Abstract
The COVID-19 pandemic, caused by the SARS-CoV-2 coronavirus, has taken a catastrophic toll on society, health-care systems and the economy. Notably, COVID-19 has been shown to be associated with a higher mortality rate in men than in women. This disparity is likely to be a consequence of a failure to invest in men’s health, as it has also been established that men have a lower life expectancy and poorer outcomes from non-communicable diseases than women. A variety of biological, social and economic factors have contributed to the sex disparities in mortality from COVID-19. A streamlined men’s health programme — with the urologist as the gatekeeper of men’s health — is needed to help prevent future tragedies of this nature.
Similar content being viewed by others
Introduction
In December 2019, a novel coronavirus (subsequently termed SARS-CoV-2) was first reported in China; the resulting disease was coined COVID-19 (ref.1). This virus has subsequently spread globally, causing a pandemic that has had a catastrophic effect on society, health-care systems and the economy. Considerable media and public interest has focused on the disproportionately high mortality from COVID-19 in men2; this discrepancy might be due to biological, genetic and lifestyle differences between the sexes, making men more vulnerable to both infections and non-communicable diseases3,4,5. Indeed, this sex gap in mortality is not a new phenomenon and contemporary health-care policies seem to have failed to adequately address the disproportionately high levels of premature male deaths3.
In this Perspectives article, we highlight the potential factors contributing to sex discrepancies in mortality from COVID-19, provide a rationale for the development of a men’s health programme and discuss the role of the urologist in this setting.
What is COVID-19?
SARS-CoV-2 is a subgenus Sarbecovirus of the genus Betacoronavirus1. The pathogenesis of COVID-19 is still poorly understood, but it has been speculated that SARS-CoV-2 first binds to the angiotensin-converting enzyme 2 (ACE2) receptor on the surface of epithelial cells in the nasal cavity6,7 after which it propagates and spreads across the respiratory tract, causing an innate immune response with cytokine secretion and an inflammatory response8. This immune response results in an influx of pulmonary infiltrates, apoptosis of alveolar type II cells and diffuse alveolar damage, leading to subsequent fibrosis, acute respiratory distress syndrome (ARDS) and microvascular thrombi7,9,10
The first cases of COVID-19 were reported in December 2019; by the end of February 2020, 55,924 cases had been confirmed11. The infection has now spread globally, with over 234 million confirmed cases (43 million in the USA alone) and 4.7 million deaths as of October 2021 (ref.12).
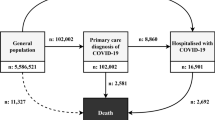
A sex-disaggregated data tracker has highlighted a gender difference worldwide13. Although cases of COVID-19 have an almost equal division between men and women, more men are hospitalized, admitted to intensive care, and die from infection (Fig. 1).
The number of confirmed cases of COVID-19 is similar between sexes (49% versus 51%) but men have a higher rate of hospitalizations, intensive care admissions and deaths. Data source: Global Health 5050. Values correct as of 5 October 2021.
Risk factors for mortality and sex
The first confirmed human cases of COVID-19 were reported in China’s Hubei province14. Several studies of patients from this region describe prognostic factors for survival after COVID-19 infection. A study of a cohort of 191 patients from two hospitals within Wuhan15 reported that the clinical factors associated with COVID-19 mortality were increasing age (69 years (interquartile range (IQR) 63–76) versus 52 years (IQR 45–58) P < 0.0001), hypertension (P = 0.0008), diabetes (P = 0.0051), cardiovascular disease (CVD) (P < 0.0001), chronic obstructive pulmonary disease (COPD) (P = 0.047) and chronic kidney disease (P = 0.024)15. Moreover, multivariate regression analysis confirmed that older age was associated with increased mortality (odds ratio (OR) 1.10, 95% confidence interval (CI) 1.03–1.17, per year increase, P = 0.0043). A prospective cohort study of 179 patients with COVID-19 who were hospitalized at Wuhan Pulmonary Hospital reported that patients who died were much older than those in the survivor group (mean age ± standard deviation; 70.2 ± 7.7 years versus 56.0 ± 13.5 years, P < 0.001) and had hypertension (61.9% versus 28.5%, P = 0.005) and CVD or cerebrovascular diseases (57.1% versus 10.8%; P < 0.001)16. Multivariate analysis showed that an age ≥65 years (OR 3.765, 95% CI 1.146‒17.394, P = 0.023) and cardiovascular or cerebrovascular comorbidities (OR 2.464, 95% CI 0.755‒8.044, P = 0.007) were associated with an increased risk of COVID-19 mortality. Accordingly, a retrospective case series of 201 patients who were admitted to a single hospital in Wuhan with COVID-19 showed that patients who developed ARDS were older (median age 58.5 (IQR 50–69) versus 48 (IQR 40–54), P < 0.001) and had a history of hypertension (23% versus 16%, P = 0.02) and diabetes (16% versus 6%, P = 0.002) compared with those who did not develop ARDS. These conclusions were also supported by a study investigating the clinical factors associated with COVID-19 disease severity and mortality in a cohort of 663 Chinese patients from a single hospital in Wuhan17. In this study, disease severity was classified according to the WHO guidelines for clinical management of COVID-19 (ref.18) and patient age ≥60 years was associated with severe and critical disease severity compared with mild and moderate categories (P < 0.001) and also increased mortality (P = 0.004). The presence of pre-existing respiratory disease (P = 0.003), CVD (P < 0.001) and endocrine diseases (P = 0.007) were independently associated with increased COVID-19 disease severity. However, only respiratory disease (P = 0.019) and CVD (P < 0.001) were also associated with increased COVID-19 mortality.
Studies examining COVID-19 in other areas of China have reported similar findings to those that have focused on the Wuhan region. Wan et al.19 studied the clinical presentation of 135 patients admitted to a single hospital in the Chongqing region. The authors assessed severity using the WHO guidelines18 and reported that severe disease was associated with increased age (median age 56 years (IQR 52–73) versus 44 years for mild disease (IQR 33–49), P < 0.001) and underlying comorbidities (70% versus 16.3%, P < 0.0001). A meta-analysis that pooled data from 11 studies in China reported that COPD was significantly associated with mechanical ventilation, critical care admission or death from COVID-19 (OR 6.44, 95% CI 1.85–22.46)20. In accordance with these data, a separate meta-analysis comprising seven studies from China observed that smoking was associated with worsening severity of COVID-19 (OR 2.16, 95% CI 1.45–3.22)21.
These studies suggest that increasing age, male sex and certain comorbidities — in particular hypertension, diabetes, CVD and COPD — are risk factors for COVID-19 mortality and worsening severity.
Data from other countries have reported similar findings. Giacomelli et al.22 performed a single-centre, prospective cohort study of patients with COVID-19 admitted to the Luigi Sacco Hospital in Milan between 21 February 2020 and 19 March 2020. Mortality was associated with increasing age, with a higher proportion of deaths than survivors in the 66−75 years (39.6% versus 19.5%), 76−85 years (20.8% versus 13.0%) and 86−95 years (10.4% versus 2.7%) age groups (all P < 0.001). CVD seemed to be associated with increased COVID-19 mortality, as a higher mortality rate was observed in those taking anti-platelet agents (P = 0.009), calcium channel blockers (P = 0.023) and angiotensin II receptor blockers (P = 0.001). Univariate and multivariate Cox analysis showed that age (adjusted hazard ratio (HR) 2.08, 95% CI 1.48–2.92 per 10 years more) and obesity (adjusted HR 3.04, 95% CI 1.42–6.49) were independently associated with an increased risk of death.
A study that compared the clinical outcomes of COVID-19 between Italy and China using records from the National Italian Institute of Health and the Chinese Centre for Disease Control and Prevention reported that the COVID-19 mortality rate was significantly higher in Italy than in China (OR 3.18, 95% CI 3.06–3.31, P < 0.001)23. Moreover, the presence of several comorbidities (diabetes (OR 1.82, 95% CI 1.22–2.15), hypertension (OR 3.46, 95% CI 2.68–4.46), chronic respiratory disease (OR 2.30, 95% CI 1.54–3.44), cancer (OR 11.73, 95% CI 5.14–28.77) and CVD (OR 1.91, 95% CI 1.45–2.50)) were associated with a higher risk of death in the Italian patients than in the Chinese population. The authors also reported that male sex (OR 1.27, 95% CI 1.11–1.46) and an age >60 years (OR 4.63, 95% CI 3.87–5.55) were associated with a higher risk of mortality in the Italian than in the Chinese population and the presence of either of these factors conferred a twofold higher risk of COVID-19 death in Italy than in China (OR 2.01, 95% CI 0.54–7.52, P = 0.00).
The reason for this discrepancy in mortality rates between countries is uncertain but it might reflect population demographics and public health-care strategies. Several studies15,22,23 have reported that increasing age is associated with a higher COVID-19 mortality and the average age of the Italian population is 46.7 years, which is 7 years older than that of the Chinese population24. A study comparing mortality rates by age group in China and Italy reported that 52.3% of the total deaths (n = 850) in Italy occurred in those aged ≥80 years, whereas this age group only accounted for 20.3% (n = 208) of deaths in China25. These data suggest that Italy had a higher COVID-19 mortality rate than China because the Italian population was older than that of China and increasing age is a risk factor for COVID-19 death.
Differences in health-care systems and polices might have also contributed to the higher mortality rate in Italy than that in China. Italy had a much higher number of reported cases than China (219,070 versus 82,918 in the time period 31 December 2019 to 10 May 2020 (ref.26)), which might be related to the higher number of deaths. Furthermore, in Italy, the high number of cases overwhelmed the health-care systems in the worse affected regions of the country, reducing the available resources for those patients who were unwell with COVID-19 (ref.24). The increased number of cases in Italy compared with China is likely to be a culmination of political and health-care policies. Soon after the outbreak of COVID-19, the Chinese government enforced a lockdown of the city of Wuhan and other cities in the Hubei province, employed a travel quarantine, postponed reopening of schools and set up temperature screening checkpoints27. Moreover, the Chinese government mandated the use of face masks in public areas and set up two emergency hospitals that provided 2,400 beds28. In addition, outdoor restriction measures were put into place, whereby only one member of each household was permitted to go outside at scheduled times28. By contrast, the mayor of Milan launched a campaign with the headline ‘Milan don’t stop’, which was designed to encourage social mobility24. Although the first cases of COVID-19 were reported in Italy at the end of January, lockdown of affected areas only occurred at the end of February and schools were not closed until early March24. Thus, despite having more time to prepare and the benefit of China’s experience with the virus, Italy was slow to mitigate the transmission of the virus.
Emerging data from New York have also suggested that age and comorbidities are associated with an increased risk of COVID-19 mortality. Mikami et al.29 studied the clinical characteristics of 6,493 patients in eight hospitals in New York City. They reported that COVID-19 hospital mortality was increased in the 50–74 years (HR 2.34, 95% CI 1.47–3.71, P < 0.001) and ≥75 years (HR 4.85, 95% CI 2.75–8.56, P < 0.001) age groups. Moreover, being female was associated with decreased hospital mortality (HR 0.84, 95% CI 0.77–0.90). A separate study reported the clinical outcomes of COVID-19 at two New York hospitals30. In this study, multivariate Cox analysis showed that older age (adjusted HR 1.31, 95% CI 1.09–1.57, per 10-year increase), chronic cardiac disease (adjusted HR 1.76, 95% CI 1.08–2.86) and chronic pulmonary disease (adjusted HR 2.94, 95% CI 1.48–5.84) were independently associated with hospital mortality.
