Abstract
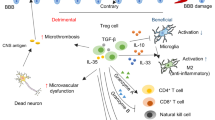
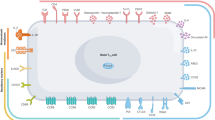
In addition to maintaining immune tolerance, FOXP3+ regulatory T (Treg) cells perform specialized functions in tissue homeostasis and remodelling1,2. However, the characteristics and functions of brain Treg cells are not well understood because there is a low number of Treg cells in the brain under normal conditions. Here we show that there is massive accumulation of Treg cells in the mouse brain after ischaemic stroke, and this potentiates neurological recovery during the chronic phase of ischaemic brain injury. Although brain Treg cells are similar to Treg cells in other tissues such as visceral adipose tissue and muscle3,4,5, they are apparently distinct and express unique genes related to the nervous system including Htr7, which encodes the serotonin receptor 5-HT7. The amplification of brain Treg cells is dependent on interleukin (IL)-2, IL-33, serotonin and T cell receptor recognition, and infiltration into the brain is driven by the chemokines CCL1 and CCL20. Brain Treg cells suppress neurotoxic astrogliosis by producing amphiregulin, a low-affinity epidermal growth factor receptor (EGFR) ligand. Stroke is a leading cause of neurological disability, and there are currently few effective recovery methods other than rehabilitation during the chronic phase. Our findings suggest that Treg cells and their products may provide therapeutic opportunities for neuronal protection against stroke and neuroinflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The microarray data for brain Treg cells are available in the Gene Expression Omnibus (GEO) database under accession number GSE109259. Source Data for figures are provided with the paper.
References
Panduro, M., Benoist, C. & Mathis, D. Tissue Tregs. Annu. Rev. Immunol. 34, 609–633 (2016).
Sekiya, T., Nakatsukasa, H., Lu, Q. & Yoshimura, A. Roles of transcription factors and epigenetic modifications in differentiation and maintenance of regulatory T cells. Microbes Infect. 18, 378–386 (2016).
Li, C. et al. TCR transgenic mice reveal stepwise, multi-site acquisition of the distinctive fat-Treg phenotype. Cell 174, 285–299.e12 (2018).
Burzyn, D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013).
Kuswanto, W. et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 44, 355–367 (2016).
Shichita, T. et al. Pivotal role of cerebral interleukin-17-producing γδT cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950 (2009).
Ito, M. et al. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 6, 7360 (2015).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Liesz, A. & Kleinschnitz, C. Regulatory T cells in post-stroke immune homeostasis. Transl. Stroke Res. 7, 313–321 (2016).
Stubbe, T. et al. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J. Cereb. Blood Flow Metab. 33, 37–47 (2013).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Anderson, M. F., Blomstrand, F., Blomstrand, C., Eriksson, P. S. & Nilsson, M. Astrocytes and stroke: networking for survival? Neurochem. Res. 28, 293–305 (2003).
Liddelow, S. & Barres, B. SnapShot: astrocytes in health and disease. Cell 162, 1170–1170.e1 (2015).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Cregg, J. M. et al. Functional regeneration beyond the glial scar. Exp. Neurol. 253, 197–207 (2014).
Anderson, M. A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Thornton, A. M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 (2010).
de las Casas-Engel, M. et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J. Immunol. 190, 2301–2310 (2013).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Yang, Y. et al. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J. Neurosci. 37, 4692–4704 (2017).
Zaiss, D. M. W., Gause, W. C., Osborne, L. C. & Artis, D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 42, 216–226 (2015).
Pekny, M. & Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta 1862, 483–491 (2016).
Okada, S. et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12, 829–834 (2006).
Hoshino, K. et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 190, 1541–1548 (1999).
Oboki, K. et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl Acad. Sci. USA 107, 18581–18586 (2010).
Luetteke, N. C. et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 126, 2739–2750 (1999).
Hedrick, M. N. et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J. Clin. Invest. 119, 2317–2329 (2009).
Sekiya, T. et al. Suppression of Th2 and Tfh immune reactions by Nr4a receptors in mature T reg cells. J. Exp. Med. 212, 1623–1640 (2015).
Shichita, T. et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 18, 911–917 (2012).
Shichita, T. et al. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat. Med. 23, 723–732 (2017).
Funato, H. et al. Effects of a calcium antagonist, lacidipine, on experimental focal cerebral ischemia in rats. Jpn. J. Pharmacol. 75, 415–423 (1997).
