Abstract
The origin of vertebrate paired appendages is one of the most investigated and debated examples of evolutionary novelty1,2,3,4,5,6,7. Paired appendages are widely considered as key innovations that enabled new opportunities for controlled swimming and gill ventilation and were prerequisites for the eventual transition from water to land. The past 150 years of debate8,9,10 has been shaped by two contentious theories4,5: the ventrolateral fin-fold hypothesis9,10 and the archipterygium hypothesis8. The latter proposes that fins and girdles evolved from an ancestral gill arch. Although studies in animal development have revived interest in this idea11,12,13, it is apparently unsupported by fossil evidence. Here we present palaeontological support for a pharyngeal basis for the vertebrate shoulder girdle. We use computed tomography scanning to reveal details of the braincase of Kolymaspis sibirica14, an Early Devonian placoderm fish from Siberia, that suggests a pharyngeal component of the shoulder. We combine these findings with refreshed comparative anatomy of placoderms and jawless outgroups to place the origin of the shoulder girdle on the sixth branchial arch. These findings provide a novel framework for understanding the origin of the pectoral girdle. Our evidence clarifies the location of the presumptive head–trunk interface in jawless fishes and explains the constraint on branchial arch number in gnathostomes15. The results revive a key aspect of the archipterygium hypothesis and help reconcile it with the ventrolateral fin-fold model.
Similar content being viewed by others
Main
The two major theories of the origin of vertebrate appendages differ in their ability to explain evolutionary patterns. The ventrolateral fin-fold hypothesis proposes that paired fins arose from ventrolateral keels extending the length of the trunk, which became subdivided into pectoral and pelvic fins. The archipterygium hypothesis argues that the girdles derived from an ancestral skeletal gill arch and that the fin endoskeleton formed from gill rays. The fin-fold hypothesis is seen as the more ‘successful’ of the two theories4,16, with support from developmental genetics17 and widespread evidence of stem-group gnathostomes possessing ventrolateral fin folds in some form5,7. However, the fin-fold hypothesis does not explain the origin of the pectoral girdle, which resulted in the subdivision of the head into a separate skull and shoulder. Furthermore, it predicts the simultaneous origin of pectoral and pelvic fins, which is currently contradicted by fossil data5,7. The archipterygium hypothesis explains the pectoral girdle and separate origins of pectoral and pelvic fins by basing their origins on pre-existing structures. Clues from developmental genetics11,12,13 have renewed interest in the archipterygium hypothesis as a viable theory.
A key challenge to testing the archipterygium hypothesis with evidence from the fossil record is the rarity of fossilized gill arches. Gill arches are cartilage-derived endoskeletal structures that were either unossified or weakly ossified and are therefore not preserved in the earliest fossil taxa. The closest fossil sister group of jawed vertebrates is the Osteostraci, which ranges from the Wenlock epoch of the Silurian period to the Late Devonian period (approximately 432 to 378 million years ago (Ma)). Osteostracans possessed distinct pectoral fins, but these were attached to a unified craniothoracic block of cartilage that was surmounted by tessellated dermal bone. Fossilized pharyngeal arches are completely unknown in osteostracans, obscuring reconstructions of pharyngeal conditions18 preceding the origins of jaws and a pectoral girdle. The phylogenetically earliest jawed vertebrates are the placoderms, heavily armoured predatory fishes and contemporaries of osteostracans. Placoderm gill arches are rarely preserved and incompletely understood19,20. However, a crucial piece of evidence has long been overlooked. Despite the rarity of the arches themselves, their attachments are well-preserved as discrete facets near the perimeter of well-ossified braincases of both osteostracans and placoderms18. In osteostracans, however, the articulation facets are quite remote from the core of the braincase, situated near the perimeter of a broad cephalic shield that defines an enlarged oralobranchial chamber (see below). Alongside these are well-established anatomical landmarks in the form of cranial innervation and blood supply patterns, recorded as grooves or ossified canals within these braincases and consistent across vertebrates. This record of both hard and inferred soft-tissue anatomy provides a framework for investigating the role of the pharynx in the skeletal and bodyplan transformations leading to the origin of paired pectoral fins and a distinct pectoral girdle.