Within the UK, several large observational studies have investigated factors associated with COVID-19 mortality. The openSAFELY study31 analysed the health records of 17,278,392 National Health Service (NHS) patients, 10,926 of whom died of COVID-19. COVID-19 mortality was associated with male sex (adjusted HR 1.59, 95% CI 1.53–1.65); increasing age (ages 60–69 years (adjusted HR 2.40); 70–79 years (adjusted HR 6.07); ≥80 years (HR 20.60)); social deprivation (adjusted HR 1.79, 95% CI 1.68–1.91); uncontrolled diabetes (adjusted HR 1.95, 95% CI 1.83–2.08); obesity (adjusted HR 1.92, 95% CI 1.72–2.13); chronic heart disease (adjusted HR 1.17, 95% CI 1.12–1.22); liver disease (adjusted HR 1.75, 95% CI 1.51–2.03); stroke/dementia (adjusted HR 2.16, 95% CI 2.06–2.27); rheumatoid/lupus/psoriasis (adjusted HR 1.19, 95% CI 1.11–1.27) and severe asthma (adjusted HR 1.13, 95% CI 1.01–1.26). A prospective observational cohort study in 208 acute hospitals in the UK reported that increasing age was associated with COVID-19 mortality (50–59 years (HR 2.63, P < 0.001), 60–69 years (HR 4.99, P < 0.001), 70–79 years (HR 8.51, P < 0.001) and ≥80 years (HR 11.09, P < 0.001))32. Other factors reported to be associated with increased COVID-19 mortality were chronic cardiac disease (HR 1.16, 95% CI 1.08–1.24, P < 0.001), chronic non-asthmatic pulmonary disease (HR 1.17, 95% CI 1.09–1.27, P < 0.001), chronic kidney disease (HR 1.28, 95% CI 1.18–1.39, P < 0.001), obesity (HR 1.33, 95% CI 1.19–1.49, P < 0.001), chronic neurological disorder (HR 1.17, 95% CI 1.06–1.29, P = 0.001), dementia (HR 1.40, 95% CI 1.28–1.52, P < 0.001), malignancy (HR 1.13, 95% CI 1.02–1.24, P = 0.017) and liver disease (HR 1.51, 95% CI 1.21–1.88, P < 0.001). Female sex was associated with lower mortality (HR 0.81, 95% CI 0.75–0.86, P < 0.001). A population cohort study using data from England assessed whether the presence of diabetes affected the risk of COVID-19 mortality33. The authors reported that the factors associated with increased COVID-19 mortality were of male sex (OR 1.94, 95% CI 1.89–1.99, P < 0.001), increasing age (70–79 years OR 2.64, 95% CI 2.53–2.76, P < 0.001 and ≥80 years OR 9.20, 95% CI 8.83–9.58, P < 0.001), social deprivation (OR 1.88, 95% CI 1.80–1.96, P < 0.001), black ethnicity (OR 1.71, 95% CI 1.61–1.82, P < 0.001), Asian ethnicity (OR 1.35, 95% CI 1.28–1.42, P < 0.001), type 1 diabetes (OR 3.51, 95% CI 3.16–3.90, P < 0.001) and type 2 diabetes (OR 2.03, 95% CI 1.97–2.09, P < 0.001).
Reflecting the data from China, these studies suggest that the main risk factors for COVID-19 mortality and severity are male sex, increasing age, cigarette smoking and certain comorbidities (hypertension, CVD, COPD and diabetes).
Within this context, that men have a higher mortality rate from COVID-19 is unsurprising, given that men have a higher incidence of CVD, hypertension, type 2 diabetes, COPD, and tobacco use than women. A WHO report34 observed that worldwide, the prevalence of smoking is higher for men than for women (40% versus 9%) and that men account for 80% of all smokers. This trend is consistent on all continents, with a higher percentage of men than women being smokers in Africa (33.3% versus 8.2%), Asia (46.1% versus 9.6%), the Americas (37.9% versus 18.0%), Middle East (44.6% versus 9.0%), Eastern Europe (47.2% versus 20.9%) and Western Europe (30.1% versus 23.4%)35. Cigarette smoking is a risk factor for COPD36, CVD37,38 and several malignancies (lung, hepatic, oral cavity, bladder, oesophageal, pancreatic, gastric, renal and lymphoma cancers)39. A 2018 meta-analysis of 156 studies on COPD data reported a higher prevalence of the disease in men than in women (9.23% versus 6.16%)40. Thus, the higher prevalence of cigarette smoking and COPD in men than in women might also predispose to worse COVID-19 outcomes.
The National England and Wales Diabetes Audit41 reported a male predominance in both type 1 (57% versus 43%) and type 2 diabetes (58% versus 42%) diagnoses during 2019–2020. The incidence of type 1 diabetes in childhood has been reported to disproportionately affect men compared with women (55% versus 45%)42,43 and a nationwide Swedish study reported a significantly higher male incidence of type 1 diabetes (20.5/100,000/year) than the incidence in females (12.7/100,000/year, P < 0.001)44. A nationwide Chinese study comprising 46,239 adults observed an age-standardized prevalence of type 2 diabetes at 10.6% among men and 8.8% among women (P < 0.001)45. Moreover, multivariate analysis showed that the male sex was associated with an increased risk of type 2 diabetes (OR 1.26, 95% CI 1.12–1.43, P < 0.001)45. A population study compared the overall rates of diabetes in Ontario, Canada during 1995–2005. The authors observed that the incidence and prevalence of diabetes were higher in men than in women (both P < 0.0001)46. Indeed, the worldwide age-standardized adult diabetes prevalence in men has been reported to be 9.0%, compared with 7.9% in women47.
Furthermore, evidence suggests that men are more susceptible to developing diabetes at a lower BMI than women. Analysis of a diabetes register in Scotland that included 51,920 men and 43,137 women showed that the mean BMI at diagnosis of diabetes was 31.83 kg/m2 in men and 33.69 kg/m2 in women48. Moreover, the authors observed an inverse relationship between age and BMI at diagnosis of type 2 diabetes and the slope was steeper in women than in men (slope estimate for men was −0.12 kg/m2 per year compared with −0.18 kg/m2 per year in women, P < 0.0001).
The cause of these sex discrepancies in diabetes have been speculated to be related to sex differences in insulin sensitivity and fat distribution. MRI-based comparison of visceral and subcutaneous adipose tissue in men and women demonstrated a significantly higher visceral adipose tissue volume (mean litres ± standard deviation) in men than in women in both white (3.40 ± 2.12 versus 1.69 ± 1.24, P < 0.05) and African American (2.48 ± 1.66 vs 1.72 ± 1.03, P < 0.05) cohorts49. Furthermore, females were noted to have a higher volume (mean litres ± standard deviation) of subcutaneous adipose tissue than men in both the white (4.86 ± 2.0 versus 3.92 ± 2.11, P < 0.05) and African American (6.58 ± 3.42 versus 3.92 ± 2.40, P < 0.05) cohorts49. These results have been supported by a separate study that also compared adipose tissue distribution in different sexes using MRI, which showed that women had a significantly higher volume (litres) of subcutaneous adipose tissue than men (39.6 ± 11.6 versus 30.7 ± 7.5, P < 0.01) but significantly lower volumes of visceral adipose tissue and lean tissue (2.5 ± 1.1 versus 4.8 ± 2.1, P < 0.01 and 42.8 ± 4.7 versus 58.2 ± 6.2 l, respectively; P < 0.01)50. A study in which annual measurements of body composition and fat distribution were assessed in 153 women reported that, although increasing age was associated with increased subcutaneous fat (P < 0.001), visceral fat only significantly increased in women who became post-menopausal (80.8 to 101.7 cm2, P < 0.05)51. Animal data have demonstrated that visceral as opposed to subcutaneous adipose tissue confers to metabolic changes that can increase susceptibility to diabetes. For example, in a mouse study, after transplantation of epididymal fat pads into either the parietal peritoneum (draining into the caval or systematic venous system) or the mesenterium (the portal venous drainage system), mice with a fat transplant in the mesenterium had a significantly higher glucose concentration after an intraperitoneal glucose load than the mice that received a fat transplant in the peritoneum (P < 0.001)52. This glucose concentration corresponded with an increase in portal but not systemic IL-6 concentrations. Application of a hyperinsulinaemic–euglycaemic clamp to both sham-operated and fat-transplanted mice resulted in an insulin-induced suppression of endogenous glucose production in sham-operated mice, but this effect was blunted in mice with a mesenterium fat transplant (P < 0.05). Collectively these data suggest that mice with fat pad transplants in the mesenterium developed hepatic insulin resistance and impaired glucose tolerance, which was associated with an increase in cytokine IL-6. Thus, men might develop diabetes at a lower BMI than women because they have a higher proportion of visceral adipose tissue, which might increase their insulin resistance.
Although not specific to sex, male obesity rates are rising and the average BMI has increased by 3.3 kg/m2 within a 39-year period (1975–2014)3,53 This increase is especially relevant as one European study highlighted that obesity was associated with an increased use of mechanical ventilation following COVID-19 infection54. Simonnet et al. investigated the association between BMI and the requirement for invasive mechanical ventilation in patients infected with COVID-19 admitted to the intensive care unit at the Roger Salengro Hospital between 27 February 2020 and 5 April 2020 (ref.54). The authors observed that the patients who required invasive mechanical ventilation had a significantly higher median BMI than those who did not (31.1 kg/m2 versus 27 kg/m2, P < 0.001).
A WHO study reported that, globally, the mean systolic blood pressure (age-standardized estimate) was 127.0 (125.7–128.3) in men compared with 122.3 (121.0–123.6) in women55. In accordance with these data, a study using data from The National Health and Nutrition Examination Survey reported that, in the USA, the prevalence of hypertension was higher in men than in women in all age groups56. However, this gap in hypertension prevalence between the sexes narrowed with age; in the 18–39-year group, the difference in prevalence rate between men and women was 18.2%, whereas in the >60 years group the difference reduced to just 1.3%. Waddell et al. investigated sex differences in age-related stiffening of large arteries in a cohort of 123 participants at a single institution57. The authors observed that the brachial and carotid pulse pressures were significantly lower in young women (mean age 23 years) than in men of the same age (P < 0.01) but significantly higher in women than men in the elderly population (mean age 62 years) (P < 0.05). The same study also compared systemic arterial compliance (SAC) in both sexes and observed no sex difference in SAC in the young cohorts (mean age 23 years) but in the older group (mean age 62 years), women had a lower SAC than men (0.27 ± 0.03 versus 0.57 ± 0.04, P < 0.001). The authors reported that follicle-stimulating hormone (FSH) levels were significantly increased in the older group compared with the younger group and FSH correlated strongly with indices of central arterial function (r = 0.39−0.65). Thus, increasing age is associated with a more pronounced stiffening of large arteries in females than in males and this discrepancy corresponds with changes in FSH.
Sex differences in hypertension prevalence might also be related to lifestyle factors. In addition to the higher prevalence of smoking in men than in women, men also have a higher salt intake. In 2014, the national diet and nutrition survey in the UK reported that men had a mean daily salt intake of 9.1 g/day, whereas women consumed an average of 6.8 g/day58. Moreover, the mean sodium intake in the USA estimated using 24-h urinary excretion was 4,205 mg/day in males and 3,039 mg/day in females59.