Balkaya, M., Kröber, J. M., Rex, A. & Endres, M. Assessing post-stroke behavior in mouse models of focal ischemia. J. Cereb. Blood Flow Metab. 33, 330–338 (2013).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Wu, J., Wrathall, J. R. & Schachner, M. Phosphatidylinositol 3-kinase/protein kinase Cδ activation induces close homolog of adhesion molecule L1 (CHL1) expression in cultured astrocytes. Glia 58, 315–328 (2010).
DeVos, S. L. & Miller, T. M. Direct intraventricular delivery of drugs to the rodent central nervous system. J. Vis. Exp. 75, e50326 (2013).
Seki, A. & Rutz, S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J. Exp. Med. 215, 985–997 (2018).
Sharov, A. A., Schlessinger, D. & Ko, M. S. ExAtlas: An interactive online tool for meta-analysis of gene expression data. J. Bioinform. Comput. Biol. 13, 1550019 (2015).
Yoshida, R. et al. A new method for quantitative analysis of the mouse T-cell receptor V region repertoires: comparison of repertoires among strains. Immunogenetics 52, 35–45 (2000).
Lefranc, M. P. et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 37, D1006–D1012 (2009).
Dash, P. et al. Paired analysis of TCRα and TCRβ chains at the single-cell level in mice. J. Clin. Invest. 121, 288–295 (2011).
Acknowledgements
We thank K. Sawamoto and K. Tanaka for comments, N. Shiino, C. Ohkura, Y. Tokifuji and Y. Hirata for technical assistance, and T. Srirat for checking the English. This work was supported by JSPS KAKENHI (S) JP17H06175, Challenging Research (P) JP18H05376, and AMED-CREST JP18gm0510019 and JP18gm1110009 to A.Y., AMED-PRIME JP18gm5910023, and AMED JP18ek0210100 to T.S., the Takeda Science Foundation, the Uehara Memorial Foundation, the Kanae Foundation, and the SENSHIN Medical Research Foundation, and Keio Gijuku Academic Developmental Funds. M.I. and M.I.-K. were supported by JSPS post-doctoral fellowship.
Reviewer information
Nature thanks S. Liddelow, T. Masuda, M. Prinz, A. Rudensky and the anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
M.I. designed and performed experiments, analysed data and wrote the manuscript; S.M.-O., R.S., Y.N. and K.K. performed mouse analysis; K.M., T.N. and O.Y. provided mice, M.I.-K. and H.N. participated in data analysis and discussion; T.S., S.C. and T.K. provided technical advice about experimental design; A.Y. initiated and directed the entire study, designed experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 T cell accumulation in the brain after ischaemic brain injury.
a, 3,3′-diaminobenzidine (DAB) staining for CD3+ cells in ischaemically injured brains at the indicated time points after stroke onset. Data are representative of two independent experiments. b, CD4+ cell numbers in the injured brain at the indicated time points after stroke onset as determined by flow cytometric analysis (n = 4 for day 0, n = 3 for day 3, n = 3 for day 6, n = 3 for day 11, n = 5 for day 14, n = 4 for day 21, n = 5 for day 60). Data are representative of two independent experiments. c, Immunohistochemical staining for MAP2, CD3 and FOXP3 in ischaemically injured brains (purple square, ischaemic border region; blue square, ischaemic core region) on day 14 after stroke onset. Yellow arrows indicate FOXP3+ cells in the MAP2+ region. Data are representative of three independent experiments. d, FTY720 (1 mg kg−1) was administered to mice daily on days 6–13 after stroke onset. Flow cytometric analysis of the numbers of GR-1+, F4/80+, CD3+, CD19+, CD4+, CD8+, IFNγ+, IL-17+ and FOXP3+ on day 14 (n = 4 control mice, n = 5 FTY treatment mice). Data are representative of two independent experiments. P values were determined by two-tailed Student’s t-test (CD3+, CD19+) or two-way ANOVA with Bonferroni’s multiple comparisons test (IFNγ+, IL-17+ and FOXP3+). e, Flow cytometric analysis of FOXP3+ cells in ischaemic brains from DEREG or wild-type littermates after administration of diphtheria toxin (DT) (12.5 μg kg−1) or PBS on days 6, 7, 9 and 11 after stroke onset. Representative dot plots of CD4+ T cells on day 14 are shown. Data are representative of two independent experiments, and shown as the mean ± s.e.m.