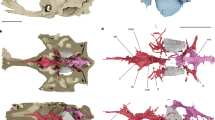
The type and only specimen of K. sibirica (FN Chernyshev Central Research Geological Museum in St Petersburg, Russia, TsNIGR 7656) is a three-dimensionally preserved skull roof and braincase (Fig. 1). The skull of Kolymaspis is anteroposteriorly elongate, with a pronounced premedian ‘snout’ (upper lip, sensu ref. 21) and large, dorsolaterally directed orbits. The dermal skull roof is nearly complete, with the separately ossified rostropineal plate and rhinocapsular ossification in articulation (Fig. 1). The dermal skull roof is coated in stellate tubercles, consistent with ‘acanthothoracid’ placoderms22,23,24. In ventral view, the braincase is broad and deeply concave; the parachordal region is laterally demarcated by raised longitudinal crests (laterobasal angles; Fig. 1). The parachordal plates here terminate posteriorly without forming a marked occipital process; the occipital glenoid facets (attachments to the spinal column) were flush with the posterior margin of the braincase, in a condition similar to Brindabellaspis25,26. They are wide, dorsoventrally flat, openings flanking the notochordal canal. Posteriorly, the braincase flares laterally into stout craniospinal processes (Fig. 1). This process is most complete on the left (observer right) side. The distal part of the craniospinal process is an open, rimmed facet (Fig. 1), indicating it was the articulation point for a second cartilage. This corresponds with Brindabellaspis, which also has a terminal facet on the craniospinal process25 (Extended Data Fig. 1). However, this feature is unknown in any other placoderms, in which the facet is either absent and the craniospinal process is wholly covered in perichondral bone (as in Romundina; Fig. 2c), or capped in dermal bone as in arthrodires. In posterior view, the occipital surface also resembles Brindabellaspis in being broad, centrally concave and lacking identifiable cavities for paired epaxial musculature (muscles raising the skull). The foramen magnum is nearly twice the diameter of the notochordal canal, consistent with stem-group gnathostome conditions, and the two are contiguous openings positioned near the ventral margin of the braincase (Fig. 1).
a, Dorsal view. b, Ventral view. c, Interpretive illustration of ventral view. d, Left lateral view. e, Posterior view. a.ic, foramen for internal carotid artery; art.crs, articular facet on end of craniospinal process; art.fac, articular facets for branchial arches; crs.p, craniospinal process; cu.fo, cucullaris muscle fossa; eyst, eystalk attachment; fo.mag, foramen magnum; gle.fo, fossa for occipital glenoid facets; hyp.fo, hypophyseal fossa; lba, laterobasal angle; N.II, optic tract canal; na, naris; not.c, notochordal canal; o.dend, endolymphatic duct opening; o.pin, pineal opening; orb.l, left orbit; orb.r, right orbit; Prm, premedian plate; rhi.fi, rhinocapsular fissure. Dark beige material is dermal (exoskeletal) bone and light beige material is perichondral (endoskeletal) bone.
a, Osteostracan Nectaspis (composite based on ref. 47). b, Acanthothoracid placoderm Kolymaspis. c, Acanthothoracid placoderm Romundina (original based on data from ref. 48 and new data). Transparent blue structures represent reconstructed branchial arches. art.ba1–6, serially numbered branchial arch attachments (corresponds to art.fac in Fig. 1); art.hyo, hyoid arch articulation; a.subcl, canal for subclavian artery; crt.j, craniothoracic joint; f.pect, pectoral fin; N.VII, facial nerve canal; N.IX, glossopharyngeal canal; N.X1–4, vagus nerve canal branches (numbered 1–4); shld.grd, shoulder girdle. Not to scale.
These observations of the Kolymaspis braincase and comparisons to other taxa enable us to identify the ancestral position of head–shoulder separation in jawless fishes and propose specific musculoskeletal transformations in the origin of the gnathostome pectoral girdle. The placoderm craniospinal processes articulate with the pectoral girdle (shld.grd; Fig. 2). The open articular facet on the craniospinal process of Kolymaspis and Brindabellaspis (hereafter referred to collectively as brindabellaspidids25) points to an endoskeletal element here, forming a junction with the pectoral girdle. This is notable because this endoskeletal element would lie in series with the pharyngeal arches (Fig. 2) and a key anatomical landmark of the head–shoulder boundary in gnathostomes: the cucullaris muscle, responsible for depressing the skull towards the shoulder girdle27,28,29. There is a wealth of anatomical and developmental evidence that the cucullaris muscle is of branchial origin16,30,31,32. This gives rise to the prediction that it may have ancestrally joined a branchial arch. We propose that this endoskeletal element is a serial homologue of an upper branchial element (epibranchial or pharyngobranchial, given its topological position) and therefore that the shoulder girdle of these taxa incorporated the dorsal element of a gill arch. Although placoderm braincases possess only two clear articular facets for branchial arches, rare skeletal material shows that they possess at least five skeletal arches (the posteriormost arch may be specialized, as in some chondrichthyans)20. No placoderms are known to possess more than this number of arches. This anatomical interpretation implies that the sixth branchial arch would most probably have been the one incorporated into the pectoral girdle, if our interpretation is correct.