The higher prevalence of hypertension, smoking and diabetes might also account for the higher levels of coronary heart disease reported in men than in women. The WHO mortality database reported a fourfold higher coronary heart disease mortality rate in men than in women aged 30–64 years and twofold in those aged 65–89 years60. Furthermore, a study of National Health and Nutrition Examination Survey data reported that the US prevalence of CVD in men was 61,500,000 (51.2% of the population) compared with 60,000,000 (44.7% of the population) in women59. The UK Biobank was a large prospective study of 502,628 participants recruited between 2006 and 2010 (ref.61). The study reported that the incidence rates of myocardial infarction per 10,000 person years were 7.76 (95% CI 7.37–8.16) in women and 24.35 (95% CI 23.57–25.16) in men61. Data also show that men develop CVD at an earlier age than women. In a prospective cohort study from the Netherlands that included 8,419 participants and studied the average age of the first manifestation of coronary heart disease62, men between the ages of 55 and 64 years had a significantly higher lifetime risk of the first manifestations of coronary heart disease than women (27.2 % versus 16.9%, P < 0.001). In the INTERHEART global case–control study, which included 27,098 participants from 52 countries, the median age of the first acute myocardial infarction was higher in women than in men (65 versus 56 years; P < 0.0001)63. This delay in CVD onset has been attributed to the cardioprotective effects of oestrogen in premenopausal women. Several studies have shown that bilateral oophorectomy confers a higher risk of CVD than an age-matched control group of women who have not undergone the procedure64,65. Rivera et al.64 reported that women who underwent bilateral oophorectomy before the age of 45 years had an increased mortality associated with CVD compared with age-matched controls (HR 1.44, 95% CI 1.01–2.05, P = 0.04). Furthermore, those treated with oestrogen replacement therapy following surgery and up to the age of 45 years or longer had no significant increase in mortality compared with a control cohort (HR 0.65, 95% CI 0.30–1.41, P = 0.28). However, women who had not received oestrogen treatment or did not continue the treatment up to the age of 45 years were noted to have an increased mortality compared with a reference cohort (HR 1.84; 95% CI 1.27–2.68, P = 0.001). A meta-analysis66 reported that the risk of CVD was higher in women who had premature menopause (defined as menopause at <40 years; HR 1.55, 95% CI 1.38–1.73, P < 0.0001), early menopause (defined as at age 40–44 years; HR 1.30, 95% CI 1.22–1.39; P < 0.0001), and relatively early menopause (defined as at age 45–49 years; HR 1.12, 95% CI 1.07–1.18; P < 0.0001), compared with those who underwent menopause aged 50–51 years. Oestrogen has been observed to stimulate vasodilation and maintain endothelial integrity in vascular injury, preventing the development of atherosclerotic plaques67. Given that atherosclerosis is a chronic process, it is not surprising that early menopause is associated with CVD. Hence, oestrogen might be cardioprotective for women and, therefore, make men more susceptible to CVD.
Thus, overall, men seem to be more susceptible to death from both non-communicable diseases and COVID-19 because they are at a higher risk of underlying CVD, respiratory conditions and other major risk factors for atherosclerosis than women. This increased risk is due to a combination of biological factors, such as the beneficial effects of oestrogen in women, but might also be attributable to an increased incidence of lifestyle and modifiable risk factors in men versus women.
The sex gap in health-care utilization
The sex gap in COVID-19 is currently making headlines, but, notably, differences in life expectancy according to sex were reported as early as the nineteenth century68 and still persist, despite the establishment of focused Men’s Health charities (for example, the Men’s Health Forum, which is aimed at improving both men’s health and male health services69) and equality legislation (such as The Equality Act of 2006, which mandated all public health sectors to promote gender equality70)71. Globally, men’s life expectancy is 5.1 years lower than women’s72. A 2018 WHO report highlighted that, within Europe, this gap in life expectancy is due to a higher frequency of premature deaths (deaths occurring between the ages of 30 and 69 years) as a result of CVD, cancer, chronic respiratory diseases and diabetes3,73.
A further contributing factor is the utilization of health-care services by men. Women attend primary care services more frequently than men but increased hospital admissions in men have been reported4,74. A cross-sectional study of 446 UK General Practices reported that the crude consultation rate was 32% lower in men than in women and the greatest sex gap in primary care consultations was seen among patients aged between 16 and 60 years75.
Although overall admission rates for the NHS within the period 2018–2019 were higher for females than for males, when analysis is limited to patients aged between 45 and 84 (which excludes maternal services) the hospital admission rate for men was marginally higher than that for women (5,872,710 versus 5,830,144)76. However, the overall proportion of patients requiring critical care admission was higher in men than in women (57% versus 43%)76.
An Australian survey77 assessing health attitudes and behaviours of 1,456 adults reported that women were more likely to have their blood pressure checked regularly than men (72% versus 60%, P < 0.001) and women were more likely to be aware of the influences of disease prevention strategies, lifestyle and genetics on health (P < 0.01). Moreover, women were more inclined than men to pursue advice on disease prevention (94% versus 89%, P < 0.001), and more likely to participate in health prevention promotion strategies (P < 0.001). A significantly higher number of men than women indicated that they were not interested in receiving information on illness prevention (12% versus 6%, P < 0.001). In a US study, Green et al.78 performed a household interview survey for members of a non-profit health maintenance organization. This study included 2,603 (1,401 female and 1,202 male) participants and the authors reported that, although women are more likely to report mental (P < 0.001) and physical (P < 0.001) health symptoms than men, self-reported measures of health concerns were not significantly different. However, women were observed to have significantly higher measures of interest in their health than men (P < 0.001).
The QUALICOPC study, which was conducted in Canada in 2013, investigated the health-care seeking practices and behaviours of 7,260 primary care patients. Thompson et al.79 studied patient experiences as reported in survey questionnaires, finding that women reported that they would consult their primary care doctor more readily than men in response to both mental (P < 0.001) and physical (P < 0.001) concerns. Multiple regression analysis demonstrated that age (P < 0.001), trust in their physician (P < 0.001), presence of chronic conditions (P = 0.001) and patient confidence in their own ability to prevent illness (P = 0.001) were significantly associated with increased male medical consultation rates for mental health concerns. However, none of the aforementioned factors was able to predict the male medical consultation rate for physical health concerns.
A literature review based on Centers for Disease Control and Prevention data on male health-care utilization in the USA reported that more men than women (22.8% versus 11.8%) did not attend a doctor’s surgery, emergency department or have a doctor home visit during 2006. Moreover, women were more likely to attend preventive care visits than men (74.4 visits per 100,000 versus 44.8 visits per 100,000)80.
Furthermore, The UK bowel screening programme was reported to have a significantly higher female uptake than male (54.4% women and 49.6% of men, P < 0.00001)81, which suggests that men are less likely to engage in health-care programmes than women.
Masculinity conventions
Underutilization of health-care services by men has been associated with masculinity conventions of self-reliance, strength and control3,82, which have also been implicated in high male suicide rates (in 2019, the global age-standardized suicide rate was 12.6 per 100,000 men compared with 5.4 per 100,000 women73,83,84. In a study that performed focus interviews with men to identify attitudes and behaviours to health care, the authors noted that participants did not value health-care utilization as a “typical” male activity and that many men ignored health issues because they conceived them as a “failure”, disclosure of which demonstrated vulnerability85. However, the same study also illustrated some divergent views, and some men reported considering health-care initiatives or lifestyle changes as feminine, whereas others had started health-promoting activities (for example, going to rehabilitation sessions). Overall, the consensus in the group was that hegemonic masculinity conventions were intrinsically linked with attitudes to health care, but, overall, insufficient services were tailored for men’s health-care needs, including disease screening.
A separate study, which conducted semi-structured interviews of 6 men diagnosed with testicular cancer in Scotland found that the vast majority of patients had not practised testicular self-examination before their diagnosis, which was attributed to ignorance of the examination process86. One man was reluctant to seek help when he noticed a testicular lump owing to embarrassment and concerns about feeling foolish if nothing was wrong. Another participant expressed reluctance to seek help because they were afraid to discuss or be examined in a private and sensitive area. Although this study is limited by the small sample size, it does raise some important issues. Many men do not practice testicular self-examination — not necessarily owing to machismo or masculine attitudes, but rather owing to misunderstanding of how to perform the examination. Moreover, many men do not seek help as they fear being embarrassed and vulnerable. Thus, men’s attitudes are not homogeneous and many other reasons — rather than simply hegemonic masculinity conventions — might explain an underutilization of health-care services.
Health-care infrastructure
The infrastructure of the health-care system itself might also be a contributing factor to the underutilization of health resources. A meta-analysis of 10 studies analysing health-care provider behaviours in medical encounters showed that patient compliance (defined as appointment keeping or compliance with treatment regimen) correlated positively with the amount of information (general discussion, overview of drugs treatment, procedures and examination, and specific details regarding the illness) provided by the health-care provider (P < 0.0005)87. Notably, health-care providers communicated less (Pearson’s r 0.15, P < 0.0005) and provided less information to male than to female patients (Pearson’s r 0.22, P < 0.01). In accordance with these data, a study88 using data from the American national ambulatory medical care survey (a survey of visits made to office-based physicians in the USA) observed that females were more likely to receive a general examination and/or medical history (P < 0.001), blood pressure check (P < 0.001), blood tests (P < 0.001) and drugs prescriptions (P < 0.05) than men. Interestingly, these trends persisted after controlling for confounding factors such as patient age, diagnosis and illness severity. Furthermore, an assessment of preventive medicine practice amongst 116 primary care physicians showed that only 29% of doctors were likely to instruct male patients to perform a testicular examination, compared with 86% who would advise female patients to perform a regular breast examination89. Why these differences arise is unclear and no studies have analysed contributing factors; the causes might be physician sex bias, patient requests for care and institutional or societal conventions (for example, a lack of health policies and/or tariffs targeting the male sex or masculinity stereotypes of self-reliance dissuading men from requesting tests and treatments from their physicians). Further research is needed to clarify the factors causing sex differences in medical care provision; one method of ameliorating these discrepancies would be to target the male sex through specific health programmes.
Unlike women, where sex-specific concerns are handled by gynaecologists, men lack a reference medical speciality that is analogous to gynaecology and do not benefit from gender-specific health-care programmes (such as cervical and breast cancer screening). Andrology services are available only in selected hospitals and typically focus on a small number of male-specific conditions, such as erectile dysfunction (ED), male infertility and testicular cancer. This contrasts with gynaecological services, which are present in most hospitals and manage female infertility, lower urinary tract symptoms (LUTS), ovarian, endometrial and cervical cancer, and a range of disorders related to menstruation, menopause, family planning, sexual dysfunction and sexually transmitted infections. No male-specific screening programmes are run within the UK and, although prostate cancer screening is practised in some countries90, the diagnostic pathways are variable and can comprise only a PSA blood test and prostate biopsies, despite the publication of both the PROMIS91 and the PRECISION92 trials that highlighted that non-parametric MRI increases the detection of clinically significant prostate cancer. A European Association of Urology (EAU) white paper on prostate cancer93 highlighted the main criticisms of prostate cancer screening, which are the overdiagnosis and potential overtreatment of prostate cancer, but this is a health-care provider interpretation and there are no large studies on the patient perspectives of overdiagnosis. Thus, further research is needed to determine whether prostate cancer screening using MRI, PSA testing and prostate biopsies is financially feasible, acceptable to patients and reduces prostate cancer mortality. Given that prostate cancer has the third highest cancer incidence globally and contributed to 3.8% of all cancer deaths in 2020 (ref.94), a new approach to detecting early prostate cancer is needed and represents a potential male health screening programme that can reduce male mortality.
Health literacy
The influence of these institutional systems is arguably even more important when we consider that increasing evidence suggests that men have worse health literacy than women. Health literacy is defined as the cognitive and social skills that determine the motivation and ability of individuals to gain access to, understand and use information in ways that promote and maintain good health95,96. A 2007 study assessed health literacy in 759 adults in the UK using a modified Test of Functional Health Literacy in Adults97. The authors observed that higher scores on the health literacy scale were associated with a greater likelihood of eating at least five servings of fruit and vegetables a day (OR 1.02, 95% CI 1.003–1.03) and non-smokers (OR 1.02, 95% CI 1.0003–1.03). Men were more likely than women to fall into the limited health literacy category (OR 2.04, 95% CI 1.16–3.55, P < 0.05). A 2015 study included a cross-sectional survey assessing the health literacy of 585 Korean adults using a self-reported health literacy questionnaire. The authors reported that Korean men were more likely to have an inadequate understanding of how to understand and fill out medical forms (P = 0.037) and also more likely to have more difficulty understanding the directions on medication bottles than women (P = 0.023). Moreover, women were noted to have a better understanding than men of documentation from a health-care provider (P = 0.007). In an indirect measurement of health literacy, a population-based survey of 2,216 adults to assess public awareness of cancer warning signs in a British population found that women recognized more red flag symptoms of cancer than men (7.4 versus 7.0, P < 0.001)98.