Extended Data Fig. 2 The effect of T cells on reactive astrocytes and neurogenesis.
a, Immunohistochemical staining for GFAP in injured brains and GFAP+ areas in the ischaemic brains at the indicated time points after stroke onset. Representative photos are shown (n = 3 for day 0 and 3, n = 4 for day 6, n = 5 for days 9, 11 and 14). Data are representative of a single experiment. b, Immunohistochemical staining for GFAP in ischaemic brains from wild-type mice injected on days 6 and 10 after stroke onset with FTY720 (1 mg kg−1) or anti-CD4 antibody (15 mg kg−1). GFAP+ areas in the ischaemic brains were determined at day 14 after stroke onset. Representative photos are shown (n = 5 control mice, n = 7 FTY-treated mice, n = 6 anti-CD4 antibody-treated mice). Data are representative of two independent experiments. c, GFAP+ areas on day 14 after stroke in ischaemic brains from DEREG or wild-type littermates after administration of diphtheria toxin (DT) or PBS on days 7, 9, and 12 after stroke onset (n = 4 PBS-treated wild type, n = 3 DT-treated wild type, n = 5 PBS-treated DEREG, n = 6 DT-treated DEREG). Data are representative of a single experiments. d, Flow cytometric analysis of the frequency of Treg cells among CD4+ T cells and GFAP+ areas in ischaemic brains from DEREG or wild-type littermates and neurological score after intraventricular (i.c.v.) administration of diphtheria toxin (125 ng kg−1) or PBS on days 8, 10 and 12, and analysis at day 14 after stroke onset (n = 6 DT-treated wild type, n = 5 DT-treated DEREG mice). Data are representative of two independent experiments. e, Immunohistochemical staining for TUNEL, NeuN and GFAP in the ischaemically injured brains from DEREG or wild-type littermates were measured on day 14. White squares indicate parts of motor cortex regions and were magnified in Fig. 1g. f, Astrocytes were isolated from the day-14 post-ischaemic brains of six DEREG (+DT) or six wild-type (+DT) littermates, and the relative expression levels of neurotoxic markers were evaluated by qPCR. Data are obtained from a pooled sample from six mice, and representative of two independent experiments. g, Heat map of the microarray analysis of the axon growth permissive molecules and A2 markers in the brains of diphtheria-toxin-treated DEREG or wild-type littermates on day 14 after MCAO or a sham operation. Data are obtained from a single experiment. h, Treg cells (1 × 106) from spleens and lymph nodes from intact mice were transferred into Cd3e−/− mice on day 5 after stroke onset. Immunohistochemical staining for FOXP3 and MAP2 in the ischaemic brains (ischaemic border region and ischaemic core region) at day 14 after stroke onset. Data are representative of two independent experiments. i, Treg cells (1 × 106) from spleens and lymph nodes from intact mice were transferred into Treg cell-sufficient wild-type mice on day 5 after stroke onset. The GFAP+ areas (n = 10 PBS-treated mice, n = 9 Treg cell-transferred mice) and the number of FOXP3+ cells (n = 6 PBS-treated mice, n = 5 Treg cell-transferred mice) in the ischaemic brains were measured. GFAP data were pooled from two independent experiments. Other data are representative of two independent experiments. P values were determined by two-tailed Student’s t-test (d, i) or one-way ANOVA (b, c). Data are mean ± s.e.m.
Extended Data Fig. 3 Characterization of brain Treg cells.
a, Flow cytometric analysis of CD44 and CD62L activation markers (left) and numbers of CD4+ cells (right) from day-14 post-ischaemic brains of wild-type or Rag2−/− OTII transgenic mice (n = 4). Data are representative of two independent experiments, and shown as mean ± s.e.m. b, Flow cytometric analysis of TCR vβ repertoires of Treg cells in brains from ischaemic mice and lymph nodes from intact mice. Data were obtained from a single experiment. c, Two-dimensional plot showing the frequency of the top ten TCRα and TCRβ CDR3 sequences expressed by Treg cells in day-14 post-ischaemic brains from 42 mice and spleens from 3 intact mice. Data were obtained from a single experiment. d, TCRα and TCRβ CDR3 sequences for individual cells (TCRα; mouse #1: n = 14, mouse #3: n = 10, mouse #4: n = 35, TCRβ; mouse #4: n = 65). Data were obtained from a single experiment.