The sixth branchial arch is key in comparisons with jawless outgroups and enables independent support of our topological observations. Osteostracans differ from all known jawed vertebrates in the absence of a distinct head–shoulder separation, which is generally regarded as the ancestral gnathostome condition27,28. There are also no obvious points of homology that mark this separation in osteostracans. However, our hypothesis locates this presumptive division at the level of the sixth branchial arch. Notably, osteostracans frequently preserve a canal for the subclavian artery, the main arterial branch that supplies the pectoral fins. This artery stems from a cluster of arteries supplying the most posterior efferent branchial arteries serving the posterior pharyngeal arches18,33,34. The main trunk of the subclavian artery is seen in several specimens, showing that it extends along the interbranchial ridge of the sixth and seventh branchial arches33,34 (Fig. 2a and Extended Data Fig. 2). Locating the shoulder on the sixth branchial arch also provides a precise explanation for a puzzling phenomenon in which most gnathostomes appear to be constrained to no more than five gill arches (hexanchiform sharks notwithstanding—these appear to involve duplication of an intermediate arch35), whereas jawless fishes range from five to several dozen separate gill compartments15. If the ancestral pectoral girdle incorporated the sixth branchial arch, this would strongly bias the standard complement of jawed vertebrate arches to no more than five.
These observations in a phylogenetic context (Fig. 3 and Extended Data Figs. 3 and 4) enable us to propose a new hypothesis for the origin of the pectoral girdle. We propose that the pectoral girdle is established on the position of the sixth branchial arch in the jawless ancestor of jawed vertebrates, and that this structure formed the primary basis of a separate head and shoulder. The initial incorporation of the gill arch provided support for the rear wall of the pharynx, joined to the skull by a kinetic, moveable linkage (Fig. 3). This link persisted in placoderms as a craniothoracic joint, and in some taxa (such as the brindabellaspidids) a vestige of the endoskeletal component remained (Fig. 3). In modern gnathostomes, the endoskeletal elements of this sixth branchial arch are completely lost (see next paragraph on origin of scapulocoracoid). However, exoskeletal (dermal) components of the pectoral girdle (for example, cleithrum and clavicle) may have their origins from branchiomeric dermal plates covering this arch (that is, from a branchial operculum). Evidence from Romundina indicates that some placoderms possessed dermal branchial coverings posterior to the submarginal plate, which is the main opercular bone in placoderms (Extended Data Fig. 5). A similar condition is possible in the enigmatic new taxon Xiushanosteus from the early Silurian of China36. If one reinterprets the larger, more posterior post-suborbital plate in Xiushanosteus as a submarginal, then the smaller plate originally identified as a submarginal becomes a posterior submarginal similar to Romundina.
Hypothetical intermediate is shown, for clarity of comparative anatomy; specific geometries may have varied substantially. Gill arch morphologies in osteostracan and placoderms are hypothetical and are shown to indicate location of articulations and constraints on overall pharynx architecture. Blue, branchial arches; orange, sixth branchial or thoracic arch; pink, pectoral fin attachment or scapulocoracoid. See Supplementary Information for complete phylogeny. Dashed lines indicate inferred pectoral girdle. Osteostracan is a composite based on ref. 47; Romundina is based on ref. 49 and new data; Eusthenopteron is a composite based on ref. 50.
We can tentatively suggest new points of homology between the heads of osteostracans and jawed vertebrates and suggest specific skeletal transformations that occurred during the origin of the pectoral girdle. First, the postbranchial lamina (rear wall of the gill chamber) is a putative homologue of a plate of branchial association (Fig. 3); this is consistent with its position and demonstrated ability to support development of tooth-like denticles37, suggesting that it at least partly derives from cranial neural crest28. The formation of a postbranchial lamina occurred as the gill openings changed from pore-like openings of jawless fishes into deep-sided clefts of jawed taxa. This was concomitant with changes to the structure of the braincase, in which the broad lateral brim was withdrawn medially, exposing the expanded clefts laterally. The sixth arch lost respiratory tissue (gills) and became the basis of a craniothoracic joint supporting feeding or buccal pumping. We do not necessarily invoke the gill arches as the anatomical precursor of the pectoral fin skeleton or the scapulocoracoid, as predicted by the archipterygium hypothesis. Recent fate-mapping studies in skate (Chondrichthyes) show that the scapulocoracoid is composed of trunk mesoderm13 (compared to a zone of mixed cranial neural crest and trunk mesoderm in the gill arches). Additionally, a pectoral fin and proximal attachment was already present and anatomically separate from the gill arches in the osteostracans. Gill arches and pectoral elements thus fail the conjunction test of homology. Nevertheless, the close anatomical proximity of these structures would have allowed them to join a common dermal support (Fig. 3).