These studies suggest that men have a lower level of health literacy than women, which might translate to poorer lifestyle choices with regard to health and also unawareness of potential signs of serious diseases such as cancer. This health illiteracy might be contributing to men’s underutilization of health services, as they might be unaware of what services are available, the symptoms and signs that would warrant seeking medical advice and the benefits of lifestyle changes and optimization of morbidities. Thus, one might argue that men are a more vulnerable population than women owing to their health illiteracy and should be targeted to improve their understanding and uptake of health services.
Thus, in addition to the effects of comorbidities and CVD risk factors, men are less likely to use health-care resources than women and this reluctance can be linked to structural barriers, health literacy and personality subtypes. Although male reluctance to use health-care services has been attributed to conventions of masculinity, this theory is an oversimplification and some men do not seek medical care because of fear rather than a show of strength.
Intersectional analysis
Increasing amounts of research are evaluating how social determinants, gendered cultural norms and expectations, and environmental factors can shape male health behaviours and use of health-care services99,100,101,102,103,104. These factors seldom act in isolation; thus, intersectional analysis is aimed at identifying how social, cultural, contextual and identity aspects can affect health outcomes103. A number of intersectional studies have highlighted how psychosocial factors in conjunction with demographic factors can confer an increased risk of health issues in men. For example, a cross-sectional study investigating psychosocial factors associated with HIV risk in men who have sex with men illustrates this concept105. The study involved 2,881 telephone interviews from participants in 4 US cities (New York, Los Angeles, Chicago and San Francisco) and reported that drug use (OR 2.2; 95% CI 1.7–2.8, P < 0.05) and partner violence (OR 1.5, 95% CI 1.2–1.9, P < 0.05) were associated with HIV seropositivity. Moreover, an increased number (OR for one problem 1.8 (95% CI 1.4–2.3), two problems 2.7 (95% CI 2.0–3.6), ≥ three problems 2.2 (95% CI 1.4–3.5, P < 0.001) of psychosocial health problems (multiple drug use, depression, childhood sexual abuse and partner violence) were associated with increasing prevalence of HIV infection. A separate study104 analysed data from 8,490 gay, bisexual and other (those not defining themselves as either gay or bisexual) men who have sex with men in Canada. The study investigated associations with demographics and psychosocial factors (specifically recreational drug use, the weekly practice of alcohol binge drinking; suicidal ideation or attempts, anxiety and/or depression necessitating mental health treatment). Multivariable analysis demonstrated a significant association (P < 0.05) between sexuality (specifically, identifying as gay rather than bisexual (the authors did not report any associations with the ‘other’ cohort of men); adjusted OR 1.68, 95% CI 1.37–2.05), being ≤45 years old (<30 years: adjusted OR 1.51, 95% CI 1.27–1.80; 30–45 years: adjusted OR 1.36, 95% CI 1.15–1.63), absence of a university degree (adjusted OR 1.19, 95% CI 1.02–1.38), a salary of <$60,000/year (adjusted OR 1.32, 95% CI 1.22–1.554), and the presence of two or more psychosocial issues.
However, intersectional analyses have been criticized, because they assume that different factors lie in isolation and are additive when, in fact, they might be intrinsically linked; for example, ethnicity and gender are separate entities, even though both are likely to contribute together to influence health behaviours99. Furthermore, some frameworks of intersectional analysis focus on individuals rather than populations or communities and, therefore, the applicability of such data in health policy is questionable99. Newer intersectional analysis models are emerging (such as the intersectional uniqueness paradigm), but these models lack an extensive body of supporting literature99.
Are men inherently vulnerable to infection and mortality?
Although case numbers of COVID-19 are roughly equal between men and women, mortality rates are higher in men (Fig. 1). Both biological and genetic mechanisms could be contributing to the disproportionate male mortality from COVID-19.
Immunology
The mortality rate associated with viral infections in general is higher in men than in women106; this discrepancy has been related to differences in immunological responses between sexes. Sex differences exist in both the innate and adaptive immune response. Interferon-α (IFNα) is required for immunological defence against viral infections, in which it acts via activation of dendritic cells, stimulation of IFNγ and activation of both CD8+ T cells and natural killer cells107. A sex-dependent pathway has been observed for the induction of IFNα, whereby production of IFNα by peripheral blood leukocytes in blood samples from women was higher than in men after stimulation with a TLR7 ligand (P < 0.0000001)107. Likewise, assessment of sex-related differences in cytomegalovirus (CMV) cytokine secretion in healthy CMV-seropositive men and women revealed that the median IL-2 concentration in response to CMV was significantly higher in females than in males (60 pg/ml versus 31.5 pg/ml, P = 0.001). Furthermore, the female cohort had a higher proportion of responders (patients with an IL-2 concentration >25 pg/ml) — 95% of women and 60% of men (P = 0.02)108.
An in vitro study reported that cultured peripheral blood mononuclear cells from females stimulated with phytohaemagglutinin produced significantly more B cells (29.6 ± 3.6 versus 23.8 ± 4.3, P < 0.05) and total T lymphocyte cells (79.0 ± 1.3 versus 73.4 ± 1.9, P < 0.05) than peripheral blood mononuclear cells from men109. These data suggest that females produce a greater humoral response than males; this conclusion has been supported by vaccination studies that have observed that women produce higher antibody responses. For example, a study comparing sex differences in response to the influenza vaccination reported that women given the full dose of the vaccine produced higher concentrations of serum haemagglutination inhibition antibody than men for all the influenza virus strains110. Furthermore, the same study observed that women given half the dose of the influenza vaccination produced comparable antibody responses to men given the full dose of the vaccine (25.4 versus 25.6)110. Similarly, geometric mean titres of haemagglutination inhibition after influenza vaccination were significantly higher in females than in males (110.7 versus 67.5, P < 0.0002)111. Females have also been shown to produce significantly higher antibody titres in response to the hepatitis A vaccination (P < 0.01)112 and smallpox vaccination (158.5 versus 124.1, P < 0.0001)113.
The X chromosome and immunity
The X chromosome encodes genes, such as TLR7 and TLR8, FOXP3 and CD40L, that are involved in recognizing viral pathogens, regulation of T cells and immunoglobulin class switching114. Males possess only one X chromosome, which is inherited from their mother, whereas females carry and express two X chromosomes. The additional X chromosome in women is thought to provide increased immunological protection compared with the single X chromosome expressed in men115. The X-linked glycosylated 91-kDA glycoprotein gene (gp91phox) is a subunit of nicotinamide adenine dinucleotide phosphate oxidase complex, which is integral to the production of microbicidal reactive oxygen species116,117. In a comparison of responses of heterozygous (±) mice, gp91phosx-deficient mice (−/−) and wild-type mice (+/+) (all female, to control for the confounding effects of sex hormones) to polymicrobial sepsis initiated by caecal ligation and puncture, wild-type animals had the highest mortality (10% overall survival), significantly lower than both mosaic (50% overall survival) and gp91phosx-deficient mice (40% overall survival) (P < 0.05)118. The longest median survival time (100 h) was observed in heterozygous mice, whereas gp91phosx-deficient and wild-type mice had a median survival time of 65 h and 40 h, respectively. The gp91phosx-deficient mice cohort had a significantly higher concentration of circulating blood neutrophil numbers than the wild-type and heterozygous groups (P < 0.05) and caecal ligation and puncture resulted in a significant decrease in circulating CD4 and CD8 T cells in wild-type and gp91phosx-deficient mice, but not in the heterozygous mice (P < 0.05). Furthermore, sepsis caused a decrease in bone marrow B cells in gp91phosx-deficient and wild-type mice but not heterozygous mice (P < 0.05). Following caecal ligation and puncture, serum levels of IL-6 were lower in heterozygous and gp91phosx-deficient mice than in wild-type animals (P < 0.05), but lung concentrations of IL-6 were significantly higher in heterozygous subjects than in wild-type or gp91phosx-deficient mice (P < 0.05). This study highlights how cellular mosaicism for gp91phox confers a different immunological response and reduced mortality rate following polymicrobial sepsis compared with wild-type or deficient states.
Irak1 is another X-linked immunomodulation gene that is involved in regulating inflammatory signalling pathways; data suggest that Irak1 deficiency is associated with a decrease in sepsis mortality119. Comparison of mortality rates and cytokine responses in Irak1-deficient mice, mice with Irak1 cellular mosaicism and wild-type mice following caecal ligation and puncture showed that wild-type mice had a significantly higher mortality rate than Irak1-deficient and Irak1-mosaic mice (P < 0.01). Moreover, serum, lung and splenic IL-6 concentrations were significantly lower in Irak1-mosaic mice than in wild-type subjects (P < 0.05). No statistical differences were observed in bacteraemia among the groups, suggesting that the difference in mortality rates between mice cohorts was related to differences in immunological and inflammatory responses rather than bacterial load. Thus, cellular mosaicism for IRAK1 related to the presence of two copies in females with two X chromosomes might offer immunological protection compared with single X chromosome gene representation.
Taken together, these two animal studies suggest that cellular mosaicism for X-linked genes and, therefore, the additional X chromosome in women, might provide immunological protection.
Receptor expression
The SARS-CoV spike protein binds to the ACE2 receptor on ACE2-expressing cells to facilitate cell entry and viral replication120,121. Virus infectivity studies using SARS-CoV-2 on HeLA cells showed that SARS-CoV-2 used ACE2 as a cellular entry point in humans120. Furthermore, anti-serum against human ACE2 inhibited both SARS-CoV-2 and SARS-CoV spike proteins122. Thus, as ACE2 is probably the entry point for SARS-CoV-2 into cells1, sex disparities in ACE2 could be clinically relevant. A comparison of serum ACE2 concentrations between sexes in a cohort of patients with heart failure (n = 2,022), reported that men had a significantly higher mean serum ACE level than women (5.38 versus 5.09, P < 0.001) and that male sex was the strongest predictor of elevated plasma concentrations of ACE2 (P < 0.001)123. The authors postulated that the higher serum concentrations of ACE2 in men than in women might explain the sex discrepancies seen in COVID-19 mortality. However, the conclusions that can be drawn from this study are limited, as it only analysed patients with heart failure and did not include patients with COVID-19. An analysis of five large-scale bulk transcriptomic datasets of normal lung tissue and two single-cell transcriptomic datasets from patients with lung cancer showed no significant differences in ACE2 gene expression between racial groups (Asian versus white), age groups (>60 years versus <60 years) and sex (male versus female)124. Interestingly, data from a rat study that compared lung ACE2 expression in male and female rats at three distinct ages (3 months, 12 months and 24 months) reported no sex differences in ACE2 expression in the 3-month and 12-month cohorts, but a significantly higher ACE2 level in female rats aged 24 months than in age-matched males (P < 0.05)125. This difference could reflect an age-related change in ACE2 expression that has not been investigated in humans, which might explain why children are less susceptible to severe disease than adults. For example, a retrospective case series of 2,135 children with COVID-19 reported to the Chinese Centre for Disease Control and Prevention between 16 January 2020 and 8 February 2020 reported that only 6% of cases were severe126. In accordance with this study a retrospective case series127 of 1,099 patients infected with COVID-19 hospitalized in China between 31 December 2019 and 20 January 2020, reported that only 0.7% of those diagnosed with severe COVID-19 were aged less than 15 years and the median age of the patient cohort with severe COVID-19 was 52 years.