Extended Data Fig. 4 5-HT7 is expressed on brain Treg cells and required for the function of brain Treg cells.
a, Scatter plot of Treg signature genes (brain Treg versus spleen Treg cells). b, Heat map of the microarray analysis of Treg cells from the brain, VAT, injured muscle, colon, pancreas and spleen. c, KEGG pathway analysis of Treg versus Tconv cells, and brain Treg versus spleen Treg cells. d, Heat map of nervous-system-related genes of Treg cells in the brain, VAT, injured muscle, colon (RORC+ and RORC−), lymph nodes and spleen. e, Scatter plot of nervous-system-related genes (brain Treg versus spleen Treg cells). f, Expression levels of Htr7 in the day-14 post-ischaemic or sham brains of diphtheria-toxin-treated DEREG or wild-type littermates, and brain T cells from wild-type ischaemic mice or spleen T cells from wild-type untreated mice. The gene expression levels were determined by microarray signals. Data were obtained from a single experiment of microarray, and confirmed by qPCR experiments. g–i, Treg cells (5 × 105) from spleens and lymph nodes from intact mice were transduced by CRIPSR–Cas9 (Htr7 gRNA or control gRNA) and transferred into Cd3e−/− mice on day 5 after stroke onset. Flow cytometric analysis of 5-HT7 (g), the number of Treg cells (h) in the ischaemic brains of Cd3e−/− mice, and neurological scores (i) on day 14 after stroke onset (n = 4 for control, n = 5 for 5-HT7 knockout (KO)). Data are representative of two independent experiments. j, Flow cytometric analysis of 5-HT7 in the ischaemic brains of wild-type mice on day 14 after stroke onset. Data are representative of three independent experiments. k, The relative expression levels of Htr7 in the ischaemic brains at day 14 after stroke onset were evaluated by qPCR. Brain Treg cells were pooled from 6 mice, other data were n = 5 per group. Data are representative of two independent experiments and shown as mean ± s.e.m. P values were determined by two-tailed Student’s t-test.
Extended Data Fig. 5 Brain Treg cells proliferate in both lymph nodes and brains.
a, Flow cytometric analysis of CD4+ T cells in cervical lymph nodes and brain from Treg-cell-transferred Rag2−/− mice. Treg cells (2 × 105 cells) from the cervical lymph nodes and spleens of ischaemic or sham-treated mice were transferred into Rag2−/− mice on day 5 after stroke onset. The absolute numbers of CD4+ T cells in the brains of Rag2−/− mice were measured on day 14 after stroke onset (n = 6). Data were pooled from two independent experiments. b, Kinetics of percentages of Ki67+ Treg cells from brains and cervical lymph nodes of ischaemic mice at the indicated time points as determined by flow cytometric analysis of CD4+ T cells (n = 2 for days 0, 6, 11, 14 and 21; n = 3 for days 3 and 17). Data are representative of two independent experiments. c, Flow cytometric analysis of the frequency of Treg cells among CD4+ T cells from the cervical lymph nodes of sham-treated and ischaemic mice on day 14 after stroke onset. The frequency of Treg cells of CD4+ T cells was determined (n = 6 sham-treated mice, n = 12 ischaemic mice) Data were pooled from two independent experiments. d, The relative expression levels of Il2 in ischaemic brains at the indicated time points after stroke onset were evaluated by qPCR (n = 6 day 0, n = 3 others). Data are representative of two independent experiments. e, Flow cytometric analysis of IL-2+ cells from day 14 post-ischaemic brains and cervical lymph nodes gated as indicated. Data are representative of two independent experiments. f, Wild-type mice were injected with control IgG or anti-IL-2 antibody on days 8, 10 and 12 after stroke onset. Neurological scores were measured on day 14 (n = 8). Data are representative of two independent experiments and shown as mean ± s.e.m. P values determined by two-tailed Student’s t-test.