Our hypothesis partly revives the archipterygium hypothesis, but not as originally envisioned by Gegenbaur8. There is no known fossil evidence of a direct skeletal remnant of the ancestral gill arch in crown-group gnathostomes, only traces in the form of patterns of vascularization and musculature inherited from jawless ancestors28. Notably, the last direct vestiges of a pharyngeal arch in the shoulder girdle would have been lost in placoderms (Fig. 3), with evidence seen only in the enigmatic brindabellaspidids as described above. There is no requirement in our hypothesis, however, for either the scapulocoracoid or the pectoral fin endoskeleton to be of pharyngeal origin, as in Gegenbaur’s archipterygium. A separate head–girdle instead evolved as part of changes in the architecture of the pharynx, rather than primarily to support fins. Our work arrives independently at a previous suggestion that the pectoral girdle and fin are a morphological amalgam of cranial and thoracic regions of the body16. Fossil evidence for this has previously been suggested by Zangerl38. However, as with Gegenbaur’s original theory, Zangerl’s idea relied heavily on chondrichthyan anatomy38, referencing symmoriids and iniopterygians. These taxa are increasingly demonstrated as highly nested within chondrichthyans and well removed from the origin of gnathostomes39,40, casting doubt on their value as models for ancestral jawed vertebrates. Thus, elements of both the archipterygium and the fin-fold hypotheses are combined to explain the origin of pectoral appendages, the shoulder and a distinct gnathostome head as a total system. All these conclusions could potentially be tested by fate-mapping studies in modern osteichthyans (as these taxa retain the dermal pectoral girdle) as well as through new fossil finds of early gnathostomes.
This interpretation adds important functional details to the tight phylogenetic connection between the origin of a pectoral girdle and the origin of jaws. The craniospinal process is one of the pivot points in the four-bar linkage that makes up the placoderm jaw-closing apparatus41. This suggests that as a sixth branchial arch became established as the rearmost support and a kinetic joint, tying the origin of the pectoral girdle to a suite of changes to the pharynx involved in opening and closing the mouth and throat. Recent evidence suggests a compact, operculate pharynx as the ancestral condition for gnathostomes42, rather than the historically accepted shark-like septate model. Thus, it is reasonable to conclude that the origin of the pectoral girdle is integrated with the evolution of a compact bucco-pharyngeal apparatus for efficient gill ventilation or feeding.
Our hypothesis is testable on several lines of evidence that could eventually overturn it. We discuss these along with existing points of weakness. First, it depends on the resolution of either Kolymaspis or Brindabellaspis as the sister group taxa of all other jawed vertebrates, and thus rests on the hypothesis of placoderm paraphyly. This is currently the case in our phylogeny (Extended Data Fig. 6). However, statistical support for placoderm paraphyly is weak (see bootstrap values in Extended Data Fig. 3) and highly debated43,44. Under placoderm monophyly, our hypothesis depends at least on the phylogenetic mapping of the craniothoracic facet to the base of all jawed vertebrates. We conducted additional analyses with constraints on placoderm monophyly leading to equivocal support for our new hypothesis (Extended Data Fig. 6 and Supplementary Information). Thus, new phylogenetic tests could reveal that the condition in the brindabellaspidids is uniquely derived (that is, neomorphic). The discovery of new fossils with both supernumerary branchial arches and a discrete pectoral girdle would also challenge our hypothesis. Furthermore, an alternative interpretation of the articular facet in brindabellaspidids is that it represents a connection to a shoulder cartilage not of pharyngeal origin. In our view, these explanations are less parsimonious and do not help account for the branchiomeric derivation of the cucullaris muscle, but they could be supported by future fossil discoveries or phylogenetic analyses. Even if those specifics are rejected, our hypothesis adds important new comparative anatomical perspectives that better reconcile the disparate anatomies of osteostracans and placoderms.
Our proposal synthesizes findings from the past two decades of research into the origin of the pectoral girdle. Furthermore, it clarifies key questions of comparative anatomy that have impeded studies on the origin of the vertebrate neck and shoulders. Key among these is resolution of the identity and location of the cucullaris muscle in osteostracans, a crucial anatomical landmark in establishing the head–shoulder interface27,28,29,45. Previous studies have struggled to identify the location of the cucullaris in osteostracans, concluding that it was absent27 or placing it in an epaxial location46. We argue that it was an undifferentiated branchial levator or protractor muscle and would have been housed in the perimeter of the oralobranchial chamber. This morphology is topologically consistent with placoderm braincases which show that the cucullaris muscle is serially aligned with the branchial levator muscles. Our investigation suggests it derived from the sixth branchial levator, consistent with the predictions of recent comparative developmental studies29. Despite the loss of posterior endoskeletal branchial arches in gnathostomes, a branchiomeric muscle of the sixth branchial arch (as the cucullaris muscle) maintained a consistent topological relationship with the dermal exoskeleton (Fig. 3). This new model of musculoskeletal transformation in pectoral girdle origins thus unifies a wide array of evidence on the origin of the pectoral girdle. It adds important new details to the biomechanical basis for the origin of the girdle and clarifies the comparative anatomy of key jawless and jawed fishes. This new framework is consistent with recent proposals of a dual origin of the pectoral girdle16 and thus contributes to the reconciliation of two long-debated theories of paired fin origins.