Smoking status has also been reported to affect ACE2 expression124, with higher ACE2 expression reported in lungs from former smokers than from non-smokers’ lungs (P = 0.04). As men tend to smoke more than women (with a global discrepancy of 35:6), this observation might explain some of the sex discrepancies in COVID-19 mortality128, whereby a higher rate of smoking in men than in women might increase ACE2 expression and, consequently, increase susceptibility to and mortality from COVID-19.
Endocrine factors
Transmembrane protease serine 2 (TMPRSS2) is a cell surface protein that has been shown to be essential for viral entry and replication of the SARS-CoV-2 virus by facilitating the priming of the spike proteins of the virus129. TMPRSS2 is expressed on the surface of type II pneumocytes in human lung tissue129 and studies evaluating the transcription of TRMPRSS2 in prostate cancer cells have shown that TRMPRSS2 expression is regulated by androgens and promoted through the androgen receptor130. Furthermore, men with castrate levels of testosterone through treatment with either the luteinizing hormone (LH)-releasing hormone agonist leuprolide or oestradiol had significantly lower levels of TMPRSS2 transcripts than untreated men (P < 0.01)130.
Thus, androgens have been postulated to contribute to the severity of COVID-19 infection, providing a mechanism by which men are more likely to become severely ill with COVID-19. A study of 4,532 men with laboratory-confirmed SARS-CoV-2 infection from 68 hospitals in Veneto, Italy noted that when the data from these patients were considered within the context of the male population of Veneto (2.4 million), 0.2% of men without cancer and 0.3% of men with cancer tested positive for COVID-19. Furthermore, a sub-analysis of this study showed that men with prostate cancer who received androgen deprivation therapy had a significantly lower risk of SARS-CoV-2 infection than those not receiving androgen deprivation therapy (OR 4.05, 95% CI 1.55–10.59) or other types of malignancy (OR 4.86, 95% CI 1.88–12.56). These data highlight that androgens could also increase susceptibility to COVID-19 infection, which would make men at an increased risk of acquiring the infection.
This hypothesis was supported by data from a murine study, which showed that male mice are at a higher risk of mortality from SARS-CoV than female mice131, in which administration of SARS-CoV led to significantly higher mortality in male mice than in female mice (90% versus 20%, P < 0.001). Furthermore, ovariectomy of the females significantly increased SARS-CoV mortality compared with intact female controls, whereby 85% of ovariectomized mice died compared with just 20% in the control cohort (P < 0.01). These data suggest that oestrogen confers a protective effect against SARS-CoV; extrapolation of these findings to the COVID-19 variant might explain the some of the sex disparities in COVID-19 mortality. However, no studies have examined COVID-19 outcomes in human patients taking hormone therapy, so retrospective analysis of whether COVID-19 differentially affects either women taking oestrogen receptor antagonists or men taking exogenous oestrogen would be interesting.
Evidence suggests that 17β-oestradiol regulates many aspects of the innate and adaptive immune systems, including stimulation of pro-inflammatory cytokines, increasing neutrophil concentrations and promoting the differentiation of bone marrow precursor cells and monocytes into dendritic cells132. By contrast, androgens have been observed to suppress the immune system; in vitro studies in cultured macrophages have reported that testosterone reduces the synthesis of TNF and nitric oxide by macrophages133. Moreover, androgens have been observed to increase levels of IL-10, which has anti-inflammatory properties and which, therefore, limit the host immune response to pathogens133,134 Furthermore, testosterone has been observed to reduce numbers of CD8+ T cells132. In accordance with these data, men with idiopathic hypogonadotropic hypogonadism have been reported to have significantly higher serum levels of the pro-inflammatory cytokines135 IL-4 (13.19 pg/ml versus 5.33 pg/ml, P < 0.001) and IL-2 (21.22 pg/ml versus 13.01 pg/ml, P < 0.001) than patients with a normal hypothalamic–pituitary–gonadal axis136. Treatment with human chorionic gonadotrophin (hCG) and human menopausal gonadotropin (hMG) in these patients corresponded to an increase in testosterone (1.10 pg/ml versus 25.30 pg/ml, P < 0.001) and significant reductions in IL-2 and IL-4 (P < 0.001) when comparing pre-treatment and post-treatment levels136. In a randomized, single-blind, placebo-controlled crossover trial of testosterone replacement therapy (TRT) versus placebo in 27 symptomatic hypogonadal men (total testosterone 2.2 nmol/l)137, TRT was associated with a decrease in TNF in patients taking TRT (5.77 versus 2.9, P = 0.02) compared with untreated patients.
Other evidence for sex disparities in viral infection related to hormone levels comes from a study that compared sex differences in mice inoculated with herpes simplex virus type 1 (HSV-1) administered into the cornea138. In this study. the authors observed that HSV-1 periocular and eyelid disease was more severe in male mice and dihydrotestosterone-treated female mice than in control female mice (P = 0.026). These data highlight that HSV-1-related complications are worse in males than females and that this effect seems to be related to testosterone levels, as females treated with dihydrotestosterone had worse outcomes than control female mice not given this treatment.
A separate study investigated sex differences in mice exposed to the coxsackie B3 virus (CVB3)139. Following CVB3 infection, mortality rate or moribund rate that necessitated euthanasia of the animals was significantly higher in male than in female mice (50% versus 0%, P < 0.01). Furthermore, male mice were reported to have a higher rate of myocarditis than female mice following CVB3 infection (P < 0.005), but gonadectomy caused female mice to have a higher rate of myocarditis than males (P < 0.00005). Thus, sex hormones might have a role in the development of myocarditis in CVB3 infection.
Collectively, these data highlight that sex differences in hormones can result in altered immunological responses, which might also account for sex differences in COVID-19 outcomes.
Effect of COVID-19 on testicular function
ACE2 has been observed to regulate COVID-19 entering human cells120,122 and is highly expressed in the testis. Single-cell RNA sequencing studies have demonstrated that ACE2 is predominantly enriched in spermatogonia, Leydig cells and Sertoli cells140, which suggests that the testis is a potential target for COVID-19 infection; however, no human or animal studies have yet been performed. Although speculation around the effect of COVID-19 on male fertility is premature, some (albeit limited) data show that COVID-19 infection in humans might affect the hypothalamic–pituitary–gonadal axis. Comparison of sex hormone profiles in 81 men diagnosed with COVID-19 at Wuhan Leishenshan hospital with 100 age-matched healthy men141 showed that COVID-19 infection was associated with a higher level of serum LH than was seen in the control cohort (LH (mIU/ml) median value 5.93 versus 3.28, P < 0.0001)141. Although no significant difference was seen in testosterone levels between the two cohorts, the authors speculated that the increased LH was due to a positive feedback effect of COVID-19 causing reduced testicular function.
Ruan et al.142 compared the semen analyses of 55 men who had recovered from COVID-19 infection (the median interval between last positive pharyngeal swab reverse transcription PCR test and semen samples collection was 80 days (IQR 64–93 days)) with 145 age-matched healthy control patients. The authors reported a significantly lower sperm concentration (66.41 versus 81.34, P = 0.0428) and total motility (48.89 versus 56.38, P < 0.001) in those with a history of COVID-19 than in the control cohort. However, both sperm concentration and total motility for both cohorts were above the WHO-recommended reference ranges143 for these parameters and it is, therefore, unclear whether COVID-19 affects male fertility.
Thus, COVID-19 infection could have longstanding implications for young men of reproductive age.
An impetus for social change
One of the enduring lessons of the pandemic is the way in which it has magnified current health care inequalities and highlighted the urgent need for reform. Health-care systems — both historically and currently — have failed the male sex. Further investment needs to be made to improve health-care engagement and to target potentially vulnerable populations. Data are available to highlight that men not only have a lower life expectancy but also have a poorer quality of life than women4; collectively, these factors are likely to be contributing to worse COVID-19 outcomes.
Confronting these political and health-care issues is our professional and ethical duty. Men’s health has been somewhat overshadowed by efforts to improve women’s health, both in health-care policies and in sponsored programmes. For example, The Gates Foundation has a maternal, newborn and child health strategy but no specific strategies related to men’s health144. And the Gates Foundation gender equality strategy is entirely focused on removing barriers in women’s and girls’ health, income and education145,146 The WHO have developed two global strategies to promote women’s health (‘Every Women Every Child’ (2010) and ‘The Global strategy for women’s, children’s and adolescents’ health’ (2016–2030)147) but none specifically targeting men’s health. Moreover, the WHO ‘Gender, health and the 2030 agenda for sustainable development’ seems to be female orientated, as the major health indicators specified include maternal mortality and coverage of essential health services including reproductive and maternal health, but no reference is specifically made to men’s health147.
This focus primarily on women’s health could, paradoxically, have negative implications for women, as studies have shown that the loss of a husband is linked to both psychological and emotional distress in widows and has adverse effects on physical health, including an increased mortality risk148. Furthermore, in the USA, more than half of all elderly women living in poverty became poverty-stricken following the loss of their spouse, illustrating the economic ramifications of premature male death on their female partners146. Similarly, studies in Australia have reported that widowhood is associated with an increased risk of poverty for women and the UK retirement survey reported that the loss of a partner had negligible effects on income for men but resulted in a decline to 61% of previous earnings for women149. Indeed, the loss of a husband can have implications beyond financial status and in India, widows have been reported to face “social stigmatisation and exclusion”150 including restrictions to employment owing to caste restraints150. Thus, the death of a husband can result in the loss of the breadwinner in some families and financial and social decline. However, a woman’s reliance on a husband for financial and social support suggests sexual inequality and, therefore, this discrimination against women should also be addressed within society.
The process of improving male engagement should be achieved through a structured men’s health-care programme. For example, the Football Fans in Training (FFIT) programme was a randomized controlled trial that assessed the efficacy of a male-specific weight loss programme in the setting of professional football clubs. The authors reported a significant improvement in the intervention group compared with the control group with regard to blood pressure (mean difference from baseline: −7.50 mmHg versus −3.50 mmHg, P < 0.0001 in systolic pressure and −3.710 mmHg versus −3.10 mmHg, P < 0.0001 in diastolic pressure) and BMI (mean difference from baseline: −1.87 kg/m2 versus −0.14 kg/m2, P = 0.0003)151.
The FFIT programme was found to offer sustained beneficial effects and a follow-up study reported that at 3.5 years significant improvements were seen in the intervention group in terms of blood pressure (mean difference from baseline: −3.13 mmHg (95% CI −5.15 to −1.11), P = 0.0080 in systolic pressure and −1.56 mmHg (95% CI −2.80 to −0.32), P = 0.308 in diastolic pressure) and BMI (mean difference from baseline: −0.96 kg/m2 (95% CI −1.31 to −0.60), P < 0.001)152.
In a separate study, a men’s health promotion strategy shaped like a mechanic’s workshop called ‘The Men’s Health Pitstop’ was shown to potentially increase men’s engagement with health care. In this study, a nine-station health assessment programme was developed that was centred around automobiles, for example, the mental health station was staffed by a psychologist and the theme was ‘shock absorbers’. The authors observed that 40% of participants had initiated contact with their GP following the Pitstop programme, and 89% had intentions to contact their GP. Furthermore, 57% of the cohort reported making health changes as a result of the programme153. However, this study included only 315 men who were recruited from an Australian Farming event; thus, the cohort size and demographic mean that extrapolating these findings to the general male population is difficult.
