger, M. J. & Moore, K. Bone stable isotope studies in archaeology. J. World Prehist. 6, 247–296 (1992).
Hedges, R. E. M. & Reynard, L. M. Nitrogen isotopes and the trophic level of humans in archaeology. J. Archaeol. Sci. 34, 1240–1251 (2007).
Reimer, P. et al. Laboratory protocols used for AMS radiocarbon dating at the 14Chrono Centre. English Heritage Research Report Series 5-2015 https://historicengland.org.uk/research/results/reports/6272/TheQueen%E2%80%99sUniversityBelfast_LaboratoryprotocolsusedforAMSradiocarbondatingatthe14CHRONOCentre (2015).
Longin, R. New method of collagen extraction for radiocarbon dating. Nature 230, 241–242 (1971).
Ambrose, S. H. & DeNiro, M. J. The isotopic ecology of East African mammals. Oecologia 69, 395–406 (1986).
van Klinken, G. J. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 26, 687–695 (1999).
Alexander Bentley, R. Strontium isotopes from the earth to the archaeological skeleton: A review. J. Archaeol. Method Theory 13, 135–187 (2006).
Frei, K. M. & Price, T. D. Strontium isotopes and human mobility in prehistoric Denmark. Archaeol. Anthropol. Sci. 4, 103–114 (2012).
Holt, E., Evans, J. A. & Madgwick, R. Strontium (87Sr/86Sr) mapping: a critical review of methods and approaches. Earth Sci. Rev. 216, 103593 (2021).
Price, T. D., Burton, J. H. & Bentley, R. A. Characterization of biologically available strontium isotope ratios for the study of prehistoric migration. Archaeometry 44, 117–135 (2002).
Thomsen, E., Andreasen, R. & Rasmussen, T. L. Homogeneous glacial landscapes can have high local variability of strontium isotope signatures: implications for prehistoric migration studies. Front. Ecol. Evol. 8, 588318 (2021).
Price, T. D., Klassen, L. & Sjögren, K. G. Pitted ware culture: isotopic evidence for contact between Sweden and Denmark across the Kattegat in the Middle Neolithic, ca. 3000 bc. J. Anthropol. Archaeol. 61, 101254 (2021).
Hede, M. U. Holocene Climate and Environmental Changes Recorded in High-resolution Lake Sediments from Højby Sø, Denmark. PhD thesis, Univ. Copenhagen (2008).
Sugita, S. Theory of quantitative reconstruction of vegetation I: pollen from large sites REVEALS regional vegetation composition. Holocene 17, 229–241 (2007).
Sugita, S. Theory of quantitative reconstruction of vegetation II: all you need is LOVE. Holocene 17, 243–257 (2007).
Nielsen, A. B. et al. Quantitative reconstructions of changes in regional openness in north-central Europe reveal new insights into old questions. Q. Sci. Rev. 47, 131–147 (2012).
Githumbi, E. et al. Pollen-based maps of past regional vegetation cover in Europe over twelve millennia—evaluation and potential. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2022.795794 (2022).
Nielsen, A. B. & Odgaard, B. V. Quantitative landscape dynamics in Denmark through the last three millennia based on the landscape reconstruction algorithm approach. Veg. Hist. Archaeobot. 19, 375–387 (2010).
Søe, N. E., Odgaard, B. V., Nielsen, A. B., Olsen, J. & Kristiansen, S. M. Late Holocene landscape development around a Roman Iron Age mass grave, Alken Enge, Denmark. Veg. Hist. Archaeobot. 26, 277–292 (2017).
Mazier, F. et al. Testing the effect of site selection and parameter setting on REVEALS-model estimates of plant abundance using the Czech Quaternary Palynological Database. Rev. Palaeobot. Palynol. 187, 38–49 (2012).