Methods
Additional specimen
We analysed an articulated specimen of Romundina from the Geowissenschaftliches Zentrum der Universität Göttingen, Museum & Collection (GZG) specimen 100–488A.
Computed tomography scanning
We used x-ray computed microtomography to scan specimens of Kolymaspis and Romundina. We scanned the Kolymaspis specimen TsNIGR 7656 at the Natural History Museum (Imaging and Analysis Centre), UK using the Nikon Metrology HMX ST 225 system, 210 kV, 150 mA, 2.5 mm copper filter, and resulting voxel size of 70 µm. The Romundina specimen was scanned at the Cambridge University Museum of Zoology, using the Nikon Metrology HMX ST 225 system, 185 kV, 245 mA, 0.75 mm copper filter, and resulting voxel size of 43 µm.
Osteostracan tomography
To explore osteostracan vascularization patterns, we created a tomographic image series from the digitized publication of Stensiö (1927)33. We used Series F of Mimetaspis hoeli in plates 106–112, cropping each slice using Adobe Photoshop and exporting each to a separate bitmap file. We then loaded the series into SPIERS Align51 and conducted a manual registration.
Segmentation and surface model visualzation
We performed segmentation of the tomographic datasets using Materialise Mimics (https://www.materialise.com). We segmented Kolymaspis primarily using Mimics v. 18; we finalized and cleaned the masks using Mimics v. 24. We segmented Mimetaspis series F, Romundina specimen GZG 100–488A, and existing data of Brindabellaspis (Extended Data Fig. 1) using Mimics v. 25. We used cycles rendering in Blender (Blender Foundation, https://www.blender.org) to generate surface model images for publication-ready figures.
Phylogenetic data
We used the dataset of King et al. 43 as the basis of our phylogenetic analysis, edited in Mesquite v 3.7052. It contains fully annotated character names and state labels. The removal of citation histories complicates dataset comparison and incorporation of new characters and data. Subsequent datasets have extinguished the records for many characters, resulting in incomplete character ontologies. This makes it nearly impossible for subsequent investigators to expand based on existing characters or to understand the original authors’ intentions. We generated a change log using a newly developed command line tool diffmatrix (https://github.com/mbrazeau/diffmatrix/releases/tag/v2.2).
To preserve character histories and ontologies, we did not permanently delete any taxa or characters from the matrix. Rather than deleting characters or taxa we considered problematic, we preserved the overall integrity of the matrix by using exclusion settings before phylogenetic analysis. Instead of attaching the phylogenetic data file as separate character list and matrix, we have stored it as a single Nexus file in a version-control archive (GitHub) as a way of improving maintainability (https://mbrazeau.github.io/gnathostome_characters). This also includes a link to a character list web page with character descriptions and citations. Changes to the matrix are detailed in the change log at https://mbrazeau.github.io/gnathostome_characters/changelog.html. A permanent version of the final dataset used in this study is archived at https://github.com/mbrazeau/gnathostome_characters/releases/tag/1.0 and a copy of the Nexus file is provided in the Supplementary Information.
Phylogenetic analysis
We conducted a phylogenetic search using TNT (v. 1.6)53. We constrained the outgroup so that osteostracans and jawed vertebrates were each monophyletic. Because TNT constraints cannot conflict with outgroup choice, we later rerooted the trees so that Galeaspida were also monophyletic. Because all character types are symmetric this has no impact on the results. We set the tree buffer to 10,000 trees (hold 10000;) for unconstrained searches and up to 50,000 for searches under different constraints for placoderm monophyly. We conducted a ‘new technology search’ with the following command and settings to apply 50 iterations on the parsimony ratchet: xmult=level 10 ratchet 50; To further explore the resulting islands, we used additional branch-breaking (bbreak=fillonly;) to swap the trees in memory. We then computed the strict consensus tree using the nelsen command. We conducted two additional searches constraining placoderms to be monophyletic. The first constrained as monophyletic all placoderms inclusive of Entelognathus and Minjinia, while the second constrained only the ‘core’ placoderms54 (excluding Entelognathus and Minjinia from the placoderm constraint).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Scan data and relevant surface meshes of Kolymaspis and Romundina are provided on FigShare (https://doi.org/10.6084/m9.figshare.22579840). The character list is stored in the original Nexus file, including character descriptions and references. Readers can access the file at https://mbrazeau.github.io/gnathostome_characters/ or in the Supplementary Information. Changes to the matrix are detailed in the change log at https://mbrazeau.github.io/gnathostome_characters/changelog.html.