Evaluative evidence regarding men’s health programmes that employ hegemonic masculine stereotypes153 is lacking, but clearly assuming that all men enjoy sports or cars is naive and a policy centred on masculinity values might, therefore, alienate many men. Further research is needed to identify the most suitable means of engaging men and their health; however, specifically targeting and engaging men seems to be a good starting platform. The Movember campaign has generated over £346 million to fund over 800 men’s health-related projects in 21 different countries154.
National programmes focused on men’s health have proven successful. Ireland adopted a National Men’s health programme into their health budget, which focused on developing health promotion initiatives to support men to adopt healthier lifestyles155. The National Men’s health policy and action plan in Ireland developed health promotion initiatives that were sex specific and aimed at optimizing the delivery of health-care services with a focus on increasing male engagement. For example, the ‘Farmers Have Hearts’ initiative offered free CVD screening and risk factor counselling for men within the rural county of Roscommon in Ireland and resulted in a reduction in the prevalence of hypertension (56% to 40%) and high cholesterol (61% to 39%)156 in the time period 1 January 2007 to 31 December 2007 (ref.155). Moreover, this programme also included an impetus to produce male-specific health literature and paraphernalia (for example, ‘Men’s Health Matters: A Practical Guide to Healthcare for Men [2011]’) that was aimed at educating and addressing male health issues155. The National Centre for Men’s Health was created in 2008 to develop and coordinate men’s health research in Ireland and a men’s health training programme was established to optimize engagement of men in both health-care and social services157. This programme has prompted research into several aspects of men’s health, including male cancers158 and male depression and suicide159. Notably, male life expectancy in Ireland has risen a remarkable 6.4 years over the past 21 years155, illustrating how a men’s health-care programme can be a tangible method of improving the longstanding issue of premature death in men.
Project Brotherhood is a community–academic partnership in Chicago that was developed to address the health and psychosocial needs of African American men160. The group was aimed at providing health services including doctor consultations and public health and development support, such as fatherhood and manhood classes and access to free condoms, in non-medical locations such as gyms and barber shops. The underlying ethos of this community project is to empower African American men through “evidence-based practice, Afrocentric culture integration and a holistic approach to health”160. Although no scientific data are available regarding the effectiveness of this organization at improving male health utilization and outcomes, it highlights a potential holistic model that targets male culture in order to optimize health.
The urologist and men’s health
The WHO defines health as “complete physical, mental and social wellbeing and not merely the absence of disease or infirmity”161. Thus, premature male death must be approached with a holistic view. Notably, several urological disorders — including ED — have been associated with a risk of CVD and cancers in men3, which positions the urologist as a potential gatekeeper of men’s health.
Urological disorders as early signs of systemic disease
Engaging and targeting men with sexual and reproductive health problems might facilitate early diagnosis and treatment of occult disease. For example, ED is recognized as one of the first signs of occult atherosclerotic disease162. A meta-analysis of 13 studies comprising 91,831 participants reported that the relative risk of CVD events in men with ED was 1.44 (95% CI 1.27–1.63) compared with men without ED163. The Massachusetts male aging study164 observed that the incidence rate of ED was 12.4 and 29.8 per 1,000 man-years in men aged 40–49 years and 50–59 years, respectively. Moreover, male infertility might be a proxy for men’s general health165 — the metabolic syndrome (MetS) is associated with a decreased sperm concentration (P = 0.0026), total sperm count (P = 0.0034), total motility (P = 0.0291), sperm vitality (P = 0.002) and abnormal sperm DNA fragmentation (P = 0.0287)166. Analysis of the records of 11,935 infertile men demonstrated that men with an abnormal semen analysis had a 2.3 times increased overall mortality risk compared with men with normal semen parameters (HR 2.9, 95% CI 1.12–4.65, P = 0.02)167. A study comparing the Charlson Comorbidity Index score — a validated predictor of 1-year mortality — between 344 men with male factor infertility and 293 age-matched fertile controls reported that the infertile cohort had a significantly higher Charlson Comorbidity Index score than fertile men (0.33 versus 0.14, P < 0.001)165.
Hypogonadism is associated with ED, infertility, diabetes and the MetS3. In a cohort of 294 men who were monitored over a period of 8 years, low levels of total testosterone predicted incident diabetes (OR 2.7, 95% CI 1.1–6.6, P = 0.03)168. Moreover, in the TIMES2 study — a randomized, multicentre, international placebo-controlled trial assessing the effects of TRT in hypogonadal men with diabetes or the MetS169 — showed that TRT improved glycaemic control compared with placebo (the HbA1c treatment difference (TD) −0.446%, P = 0.035). Furthermore, TRT was associated with a significant decrease in lipoprotein A (TD −0.31 µmol/l, 95% CI −0.543 to −0.082, P = 0.008), total cholesterol (TD −0.336 mmol/l, 95% CI −0.558 to −0.114, P = 0.003) and LDL cholesterol (TD −0.210 mmol/l, 95% CI 0.374 to −0.047, P = 0.012) compared with placebo. Thus, androgens can optimize health outcomes (improvements in lipid profiles and glycaemic control), but are also associated with worse outcomes in infection.
A study investigating the relationship between the metabolic syndrome and LUTS in a cohort of 1,899 American men170 showed a positive association (OR 1.68, 95% CI 1.21–2.35) between the MetS in men with mild or severe LUTS (American Urological Association symptom index 2–35) compared with no or low symptoms (American Urological Association symptom index score of 0 or 1)170. Investigation of the relationship between depression and anxiety and LUTS in a cohort of 14,139 men indicated that men without LUTS had a significantly lower hospital anxiety and depression scale score than those with voiding LUTS (3.3 versus 4.2, P < 0.001) or storage LUTS (3.3 versus 3.9, P < 0.001). Furthermore, a meta-analysis comprising 11 studies reported that the presence of nocturia was associated with a 1.27-fold increased risk of mortality (RR 1.27, 95% CI 1.16–1.40)171.
Thus, the urological specialty has a unique opportunity of being able to target major CVD risk factors and other premature causes of male death at an early stage through lifestyle changes, screening for occult disease, risk stratification and early medical intervention. Indeed, screening for CVD in men who present with ED has been shown to be a cost-effective intervention for secondary prevention of both ED and CVD172,173. In accordance with these observations, a study of Medicare reimbursement for both CVD risk factors and ED diagnosis and management reported that the cost of CVD risk-factor screening in men presenting with ED was $138.20 per individual and that screening the US population over the duration of 20 years would cost $2.6 billion, but would identify 5.8 million men at risk of CVD, preventing 1.1 million acute CVD events and thereby resulting in a $21.3 billion net saving173.
Globally, the mortality rate for cancer is almost 50% higher in men than in women — the global age-standardized mortality rate is 122.7 in men compared with 83.1 in women174. Men have a higher incidence of all the top five gender-neutral cancers (lung, colon, non-melanoma of skin, stomach and liver) than women, and prostate cancer is the third most common cancer worldwide94.
Within this context, a large European study175 of 90,199 participants with 10,455 incident cancers reported that cigarette use was associated with a higher proportion of total cancer burden in men than in women (30.5%, 95% CI 27.5–34.3%). Worldwide, smoking is more prevalent in men than in women34 and, thus, cigarette cessation is a modifiable risk factor that can be targeted to reduce the incidence of cancer mortality in men.
Notably, infertile men have been observed to be at a higher risk of malignancy than fertile men. In a study of data from both a Texas fertility institution and a cancer registry, infertile men had a significantly higher risk of developing cancer (overall rather than specific types) than the general population (standardized incidence ratio 1.7, 95% CI 1.2–2.5, P < 0.05). Moreover, azoospermic men were at a 2.2-times greater risk of developing cancer (overall rather than specific types) than non-azoospermic men (HR 2.2, 95% CI 1.0–4.8, P = 0.02)176. Regarding male cancers specifically, a Danish study investigated the risk of testicular cancer in 32,442 infertile men presenting to a single fertility clinic in Denmark177. The authors observed that infertile men were more likely to develop testicular cancer than the general population (standardized incidence ratio 1.6, 95% CI 1.3–1.9, P < 0.05).
The pathophysiological mechanisms that underpin this association between male infertility and later cancer diagnosis is unknown, but have been speculated to be related to in utero exposure to endocrine-disrupting chemicals (EDCs)178. This theory of testicular dysgenesis syndrome postulates that rising incidence of hypospadias, cryptorchidism, male infertility and testicular cancer is related to prenatal exposure to EDCs, which results in abnormalities in male sexual differentiation and fetal development178. However, this theory is limited by a lack of human studies and a paucity of data analysing specific effects of individual EDCs. However, irrespective of the cause, male infertility seems to confer an increased risk of developing malignancy, highlighting that infertile men are a vulnerable population. Thus, urologists have the opportunity to counsel and screen infertile men for symptoms and signs of malignancy that could potentially result in earlier diagnoses and treatment at a less advanced stage.
Men’s health as an early intervention
In addition to lifestyle advice, a men’s health programme could educate men and screen patients for diseases, including red flag symptoms and signs of malignancy. Indeed, a streamlined urology-centric men’s health clinic could not only offer cancer and CVD screening but could also provide mental health screening, which might help to offset the high suicide rates in young men. In 2018, a total of 6,507 suicides were registered in the UK, of which 75% were men179. Moreover, the global suicide rate in the age group 15–19 years was 2.6 times higher in males than in females180. Although trials assessing the effects of early interventions in suicide prevention are lacking, a Cochrane review of 55 trials reported that cognition-based psychotherapy resulted in fewer participants repeating self-harm than conventional treatment (OR 0.70, 95% CI 0.47–1.30). Given that a history of self-harm is a major risk factor for suicide181, early psychological intervention might help to reduce the high burden of male suicidal death; if urologists can identify these patients during their consultations, early referral to a support system could be arranged.
The urological speciality is the only medical discipline that deals with male-specific benign and malignant diseases, and male sexual and reproductive health is strongly associated with the major causes of premature male death3. Within this context, urologists are in an ideal position to become the gatekeepers of men’s health.
A new model for men’s health
Within both publicly funded health-care frameworks (for example, as in the UK) and systems combining public and private funding (such as in Australia and the USA), after assessment by the primary care provider, patients often need to consult with multiple specialists for each individual health complaint
This approach is resource heavy for primary and secondary health providers and time-consuming for the patient. Moreover, it might also contribute to men’s reluctance to seek health care75. Thus, a more holistic approach is required, using male-specific diseases as a platform to target men’s health in general and to improve health outcomes (Fig. 2). Accordingly, we should consider and offer health screening for occult disease in a more systematic approach and at an earlier age than is currently implemented. For example, men presenting with ED should be screened for CVD risk factors immediately, owing to the strong association between ED and subsequent CVD events163 and, conversely, those men who have CVD risk factors should be assessed for sexual dysfunction. Depression and sexual dysfunction have a bidirectional relationship182; thus, men presenting with low mood or ED should be screened and, if necessary, treated for both. Moreover, men presenting with infertility should be assessed for hypogonadism and symptoms and signs of malignancy3. All men should undergo a CVD risk factor assessment, as treatment of modifiable and lifestyle risk factors can reduce the risk of future CVD events, malignancy, ED and infertility.
Current barriers to male health-care utilization include the absence of a reference specialist and the need to see several specialties, sometimes in different hospitals and over multiple appointments. Advantages of a men’s health policy include a multidisciplinary pathway that incorporates the diagnosis, treatment and prevention of both benign and malignant conditions. Potential outcomes from a men’s health policy (such as increasing male life expectancy, novel data acquisition and reducing the burden and costs of cancer and cardiovascular disease (CVD) morbidities through a preventive medicine approach.