Acknowledgements
The Lundbeck Foundation GeoGenetics Centre is supported by grants from the Lundbeck Foundation (R302-2018-2155, R155-2013-16338), the Novo Nordisk Foundation (NNF18SA0035006), the Wellcome Trust (WT214300), Carlsberg Foundation (CF18-0024), the Danish National Research Foundation (DNRF94, DNRF174), the University of Copenhagen (KU2016 programme) and Ferring Pharmaceuticals A/S, to E.W. This research has been conducted using the UK Biobank Resource and the iPSYCH Initiative, funded by the Lundbeck Foundation (R102-A9118 and R155-2014-1724). This work was further supported by the Swedish Foundation for Humanities and Social Sciences grant (Riksbankens Jubileumsfond M16-0455:1) to K.K. M.E.A. was supported by Marie Skłodowska-Curie Actions of the EU (grant no. 300554), The Villum Foundation (grant no. 10120) and Independent Research Fund Denmark (grant no. 7027-00147B). A.F. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany´s Excellence Strategy–EXC 2150–390870439. R. Macleod was supported by an SSHRC doctoral studentship grant (G101449: ‘Individual Life Histories in Long-Term Cultural Change’). B.S.P. has received funding from the European Union’s Horizon 2020 research and innovation programme under the ERC-StG grant agreement No 94924. M.N. is funded by the Human Frontier Science Program Postdoctoral Fellowship LT000143/2019-L4. A.R.-M. was supported by the Lundbeck Foundation (grant R302-2018-2155) and the Novo Nordisk Foundation (grant NNF18SA0035006); E.K.I.-P. was supported by the Lundbeck Foundation (grant R302-2018-2155) and the Novo Nordisk Foundation (grant NNF18SA0035006). W.B. is supported by the Hanne and Torkel Weis-Fogh Fund (Department of Zoology, University of Cambridge). A.P. is funded by Wellcome grant WT214300. B.S.d.M. and O.D. are supported by the Swiss National Science Foundation (SFNS PP00P3_176977) and European Research Council (ERC 679330). G.R. is supported by a Novo Nordisk Foundation Fellowship (gNNF20OC0062491). N.N.J. is supported by Aarhus University Research Foundation. A.J.S. is supported by a Lundbeckfonden Fellowship (R335-2019-2318) and the National Institute on Aging (NIH award numbers U19AG023122, U24AG051129 and UH2AG064706). R. Maring was funded by the Aarhus University Research Foundation through a grant awarded to M. A. Mannino for the project titled Danish and European Diets in Time (AUFF-E-2015-FLS-8-2). S.R. was funded by the Novo Nordisk Foundation (NNF14CC0001). T.S.K. is funded by Carlsberg grant CF19-0712. R.D. is funded by the Wellcome Trust (WT214300). R.N. is funded by the National Institute of General Medical Sciences (NIH grant R01GM138634). T.W. is supported by the Lundbeck Foundation iPSYCH initiative (R248-2017-2003). We are indebted to P. Bennike for her contribution during the formative years of the project until shortly before her death in 2017. We acknowledge staff at the National Museum, the Anthropological Laboratory, and the regional museums in Denmark, responsible for collecting, recording and curating prehistoric skeletal remains studied herein. The process of identifying and sampling suitable archaeological remains for the current study was dependent on numerous specialists in Danish archaeology including researchers, museum employees and citizen scientists. We thank the following in this regard (alphabetically ordered): A. H. Andersen, S. Bergerbrant, K. Christensen, K. M. Gregersen, V. Grimes, E. Johansen, O. T. Kastholm, T. Lotz, E. Lundberg, M. Mannino, J. Olsen, K. Rosenlund and H. H. Sørensen. We also acknowledge those who have assisted in the sampling process or the gathering of provenance data on prehistoric human remains which were not analysed here owing to insufficient DNA preservation. These include H. Dahl (Tybrind Vig), I. B. Enghoff (Østenkær), A. B. Gurlev (Vedbæk Havn), L. Holten (Aldersro), O. Lass (Hesselbjerg/Ferle Enge and Nivå), L. Matthes (Knudsgrund/Knudshoved) and K. Randsborg (deceased) (Nivå). We are grateful for contributions from F. Racimo, and express our gratitude to the many researchers who have supported laboratory work, analytical procedures and evaluation of results that are presented here, including M. Mannino, P. Reimer and M. Thompson. E.W. thanks St. John’s College, Cambridge, for providing a stimulating environment of discussion and learning.