Code availability
All TNT commands and scripts used in our analyses are provided in the Supplementary Information. Diffmatrix v2.2 used to generate the matrix change log is available as both source code and both macOS and Windows executables from https://github.com/mbrazeau/diffmatrix/releases/tag/v2.2.
References
Shubin, N., Tabin, C. & Carroll, S. Fossils, genes and the evolution of animal limbs. Nature 388, 639–648 (1997).
Shubin, N., Tabin, C. & Carroll, S. Deep homology and the origins of evolutionary novelty. Nature 457, 818–823 (2009).
Wagner, G. P. Homology, Genes, and Evolutionary Innovation (Princeton Univ. Press, 2014).
Coates, M. The evolution of paired fins. Theory Biosci 122, 266–287 (2003).
Wilson, M. V. H., Hanke, G. F. & Märss, T. in Major Transitions in Vertebrate Evolution (eds Anderson, J. S. & Sues, H.-D.) 122–149 (Indiana Univ. Press, 2007).
Johanson, Z. Evolution of paired fins and the lateral somitic frontier. J. Exp. Zool. B 314B, 347–352 (2010).
Gai, Z. et al. Galeaspid anatomy and the origin of vertebrate paired appendages. Nature 609, 959–963 (2022).
Gegenbaur, C. Zur morphologie der Gliedmaassen der Wirbeltiere. Morphologisches Jahrbuch 2, 396–420 (1876).
Thacher, J. Median and paired fins: a contribution to the history of vertebrate limbs. Trans. Connecticut. Acad. Sci. 3, 281–308 (1877).
Balfour, F. M. On the development of the skeleton of the paired fins of Elasmobranchii, considered in relation to its bearings on the nature of the limbs of the vertebrata. Proc. Zool. Soc. Lon. 49, 656–670 (1881).
Tabin, C. J. Why we have (only) five fingers per hand: Hox genes and the evolution of paired limbs. Development 116, 289–296 (1992).
Gillis, J. A., Dahn, R. D. & Shubin, N. H. Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc. Natl Acad. Sci. USA 106, 5720–5724 (2009).
Sleight, V. A. & Gillis, J. A. Embryonic origin and serial homology of gill arches and paired fins in the skate, Leucoraja erinacea. eLife 9, e60635 (2020).
Bystrow, A. Kolymaspis sibirica g. n., s. n.—a new representative of the Lower Devonian agnathous vertebrates. Vestnik Leningr. Univ. Geol. Geogr. 18, 5–13 (1956).
Janvier, P. in Recent Advances in the Origin and Early Radiation of Vertebrates (eds Arratia, G., Wilson, M. V. H. & Cloutier, R.) 29–52 (Verlag Dr. Friedrich Pfeil, 2004).
Diogo, R. Cranial or postcranial—Dual origin of the pectoral appendage of vertebrates combining the fin‐fold and gill‐arch theories? Dev. Dyn. 249, 1182–1200 (2020).
Tanaka, M. et al. Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature 416, 527–531 (2002).
Janvier, P. Les Céphalaspides du Spitsberg (Éditions du Centre National de la Recherche Scientifique, 1985).
Stensiö, E. A. in Traité de Paléontologie, Vol. 4 (ed. Piveteau, J.) 71–692 (1969).
Brazeau, M. D., Friedman, M., Jerve, A. & Atwood, R. C. A three-dimensional placoderm (stem-group gnathostome) pharyngeal skeleton and its implications for primitive gnathostome pharyngeal architecture. J. Morphol. 278, 1220–1228 (2017).
Dupret, V., Sanchez, S., Goujet, D., Tafforeau, P. & Ahlberg, P. E. A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature 507, 500–503 (2014).
Ørvig, T. in Problèmes Actuels de Paléontologie: Evolution des Vertébrés (ed. Lehman, J. P.) 41–72 (Colloques Internationaux Centre National de la Recherche Scientifique, 1975).
Stensiö, E. A. Contributions to the knowledge of the vertebrate fauna of the Silurian and Devonian of western Podolia. II. Notes on two arthrodires from the Downtonian of Podolia. Arkiv. Zoolog. 35A, 9 (1944).
Long, J. A. & Young, G. C. Acanthothoracid remains from the Early Devonian of New South Wales, including a complete sclerotic capsule and pelvic girdle. Mem. Assoc. Australas. Palaeontol. 12, 65–80 (1998).