Qualitative studies using patient questionnaires in a range of specialities have suggested high patient satisfaction with ‘one-stop’ clinics compared with traditional outpatient encounters183,184. A study investigating patient experiences with a one-stop haematuria clinic and a clinical pathway containing several visits showed high patient satisfaction (recorded through a questionnaire), reporting that 82% of the participants expressed preference for a one-stop clinic model183. A separate study184 investigated the effects of a one-stop clinic for minor skin surgery in 32 UK plastic surgery departments, reporting that the use of one-stop clinics was associated with a higher degree of patient satisfaction (95% versus 72%) than conventional pathways and improved compliance with 18-week waiting time targets (95% versus 85%).
Thus, one-stop men’s health clinics might improve patient satisfaction and male uptake of health-care services.
COVID-19 as an opportunity
The COVID-19 pandemic has forced rationalization and prioritization of clinical services. Elective surgical output has been universally reduced, with a focus on oncological operations and life-saving surgery. A description of the activities of the urological department in Bergamo Hospital, Italy, at the beginning of the pandemic highlighted that, in March 2020, the operating capacity was reduced to 15% of the normal activity and focused solely on oncological and emergency procedures. Moreover, 30% of the urological staff were redeployed to care for patients with COVID-19 and 7 of the 13 urologists remaining were self-isolating185. This report highlights the challenges presented by the COVID-19 pandemic, both in terms of service provision and retention of the workforce. Notably, specific recommendations from the EAU focused on response to the coronavirus pandemic have categorized the overall management of sexual health and ED in the COVID-19 period as low priority186. Although this approach might be justified in view of the immediate risks associated with COVID-19 infection compared with the benefits of andrological treatment, the long-term ramifications of delays in treatment, the effects on patients’ mental health notwithstanding, are not clear. Indeed, both ED and infertility are harbingers of underlying serious health risks and provide opportunities to mitigate risk factors at an early stage to avoid their long-term sequelae. Given the current prioritization of oncological procedures, the post-pandemic period will probably require re-organization and re-structuring of many clinical services to cope with demand, possibly to the detriment of subspecialties dealing with benign conditions such as male sexual and reproductive health186. However, we believe that this period presents an opportunity to develop a dedicated men’s health programme aimed at assessing and managing a range of health issues in a single visit, and that could be implemented worldwide.
Indeed, men’s health services are lacking worldwide. In a Delphi survey of men’s health for 128 stakeholders (policy makers, clinicians, researchers and consumers) from 28 Asian countries187, only 20.4% of respondents described men’s health being present in one or more of: national policy, public awareness programmes, health-care services, clinical practice guidelines, health screening programmes, training for health-care workers, school health education, research, social support service, national registries or law. Moreover, 85% of participants were concerned about men’s health issues, specifically smoking, hypertension and CVD.
Establishing a men’s health programme will require collaboration from a range of specialties, including urologists, cardiologists, endocrinologists, dieticians, physiotherapists and psychologists, and would not only benefit patients but could also streamline services in a cost-effective manner8. The contribution of primary care practitioners will be paramount to the success of such a programme, and a shared-care management approach with urologists would enable ongoing treatments and preventive care practice in the community. Future data acquisition will enable the development of evidence-based, sex-specific protocols and guidelines, facilitating a community men’s health programme supported by primary care doctors.
A structured men’s health-care programme could also improve health-care utilization by appealing to the male psyche and promoting men’s empowerment over their health. The implementation of this approach might vary according to race and ethnicity. For example, data are available to show that the Latino culture values men as the breadwinner or provider for the family188; thus, this community could be encouraged to support men’s health with the rationale that it can be instrumental to the wellbeing of individual families and the community as a whole. Similarly, studies have reported that dietary interventions instituted through African American churches can improve fruit and vegetable intake for African American men189,190. Thus, churches could provide an ideal location for advertising and practising men’s health-care initiatives to target the African American community. Men’s health awareness campaigns can be regularly promoted around Fathers’ Day, Men’s Health Month (June) and other high-profile campaigns, such as Movember.
The intersectionality of sex, gender, race and health outcomes is a complex but expanding field of research and the benefit of a men’s health programme would be the opportunity to collect sex-specific data. A 2019 bibliometric analysis of sex-related reporting in medical research studied more than 11.5 million papers published between 1980 and 2016, and observed that sex-related outcomes reporting increased from 36% to 69% and 59% to 67% in public health and clinical medicine research, respectively191. Thus, although sex-specific outcome reporting is improving, the literature is still lacking.
Psychological input will be mandatory for developing any men’s health programme, because our lack of understanding of how to change the patterns of thinking or behaviours of men to embrace health promotions and improve health literacy is a barrier to optimizing health outcomes192,193. Moreover, sociological data acquisition could also optimize male utilization of health-care services, helping to identify and address societal, cultural, and ethnic determinants affecting health care193.
Challenges and lessons
Developing a new model of care will have initial challenges, including acquisition of funding and the logistics of setting up a multidisciplinary service. However, the sex discrepancies in COVID-19 mortality rates, coupled with the sex gap in life expectancy from non-communicable diseases, has highlighted that the current health-care system has failed men. A novel perspective on men’s health is required. One programme attempting to address this failure is the EAU working panel on Male and Sexual Reproductive Health, which has developed the first evidence-based guidelines on male sexual and reproductive health, including guidance on how urological conditions can be harbingers for occult systematic diseases172.
A men’s health service would enshrine and enable pooling of services and resources, expedite patient access to care, and could also facilitate referral for specialist investigations or treatments, such as cardiac angiography, treatment of diabetes and imaging for raised PSA. Alternatively, these investigations could even be incorporated into current streamlined pathways, for example MRI and same-day local anaesthetic prostate biopsy for rapid-access prostatic cancer diagnostic pathways. A one-stop model could reduce treatment delay, improve cost-effectiveness and increase patient satisfaction10. Moreover, a sex-specific health-care model would incorporate sex-specific protocols, patient-reported outcomes and a patient-centred environment that could enable interaction with peers4, facilitating disease screening and dissemination of health information to patients. Moreover, auditing of the service and patient experiences would provide an opportunity for research into men’s health. This model would be dynamic, incorporating patient opinions in order to constantly adapt to optimize patient satisfaction and clinical outcomes.
The creation of a programme like this could also be used to trial new and emerging technologies to identify novel methods of improving male health-care outcomes. For example, the mobile web application, ScreenMan, which provides educational information for 15 health conditions including depression and alcohol misuse. In an evaluation of the usability of the software through a participant questionnaire using a validated system usability score, ScreenMan was categorized as having a ‘good’ system usability score (mean 76.4), but no statistically significant improvement was seen in behavioural change patterns in favour of participants intending to screen for medical conditions earlier (<6 months) following the use of the software194. Although this app did not change health-care outcomes, others could be evaluated and might be helpful for implementation within a men’s health programme.
A men’s health programme would provide the opportunity to implement new technologies to optimize existing health pathways. New technologies, such as telehealth (telemedicine), could potentially improve male engagement by removing time and location restraints to health-care services and also prove a cheaper, less labour-intensive resource194. A systematic review analysing the impact of telehealth in urological conditions observed that the majority of studies reported clinical safety and high patient satisfaction with telehealth compared with standard pathways195. Thus, telehealth should have a role in the implementation of a new approach to men’s health.
Conclusions
The disparity in increased COVID-19 mortality between men and women has highlighted underlying deficiencies in our current health-care system. Men are more vulnerable to infections than women owing to underlying biological causes, including immunological106, hormonal132 and genetic differences196, and men also tend to have poorer general health and reduced access to health care. This discrepancy has also resulted in a sex gap in life expectancy from non-communicable diseases3. Policy makers and health-care stakeholders must take heed of the success of FFIT and Ireland’s men’s health programme and develop approaches that specifically and aggressively target men, especially given their reluctance to engage with health-care services and the higher rates of health illiteracy in men than in women.
The resumption of normal health-care services once the COVID-19 pandemic has resolved is likely to be followed by a high burden of referrals; thus, development of a new model of care within a streamlined multidisciplinary setting is a logical approach to creating focused men’s health clinics that could solve the problem of sex discrepancies in health and disease.
References
Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395, 565–574 (2020).
BBC. Coronavirus: risk of death is higher for ethnic minorities — BBC News. BBC https://www.bbc.co.uk/news/health-52889106 (2020).
Tharakan, T. et al. Male sexual and reproductive health — does the urologist have a role in addressing gender inequality in life expectancy? Eur Urol. Focus 6, 791–800 (2019).
Tharakan, T., Jayasena, C. & Minhas, S. Men’s health clinics: a real need or a marketing strategy. Int. J. Impot. Res. 32, 565–568 (2020).
Tharakan, T., Salonia, A. & Minhas, S. Male life expectancy is still inferior to that of women: urologists must refine and develop the concept of men’s health. Eur. Urol. 76, 712–713 (2019).
Perico, L., Benigni, A. & Remuzzi, G. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron 144, 1–9 (2020).
Mason, R. J. Pathogenesis of COVID-19 from a cell biologic perspective. Eur. Respir. J. https://doi.org/10.1183/13993003.00607-2020 (2020).
Li, G. et al. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 112, 1–7 (2020).
Oudkerk, M. et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology 77, 1–15 (2020).
Ciceri, F. et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. 22, 95–97 (2020).
Bruce Aylward (WHO); Wannian Liang (PRC). Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). WHO https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (2020).
Coronavirus Resource Center. COVID-19 Map — Johns Hopkins Coronavirus Resource Center. JHU https://coronavirus.jhu.edu/map.html (2020).
GlobalHealth505. Covid-19 — Global Health 50/50. GlobalHealth505 https://globalhealth5050.org/covid19/ (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020).
Du, R. H. et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 55, 1–8 (2020).
Zhang, J. et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin. Microbiol. Infect. 26, 767–772 (2020).
WHO clinical management of COVID-19. WHO https://www.who.int/publications/i/item/clinical-management-of-covid-19 (2020).
Wan, S. et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 92, 797–806 (2020).
Zhao, Q. et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J. Med. Virol. 92, 1915–1921 (2020).
Guo, F. R. Smoking links to the severity of COVID-19: an update of a meta-analysis. J. Med. Virol. 92, 2304–2305 (2020).
Giacomelli, A. et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol. Res. 158, 1–7 (2020).
Lippi, G., Mattiuzzi, C., Sanchis-Gomar, F. & Henry, B. M. Clinical and demographic characteristics of patients dying from COVID-19 in Italy versus China. J. Med. Virol. 92, 1759–1760 (2020).
Goumenou, M. et al. COVID‑19 in Northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (Review). Mol. Med. Rep. 22, 20–32 (2020).
Onder, G., Rezza, G. & Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323, 1775–1776 (2020).
Chen, J. et al. COVID-19 infection: the China and Italy perspectives. Cell Death Dis. 11, 1–17 (2020).
Peng, F. et al. Management and treatment of COVID-19: the Chinese experience. Can. J. Cardiol. 36, 915–930 (2020).
Liu, W., Yue, X. G. & Tchounwou, P. B. Response to the COVID-19 epidemic: the Chinese experience and implications for other countries. Int. J. Environ. Res. Public Health 17, 1–6 (2020).
Mikami, T. et al. Risk factors for mortality in patients with COVID-19 in New York City. J. Gen. Intern. Med. 36, 1–10 (2020).
Cummings, M. J. et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 395, 1763–1770 (2020).
Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584, 430–436 (2020).
Docherty, A. B. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 369, 1–12 (2020).
Barron, E. et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 8, 813–822 (2020).
WHO. Gender, women and the tobacco epidemic. WHO https://apps.who.int/iris/bitstream/handle/10665/44342/9789241599511_eng.pdf;jsessionid=937E58FF52F52314F08381C64FD6A095?sequence=1 (2020).
Pampel, F. C. Global patterns and determinants of sex differences in smoking. Int. J. Comp. Sociol. 47, 466–487 (2006).