Extended Data Fig. 6 Brain Treg cells proliferate in response to IL-33.
a, The relative expression levels of Il33 in the ischaemic brains at the indicated time points after stroke onset were evaluated by qPCR (n = 6 day 0, n = 3 others). Immunohistochemical staining for IL-33 in the brains of Il33−/−, wild-type sham or wild-type ischaemic mice on day 14 after stroke onset. Data are representative of two independent experiments. b, Flow cytometric analysis of ST2 in the ischaemic brains of wild-type, Il33−/−, or Il1rl1−/− mice on day 14 after stroke onset. The frequency of ST2+ cells of Treg cells was determined (n = 3). Data are representative of two independent experiments. c, Flow cytometric analysis of the frequency of Treg cells among CD4+ T cells from the ischaemic mice administrated with anti-ST2 antibody on days 7, 10 and 12, and analysed on day 14 after stroke onset. The frequency of Treg cells of CD4+ T cells was determined (n = 5 control IgG-treated mice, n = 4 ST2 antibody-treated mice). Data are representative of a single experiment. d, Flow cytometric analysis of the frequency of ST2+ cells among brain cells from the ischaemic mice on day 14 after stroke onset. (n = 4). Data are representative of two independent experiments. e, Treg cells (1 × 106) from spleens and lymph nodes from wild-type or Il1rl1−/− mice were transferred into Rag2−/− mice on day 5 after stroke onset. The representative plots of CD4+ cells from the indicated tissues in the ischaemic brains on day 14. Data are representative of two independent experiments and shown as mean ± s.e.m. P values determined by two-tailed Student’s t-test.
Extended Data Fig. 7 Expression of chemokine receptors on CD4+ T cells.
a, TH1 (IFNγ+), IFNγ−FOXP3− and Treg (FOXP3+) cells were isolated from the day-14 post-ischaemic brains of 8 mice and the relative expression levels of the indicated chemokine receptors on CD4+ T cells were evaluated by qPCR. Data were obtained from pooled samples of eight mice, and representative of two independent experiments. b, Oligodendrocytes, astrocytes and microglia were isolated from ischaemic brains at the indicated time points after stroke onset and the relative expression of Ccl1 and Ccl20 was evaluated by qPCR (n = 3 sham and day-14 mice, n = 2 day 3 and 7 mice). Data are representative of two independent experiments. c, Representative plots of flow cytometric analysis of CD4+ cells and the absolute number of Treg cells from the brains of wild-type or Ccr6−/− mice in Fig. 3h at day 14 after stroke onset (n = 6 wild-type mice, n = 4 Ccr6−/− mice). Data are representative of two independent experiments. d, Representative plots of flow cytometric analysis of CD4+ cells and the absolute number of Treg cells from day-14 post-ischaemic mice injected intraventricularly with CCL1 or CCL20 proteins on days 6 and 9 post-stroke-onset in Fig. 3i (n = 6 PBS-treated mice, n = 5 CCL1-treated mice, n = 6 CCL20-treated mice). Data are representative of two independent experiments. P values determined by two-tailed Student’s t-test. Data are mean ± s.e.m.
Extended Data Fig. 8 AREG expressed homogeneously on day 14 after stroke onset.
a, Flow cytometric analysis of AREG expression levels in Treg cells in the infarct core region and the glial scar region from ischaemic brains on day 14 after stroke (n = pool of 3 mice × 5). Data are representative of two independent experiments. b, The relative mRNA expression levels (relative to Hprt1 mRNA) of tissue Treg cell signature genes from brain Treg cells on days 3 and 14 after stroke onset and spleen Treg cells were evaluated by qPCR. Data are representative of two independent experiments. c, Flow cytometric analysis of AREG, ST2 and 5-HT7 expression levels in Treg cells from ischaemic brains on days 3 and 14 after stroke onset. Data are representative of two independent experiments and shown as mean ± s.e.m. P values determined by two-tailed Student’s t-test.
Extended Data Fig. 9 Treg cells regulate astrogliosis by suppressing the IL-6–STAT3 signalling pathway.