Young, G. C. A new Early Devonian placoderm from New South Wales, Australia, with a discussion of placoderm phylogeny. Palaeontographica 167, 10–76 (1980).
Zhu, Y.-A. et al. Endocast and bony labyrinth of a Devonian “placoderm” challenges stem gnathostome phylogeny. Current Biology 31, 1112–1118.e4 (2021).
Ericsson, R., Knight, R. & Johanson, Z. Evolution and development of the vertebrate neck. J. Anat. 222, 67–78 (2012).
Matsuoka, T. et al. Neural crest origins of the neck and shoulder. Nature 436, 347–355 (2005).
Sefton, E. M., Bhullar, B.-A. S., Mohaddes, Z. & Hanken, J. Evolution of the head–trunk interface in tetrapod vertebrates. eLife 5, e09972 (2016).
Diogo, R. et al. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520, 466–473 (2015).
Lescroart, F. et al. Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium. Proc. Natl Acad. Sci. USA 112, 1446–1451 (2015).
Heude, E. et al. Unique morphogenetic signatures define mammalian neck muscles and associated connective tissues. eLife 7, e40179 (2018).
Stensiö, E. A. The Downtonian and Devonian Vertebrates of Spitsbergen, I: Family Cephalaspidae (Dybwad, 1927).
Wängsjö, G. The Downtonian and Devonian vertebrates of Spitsbergen. IX (Norsk Polarinstitutt, 1952).
Shirai, S. Identity of extra branchial arches in Hexanchiformes (Pisces, Elasmobranchii). Bull. Fac. Fish Hokkaido Univ. 43, 24–32 (1992).
Zhu, Y. et al. The oldest complete jawed vertebrates from the early Silurian of China. Nature 609, 954–958 (2022).
Johanson, Z. & Smith, M. M. Placoderm fishes, pharyngeal denticles, and the vertebrate dentition. J. Morphol. 257, 289–307 (2003).
Zangerl, R. Handbook of Paleoichthyology, Vol. 3A Chondrichthyes I (Gustav Fisher Verlag, 1981).
Coates, M. I., Gess, R. W., Finarelli, J. A., Criswell, K. E. & Tietjen, K. A symmoriiform chondrichthyan braincase and the origin of chimaeroid fishes. Nature 541, 208–211 (2017).
Frey, L., Coates, M. I., Tietjen, K., Rücklin, M. & Klug, C. A symmoriiform from the Late Devonian of Morocco demonstrates a derived jaw function in ancient chondrichthyans. Commun. Biol. 3, 681 (2020).
Anderson, P. S. L. & Westneat, M. W. Feeding mechanics and bite force modelling of the skull of Dunkleosteus terrelli, an ancient apex predator. Biol. Lett. 3, 77–80 (2007).
Dearden, R. P., Stockey, C. & Brazeau, M. D. The pharynx of the stem-chondrichthyan Ptomacanthus and the early evolution of the gnathostome gill skeleton. Nat. Commun. 10, 2050 (2019).
King, B., Qiao, T., Lee, M. S. Y., Zhu, M. & Long, J. A. Bayesian morphological clock methods resurrect placoderm monophyly and reveal rapid early evolution in jawed vertebrates. Syst. Biol. 66, 499–516 (2017).
Rücklin, M. et al. Acanthodian dental development and the origin of gnathostome dentitions. Nat. Ecol. Evol. 5, 919–926 (2021).
Naumann, B., Warth, P., Olsson, L. & Konstantinidis, P. The development of the cucullaris muscle and the branchial musculature in the Longnose Gar, (Lepisosteus osseus, Lepisosteiformes, Actinopterygii) and its implications for the evolution and development of the head/trunk interface in vertebrates. Evol. Dev. 19, 263–276 (2017).
Kuratani, S. A muscular perspective on vertebrate evolution. Science 341, 139–140 (2013).
Janvier, P. Norselaspis glacialis n.g., n.sp. et les relations phylogénétiques entre les kiaeraspidiens (Osteostraci) du Dévonien inférieur du Spitsberg. Palaeovertebrata 11, 19–131 (1981).
Dupret, V., Sanchez, S., Goujet, D. & Ahlberg, P. E. The internal cranial anatomy of Romundina stellina Ørvig, 1975 (Vertebrata, Placodermi, Acanthothoraci) and the origin of jawed vertebrates—Anatomical atlas of a primitive gnathostome. PLoS ONE 12, e0171241 (2017).
Goujet, D. & Young, G. C. in Recent Advances in the Origin and Early Radiation of Vertebrates (eds Arratia, G., Wilson, M. V. H. & Cloutier, R.) 109–126 (Verlag Dr Friedrich Pfeil, 2004).