Author information
Authors and Affiliations
Contributions
M.E.A., M.S. and A.F. contributed equally to this work. E.W. initiated the study. M.E.A., M.S., A.F., T.W., K.K. and E.W. led the study. M.E.A., M.S., A.F., M.M, R.N., T.W., K.K. and E.W. conceptualized the study. M.E.A., M.S., T.S.K., R.D., R.N., O.D., T.W., K.K. and E.W. supervised the research. M.E.A., R.D., R.N., T.W., K.K. and E.W. acquired funding for research. A.F., M.E.A., J.S., K.-G.S., M.L.S.J., T.Z.T.J., M.U.H., B.H.N, E.K., J.H., K.B.P., L.P., L.K., P. Lotz., P. Lysdahl, P.B., P.V.P., R. Maring, S.W., S.A.S, S.H.A, T.J. and N.L. were involved in sample collection. M.E.A., M.S., A.I., J.S., A.P., B.S.d.M., L.V., A.S., D.J.L., T.S.K., R.D., R.N., O.D., K.K. and E.W. were involved in developing and applying methodology. M.E.A., J.S. and L.V. led the DNA laboratory work research component. K.-G.S. led bioarchaeological data curation. M.E.A., M.S., A.R.-M., E.K.I.-P., W.B., A.I., A.P., B.S.d.M., B.S.P., R.A.H., T.V., H.M., A.V., A.B.N., P.R., G.R., A.D.R., A.J.S., A. Rosengren, R. Maring, S.R., T.S.K. and O.D. undertook formal analyses of data. M.E.A., M.S., A.F., K.-G.S., A.I., R. Macleod, A. Rosengren, B.S.P., M.F.M., A.B.N., M.U.H., N.N.J., L.P., N.L., T.W., K.K. and E.W. drafted the main text (M.E.A., M.S. and A.F. led this). M.E.A., M.S., A.F., K.-G.S., A.I., R. Macleod., A. Rosengren, B.S.P., M.L.S.J., M.N., J.S., T.D.P., M.F.M., A.B.N., M.U.H., L.S., P.O.N., P.R., A.R.-M, E.K.I.-P., W.B., A.P., B.S.d.M., F.D., R.A.H., T.V., H.M., A.V., L.V., A.S., A.J.S., A. Ruter, A.B.G., B.H.N., E.B.P., E.K., J.H., K.B.P., L.P., L.K., M.J., O.C.U., P.L., P.B., P.V.P., R. Maring, R.I., S.W., S.A.S., T.J., N.L., D.J.L., S.R., T.S.K., K.H.K., R.D., R.N., O.D., T.W. and K.K. drafted supplementary notes and materials. All authors read, commented on, and agreed upon the submitted manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Patricia Fall, Birgitte Skar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Model-based clustering.
Unsupervised model-based clustering results (ADMIXTURE) for K = 2 to K = 15 assumed components for all published shotgun-sequenced ancient individuals from Denmark - including the herein presented 93 genomes (contamination <5% and close relative pairs excluded). Imputed genomes were used where available3. For low-coverage individuals (indicated with black cross) pseudo-haploid genotypes were used.
Extended Data Fig. 2 Allele sharing of Danish Mesolithic individuals.
a, D-statistic testing whether Danish Mesolithic individuals form a clade with the earliest Danish Mesolithic individual in the dataset (NEO254, Koelbjerg Man) to the exclusion of a genetic cluster of Mesolithic hunter-gatherer individuals from Sweden (Sweden_10000BP_7500BP). b, D-statistic testing whether Danish Mesolithic individuals form a clade with a genetic cluster of Western European HG individuals (EuropeW_13500BP_8000BP) to the exclusion of a genetic cluster of Eastern European HG individuals (RussiaNW_11000BP_8000BP). Error bars indicate three standard errors.
Extended Data Fig. 3 IBD sharing among ancient individuals from Denmark.
Heatmap showing pairwise amount of total length of IBD shared between 72 ancient Danish individuals dated to older than 3,000 cal. BP. Colours in border and text indicate genetic cluster membership, and dendrograms show clustering hierarchy.
Extended Data Fig. 4 Genetic affinities of ancient individuals from Denmark.
Panels show principal component analyses based on pairwise IBD-sharing of a, 30 imputed Danish Mesolithic individuals in context of 105 European HGs (right panel shows Danish individuals coloured by age); b, 22 imputed Danish early Neolithic individuals within the context of 170 Anatolian and European Neolithic farmers c, 21 imputed Danish LNBA individuals within the context of 127 European LNBA individuals. Symbol colour and shape indicate the genetic cluster of an individual (Supplementary Data III). The extent of PCA positions of individuals from Denmark are indicated with a dotted line hull. Ancestry cluster categories defined in3.
Extended Data Fig. 5 Dietary isotopic signatures.
δ13C and δ15N values in bone/dentine samples from 100 ancient Danish individuals, coloured according to their main genetic ancestry group. A fundamental dietary and genetic shift is observed at the transition from the Mesolithic to the Neolithic c. 5,900 cal. BP (dashed line). Four anomalous individuals are highlighted. Data from3 and Supplementary Data II.