a, DEREG or wild-type littermates were administered diphtheria toxin intraperitoneally on days 6, 9 and 12, and administered PBS or AREG protein intraventricularly on days 7, 9 and 12 after stroke onset. The GFAP+ areas and neurological scores were determined on day 14 (n = 7 PBS-treated wild type, n = 9 AREG-treated wild type, n = 10 PBS-treated DEREG, n = 8 AREG-treated DEREG). Data were pooled from two independent experiments. b, Ischaemic DEREG or wild-type littermates were administered diphtheria toxin intraperitoneally on days 6, 9 and 12 after stroke onset, and administered PBS or AREG protein (50 μg kg−1) intraventricularly on days 7, 9 and 12 after stroke onset. TUNEL+ cells in the ischaemic brains were measured on day 14 (n = 7 PBS-treated wild-type mice, n = 7 PBS-treated DEREG mice, n = 3 AREG-treated DEREG mice). Data were obtained from a single experiment. c, Ischaemic DEREG or wild-type littermates were administered diphtheria toxin intraperitoneally on day 0 after stroke onset, and administered PBS or AREG protein (50 μg kg−1) intraventricularly on day 1 after stroke onset. Neurological score and infarct region were evaluated on day 3 (n = 3 PBS-treated wild-type mice, n = 5 others). Data were obtained from a single experiment. d, Heat map of the qPCR analysis of neurotoxic markers of astrocytes on day-14 post-ischaemic brains of Cd3e−/− mice injected with PBS, wild-type Treg cells, or Areg−/− Treg cells on day 5 after stroke onset (n = 3). Data are representative of two independent experiments. e, KEGG pathway analysis of day-14 post-ischaemic brains of diphtheria-toxin-treated DEREG or wild-type littermates. Data were obtained from a single experiment. f, Microglia, CD45highCD11bhigh, CD45− and astrocytes were isolated from the day-14 post-ischaemic brains of diphtheria-toxin-treated DEREG mice (n = 4). The relative expression levels of Il6 were evaluated by qPCR (relative to Hprt1). Data were obtained from a single experiment. g, ELISA for IL-6 of serum from ischaemic DEREG or wild-type littermates (n = 14 wild-type mice, n = 13 DEREG mice), Cd3e−/− (CD3KO) mice transferred with wild-type or Areg−/− Treg cells (n = 2 intact mice, n = 6 PBS-treated mice, n = 6 wild-type Treg-cell-transferred mice, n = 5 Areg−/− Treg-cell-transferred mice), and Rag2−/− (Rag2KO) mice transferred with wild-type or Il1rl1−/− Treg cells (n = 3 PBS-treated mice, n = 6 wild-type Treg-cell-transferred mice, n = 6 Il1rl1−/− Treg-cell-transferred mice). Data were pooled from two independent experiments. h, Relative Gfap and Stat3 mRNA expression levels (relative to Hprt1) in primary astrocytes stimulated with the indicated cytokines for 24 h (n = 3). Data are representative of two independent experiments. i, Microglia and astrocytes isolated from 12 adult mice were stimulated for 24 h with brain lysates from control or ischaemic mice in the presence or absence of AREG. Relative Il6 mRNA expression levels (relative to Hprt1) were evaluated by qPCR (n = 3). Data are representative of three independent experiments. j, GFAP+pSTAT3+ cells in ischaemic brains were measured on day 14 after stroke onset and representative photos are shown (n = 8 PBS-treated wild type, n = 5 PBS-treated DEREG, n = 6 AREG-treated DEREG). Data are representative of two independent experiments. k, Wild-type and Areg−/− Treg cells were transferred into Cd3e−/− mice on day 5 after stroke onset. Representative photographs of Fig. 4h of GFAP+pSTAT3+ cells in the day-14 post-ischaemic brains are shown. Data are representative of two independent experiments and shown as mean ± s.e.m. P values were determined by one-way ANOVA.
Extended Data Fig. 10 Brain slice and gating strategy of flow cytometric analysis.
a, For evaluation of ischaemic brain tissues, 1-mm-thick serial coronal slices from the brain were embedded in paraffin. The infarct area was detected by MAP2 staining of all slices (slices 1–6). The reactive astrocyte area (astrogliosis area) was detected by GFAP staining of slice 3 at the ischaemic core of the transient middle artery occlusion. b, Gating strategy of analysis of various brain cells. c, A scheme of regulation of brain damage by brain Treg cells.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Ito, M., Komai, K., Mise-Omata, S. et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565, 246–250 (2019). https://doi.org/10.1038/s41586-018-0824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0824-5
This article is cited by
-
Regulatory T cells limit age-associated retinal inflammation and neurodegeneration
Molecular Neurodegeneration (2024)
-
Understanding immune microenvironment alterations in the brain to improve the diagnosis and treatment of diverse brain diseases
Cell Communication and Signaling (2024)
-
Analysis of brain and blood single-cell transcriptomics in acute and subacute phases after experimental stroke
Nature Immunology (2024)
-
Farrerol Alleviates Cerebral Ischemia–Reperfusion Injury by Promoting Neuronal Survival and Reducing Neuroinflammation
Molecular Neurobiology (2024)
-
Single-Cell Mapping of Brain Myeloid Cell Subsets Reveals Key Transcriptomic Changes Favoring Neuroplasticity after Ischemic Stroke
Neuroscience Bulletin (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.