Jarvik, E. Basic Structure and Evolution of Vertebrates, Vol. 1 (Academic Press, 1980).
Sutton, M. D., Garwood, R. J., Siveter, D. J. & Siveter, D. J. SPIERS and VAXML: a software toolkit for tomographic visualisation and a format for virtual specimen interchange. Palaeontol. Electron. 15, 5T,14p (2012).
Maddison, D. R. & Maddison, W. P. Mesquite: a modular system for evolutionary analysis. Version 3.81 http://www.mesquiteproject.org (2021).
Goloboff, P. A. & Morales, M. E. TNT version 1.6, with a graphical interface for MacOS and Linux, including new routines in parallel. Cladistics 39, 144–153 (2023).
King, B. & Rücklin, M. A Bayesian approach to dynamic homology of morphological characters and the ancestral phenotype of jawed vertebrates. eLife 9, e62374 (2020).
Acknowledgements
The authors thank A. Gehler for access to collections and R. Garwood and M. Sutton for technical support with SPIERS. TNT is made freely available with the support of the Willi Hennig Society. Computed tomography scanning of Kolymaspis was performed with the help of F. Ahmed at the Imaging and Analysis Centre, Natural History Museum, UK; computed tomography scanning of Romundina was performed by K. Smithson at the Cambridge Biotomography Centre, Department of Zoology, University of Cambridge. Original tomography and segmentation were supported by European Research Council Starting Grant under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC Grant Agreement no. 311092 (M.D.B.) and the John Fell Fund, University of Oxford (M.F.). A.O.I. was supported by the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.D.B. Methodology: M.D.B., M.C., M.F., A.E.F.E.F. and L.H. Investigation: M.D.B., M.C., A.E.F.E.F. and L.H. Visualization: M.D.B., M.C., A.E.F.E.F., L.H. and M.F. Funding acquisition: M.D.B. and M.F. Specimen access and geological data: A.O.I. Project administration: M.D.B. Supervision: M.D.B. Writing of original draft: M.D.B. Writing (review and editing): M.D.B., M.F. and Z.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Min Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 2 Posterior section of the skull and endocast of the osteostracan Mimetaspis hoeli showing the position of the subclavian artery relative to the gill compartments.
a, Skull. b, Ventral view of the skull. c, Endocast of blood vessels and ‘sel’ canals. d, Posterior view of the endocast and branchial space. e, Dorsal view of the left side arteries and branchial space. f, Dorsal view of the right side arteries and branchial space. a and c are from the same view. Additional abbreviations: a.eff.com, common efferent artery (dorsal aorta); ibr.s, interbranchial septa; k2–k10, pharyngeal cavities; sel, sensory field canals; v, canals for vessels; v.marg, marginal vein cavities. Colour scheme: dark grey, bone; red, small canals; blue, large canals and cavities; green, branchial space. Dotted lines indicate where the continuation of the specimen would be if it was complete. The arrows indicate the orientation of the specimen: A, anterior; D, dorsal; L, left; P, posterior; R, right. Top left scale bar is for a, b and c and equals 6 mm. Bottom left scale bar is for e and equals 3 mm. Bottom right scale bar is for f and equals approximately 4 mm.
Extended Data Fig. 3 Strict consensus tree from equal weights parsimony analysis.
Outgroup (jawless vertebrates) shown in light grey. See supplementary information for details of tree number and tree score. Numbers below nodes show bootstrap values 50% or above.
Extended Data Fig. 4 Strict consensus tree from implied weights (k = 12) parsimony analysis.
Outgroup (jawless vertebrates) shown in light grey. See supplementary information for details of tree number and tree score.
Extended Data Fig. 6 Gnathostome phylogeny under two constraints on placoderm monophyly.
Left, strict consensus tree of equally weighted parsimony analysis.Centre, all placoderms inclusive of Entelognathus and Minjinia constrained as a clade; Right, “core placoderms” (excluding Entelognathus and Minjinia) constrained as a clade. Colour coding shows parsimony ancestral states mapping for character 180 (Endoskeletal craniothoracic [sixth branchial] facet). Black: present; white: absent; gray: ambiguous.
Supplementary information
Supplementary Information
This file contains additional specimen information (geological provenance and age), additional phylogenetic results, and supplementary references.
Supplementary Data
An archive folder containing the original Nexus file (MATRIX_MASTER.nex), the exported TNT file, TNT script, and a README file for instructions on reproducing the phylogenetic analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brazeau, M.D., Castiello, M., El Fassi El Fehri, A. et al. Fossil evidence for a pharyngeal origin of the vertebrate pectoral girdle. Nature 623, 550–554 (2023). https://doi.org/10.1038/s41586-023-06702-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06702-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.