Extended Data Fig. 6 Ancestry modelling of ancient individuals.
a, Heatmap of ancestry proportions for 72 ancient individuals from Denmark dated to older than 3,000 cal. BP estimated from supervised mixture models. Results for three different sets of ancestry source groups (deep, fEur, postNeol, Supplementary Data IV) are distinguished in facet rows. Genetic cluster membership for Danish target individuals is indicated by column facets. b, Spatial distribution of estimated ancestry proportions of three different HG sources for Neolithic farmer individuals from Scandinavia and Poland. c, Spatial distribution of estimated ancestry proportions of two different farmer sources for LNBA individuals from Scandinavia and Poland. d, Ancestry proportions for Scandinavian Iron Age and Viking Age individuals (postBA reference set). e, Ancestry proportions for selected ancient European individuals with ancestry related to Scandinavian LNBA individuals (source Scandinavia_4000BP_3000BP, postBA reference set, Supplementary Data IV).
Extended Data Fig. 7 Contrasting hunter-gatherer admixture dynamics in Neolithic farmer individuals from Denmark and Sweden.
a, Admixture time estimated using DATES44 as a function of age for Neolithic farmer individuals from Denmark (left) and Sweden (right). Pie charts indicate ancestry composition (light grey - farmer ancestry; dark grey - non-local hunter-gatherer ancestry; colour - local hunter-gatherer ancestry). b, Total amount of hunter-gatherer ancestry proportion as a function of admixture time for Neolithic farmer individuals from Denmark (left) and Sweden (right). Error bars indicate ± 1 standard error of admixture time estimate.
Extended Data Fig. 8 Fine-scale structure in late Neolithic and Early Bronze Age (LNBA) Scandinavians c. 4,500-3,000 cal. BP.
a–e, Geographic locations and PCA based on pairwise IBD sharing (middle) of 148 European LNBA individuals predating 3,000 cal. BP (Supplementary Data IV). Geographic locations are shown for 65 individuals belonging to the five genetic clusters observed in 38 ancient Scandinavians (a,b, LNBA phase I; c,d, LNBA phase II; e, LNBA phase III; temporal sequence shown in timeline in centre of plot). Individual assignments and frequency distribution of major Y chromosome haplogroups are indicated in maps and timeline. Plot symbols with black circles indicate the 38 Scandinavian individuals in the PCA panels. Ancestry proportions for the 38 Scandinavian individuals estimated using proximal source groups from outside Scandinavia (postNeolScand source set) are shown on the right of the respective cluster results.
Supplementary information
Supplementary Information
Supplementary Notes 1–6: 1, Overview of Danish Samples (including Figs S1.1 to S1.3); 2, Polygenic prediction of height, eye colour and hair colour (including Table S2.1); 3, Bayesian Chronological models of the transition (including Figs S3.1 to S3.6); 4, Dietary variation in Mesolithic, Neolithic and Bronze Age Denmark (including Figs S4.1 to S4.2); 5, Strontium Analysis of Danish Samples (including Figs S5.1 to S5.3, and Table S5.1); and 6, Vegetation and landscape in Post-Glacia Denmark – illustrated using a high-resolution land cover reconstruction (LOVE) from Lake Højby, Northwest Zealand (including Figs S6.1 to S6.2).
Supplementary Data 1
Basic overview of samples and genetic data.
Supplementary Data 2
Isotopic data from 100 Danish samples.
Supplementary Data 3
Isotopic data from 100 Danish samples: a, Metadata for ancient genomes from Denmark used in this study; b, Metadata for selected contextual ancient genomes from Western Eurasia.
Supplementary Data 4
Ancestry proportions for sample sets: a, Ancestry proportions for set “deep”; b, Ancestry proportions for set “fEur”; c, Ancestry proportions for set “postNeol”; d, Ancestry proportions for set “postBA”; e, Ancestry proportions for set “postNeolScand”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allentoft, M.E., Sikora, M., Fischer, A. et al. 100 ancient genomes show repeated population turnovers in Neolithic Denmark. Nature 625, 329–337 (2024). https://doi.org/10.1038/s41586-023-06862-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06862-3
This article is cited by
-
Ancient migration and the modern genome
Nature Reviews Genetics (2024)
-
Prehistoric events might explain European multiple sclerosis risk
Nature (2024)
-
Ancient DNA reveals origins of multiple sclerosis in Europe
Nature (2024)
-
Population genomics of post-glacial western Eurasia
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.