Buist, A. S., Vollmer, W. M. & McBurnie, M. A. Worldwide burden of COPD in high- and low-income countries. Part I. The Burden of Obstructive Lung Disease (BOLD) Initiative. Int. J. Tuberc. Lung Dis. 12, 703–708 (2008).
Blanco-Cedres, L. et al. Relation of cigarette smoking to 25-year mortality in middle-aged men with low baseline serum cholesterol: the Chicago Heart Association detection project in industry. Am. J. Epidemiol. 155, 354–360 (2002).
Friedman, G. D., Dales, L. G. & Ury, H. K. Mortality in middle-aged smokers and nonsmokers. N. Engl. J. Med. 300, 213–217 (1979).
Jacob, L., Freyn, M., Kalder, M., Dinas, K. & Kostev, K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: a retrospective study of 422,010 patients followed for up to 30 years. Oncotarget 9, 17420–17429 (2018).
Ntritsos, G. et al. Gender-specific estimates of COPD prevalence: a systematic review and meta-analysis. Int. J. COPD 13, 1507–1514 (2018).
NHS Digital. National Diabetes Audit. NHS https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit (2021).
Beeson, P. B. Age and sex associations of 40 autoimmune diseases. Am. J. Med. 96, 457–462 (1994).
Gale, E. A. M. & Gillespie, K. M. Diabetes and gender. Diabetologia 44, 3–15 (2001).
Blohmé, G. et al. Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15–34-year age group in Sweden. Diabetologia 35, 56–62 (1992).
Yang, W. et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 362, 1090–1101 (2010).
Lipscombe, L. L. & Hux, J. E. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995–2005: a population-based study. Lancet 369, 750–756 (2007).
NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530 (2016).
Logue, J. et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 54, 3003–3006 (2011).
Demerath, E. W. et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity 15, 2984–2993 (2007).
Ross, R. et al. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am. J. Clin. Nutr. 59, 1277–1285 (1994).
Lovejoy, J. C., Champagne, C. M., De Jonge, L., Xie, H. & Smith, S. R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 32, 949–958 (2008).
Rytka, J. M., Wueest, S., Schoenle, E. J. & Konrad, D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes 60, 56–63 (2011).
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387, 1377–1396 (2016).
Simonnet, A. et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 28, 1195–1199 (2020).
WHO. By category | Mean systolic blood pressure trends, age-standardized (mmHg) — global estimates. WHO https://apps.who.int/gho/data/view.main.12467GLOBAL?lang=en (2020).
CDC. National Health and Nutrition Examination Survey 2020. CDC https://www.cdc.gov/nchs/data/factsheets/factsheet_nhanes.pdf (2020).
Waddell, T. K., Dart, A. M., Gatzka, C. D., Cameron, J. D. & Kingwell, B. A. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J. Hypertens. 19, 2205–2212 (2001).
Bates B. et al. National Diet and Nutrition Survey — assessment of dietary sodium in adults (aged 19 to 64 years) in England, 2014. (Department of Health, 2016).
Benjamin, E. J. et al. Heart disease and stroke statistics — 2019 update: a report from the American Heart Association. Circulation 139, e56–e528 (2019).
Bots, S. H., Peters, S. A. E. & Woodward, M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob. Health 2, 1–8 (2017).
Millett, E. R. C., Peters, S. A. E. & Woodward, M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ 363, 1–11 (2018).
Leening, M. J. G. et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 349, 1–13 (2014).
Anand, S. S. et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur. Heart J. 29, 932–940 (2008).
Rivera, C. M. et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 16, 15–23 (2009).
Rosenberg, L. et al. Early menopause and the risk of myocardial infarction. Am. J. Obstet. Gynecol. 139, 47–51 (1981).
Zhu, D. et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public. Health 4, 553–564 (2019).
Mendelsohn, M. E. & Karas, R. H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 340, 1801–1811 (1999).
Office for National Statistics. English Life Tables — Office for National Statistics. ONS https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/englishlifetablesno17/2015-09-01 (1999).
Men’s Health Forum. Men’s Health Manifesto. Men’s Health Forum https://www.menshealthforum.org.uk/mens-health-manifesto (2014).
Payne S. How can gender equity be addressed through health systems? WHO https://www.euro.who.int/__data/assets/pdf_file/0006/64941/E92846.pdf (2009).
Baker, P. 25 years post-Calman: the state of men’s health in the UK. Trends Urol. Mens Health 8, 13–16 (2017).
GBD 2017 Population and Fertility Collaborators. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1684–1735 (2018).
The WHO Regional Office For Europe. The health and well-being of men in the WHO European region: better health through a aender approach. WHO www.euro.who.int (2018).
Juel, K. & Christensen, K. Are men seeking medical advice too late? Contacts to general practitioners and hospital admissions in Denmark 2005. J. Public. Health 30, 111–113 (2008).
Wang, Y., Hunt, K., Nazareth, I., Freemantle, N. & Petersen, I. Do men consult less than women? An analysis of routinely collected UK general practice data. BMJ Open 3, 1–7 (2013).
NHS Digital. Hospital admitted patient care activity 2018–19. NHS https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2018-19 (2019).
Deeks, A., Lombard, C., Michelmore, J. & Teede, H. The effects of gender and age on health related behaviors. BMC Public. Health 9, 1–8 (2009).
Green, C. A. & Pope, C. R. Gender, psychosocial factors and the use of medical services: a longitudinal analysis. Soc. Sci. Med. 48, 1363–1372 (1999).
Thompson, A. E. et al. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam. Pract. 17, 1–7 (2016).
Pinkhasov, R. M. et al. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int. J. Clin. Pract. 64, 475–487 (2010).
Logan, R. F. A. et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 61, 1439–1446 (2012).
Seidler, Z. E., Dawes, A. J., Rice, S. M., Oliffe, J. L. & Dhillon, H. M. The role of masculinity in men’s help-seeking for depression: a systematic review. Clin. Psychol. Rev. 49, 106–118 (2016).
WHO. Suicide worldwide in 2019: global health estimates. WHO https://www.who.int/publications/i/item/9789240026643 (2021).
Baker, P. & Shand, T. Men’s health: time for a new approach to policy and practice? J. Glob. Health 7, 1–5 (2017).
Coles, R. et al. What men really want: a qualitative investigation of men’s health needs from the Halton and St Helens primary care trust men’s health promotion project. Br. J. Health Psychol. 15, 921–939 (2010).
Gascoigne, P. & Whitear, B. Making sense of testicular cancer symptoms: a qualitative study of the way in which men sought help from the health-care services. Eur. J. Oncol. Nurs. 3, 62–69 (1999).
Hall, J. A., Roter, D. L. & Katz, N. R. Meta-analysis of correlates of provider behavior in medical encounters. Med. Care 26, 657–675 (1988).
Verbrugge, L. M. & Steiner, R. P. Physician treatment of men and women patients: sex bias or appropriate care? Med. Care 19, 609–632 (1981).
Misener, T. R. & Fuller, S. G. Testicular versus breast and colorectal cancer screening. Early detection practices of primary care physicians. Cancer Pract. 3, 310–316 (1995).
Patasius, A., Krilaviciute, A. & Smailyte, G. Prostate cancer screening with PSA: ten years’ experience of population based early prostate cancer detection programme in Lithuania. J. Clin. Med. 9, 1–9 (2020).
Ahmed, H. U. et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389, 815–822 (2017).
Kasivisvanathan, V. et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N. Engl. J. Med. 378, 1767–1777 (2018).
EAU. White paper on prostate cancer. EAU https://www.europa-uomo.org/wp-content/uploads/2020/05/EAU_PCa-WhitePaper-FINAL-VERSION.pdf (2020).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Dennis, S. et al. Which providers can bridge the health literacy gap in lifestyle risk factor modification education: a systematic review and narrative synthesis. BMC Fam. Pract. 13, 1–29 (2012).
Nutbeam, D. & Kickbusch, I. Advancing health literacy: a global challenge for the 21st century. Health Promot. Int. 15, 183–184 (2000).
Von Wagner, C., Knight, K., Steptoe, A. & Wardle, J. Functional health literacy and health-promoting behaviour in a national sample of British adults. J. Epidemiol. Community Health 61, 1086–1090 (2007).
Robb, K. et al. Public awareness of cancer in Britain: a population-based survey of adults. Br. J. Cancer 101, 18–23 (2009).
Wong Y. J., Liu T., Klann E. M. The intersection of race, ethnicity, and masculinities: progress, problems, and prospects. In The psychology of men and masculinities (eds Levant, R. F. & Wong, Y. J.) 261–288 (American Psychological Association, 2017).
Ragonese, C. & Barker, G. Understanding masculinities to improve men’s health. Lancet 394, 198–199 (2019).
Griffith, D. M., Metzl, J. M. & Gunter, K. Considering intersections of race and gender in interventions that address US men’s health disparities. Public Health 125, 417–423 (2011).
Griffith, D. M. Biopsychosocial approaches to men’s health disparities research and policy. Behav. Med. 42, 211–215 (2016).
Griffith, D. M. An intersectional approach to men’s health. J. Mens Health 9, 106–112 (2012).
Ferlatte, O., Salway, T., Trussler, T., Oliffe, J. L. & Gilbert, M. Combining intersectionality and syndemic theory to advance understandings of health inequities among Canadian gay, bisexual and other men who have sex with men. Crit. Public Health 28, 509–521 (2018).
Stall, R. et al. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am. J. Public Health 93, 939–942 (2003).
Ruggieri, A., Anticoli, S., D’ambrosio, A., Giordani, L. & Viora, M. The influence of sex and gender on immunity, infection and vaccination. Ann. Ist. Super. Sanita 52, 198–204 (2016).
Berghöfer, B. et al. TLR7 ligands induce higher IFN-α production in females. J. Immunol. 177, 2088–2096 (2006).
Villacres, M. C., Longmate, J., Auge, C. & Diamond, D. J. Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum. Immunol. 65, 476–485 (2004).
Abdullah, M. et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 272, 214–219 (2012).
Engler, R. J. M. et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch. Intern. Med. 168, 2405–2414 (2008).
Cook, I. F., Barr, I., Hartel, G., Pond, D. & Hampson, A. W. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine 24, 2395–2402 (2006).
Tanaka, E., Sata, M., Nakano, H., Suzuki, H. & Tanikawa, K. Antibody response to inactivated hepatitis A vaccine. Hepatol. Res. 9, 103–112 (1997).
Kennedy, R. B. et al. Gender effects on humoral immune responses to smallpox vaccine. Vaccine 27, 3319–3323 (2009).
Van Lunzen, J. & Altfeld, M. Sex differences in infectious diseases-common but neglected. J. Infect. Dis. 209, S79–S80 (2014).
Gabriel, G. & Arck, P. C. Sex, immunity and influenza. J. Infect. Dis. 209, S93–S99 (2014).
Yu, L., Quinn, M. T., Cross, A. R. & Dinauer, M. C. Gp91phox is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl Acad. Sci. USA 95, 7993–7998 (1998).
Banerjee, E. R. & Henderson, W. R. Defining the molecular role of gp91phox in the immune manifestation of acute allergic asthma using a preclinical murine model. Clin. Mol. Allergy 10, 1–14 (2012).
Chandra, R. et al. Female X-chromosome mosaicism for NOX2 deficiency presents unique inflammatory phenotype and improves outcome in polymicrobial sepsis. J. Immunol. 186, 6465–6473 (2011).
Chandra, R. et al. IRAK1-dependent signaling mediates mortality in polymicrobial sepsis. Inflammation 36, 1503–1512 (2013).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Li, W. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 181, 271–280 (2020).
Sama, I. E. et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur. Heart J. 41, 1810–1817 (2020).
Cai, G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. Preprint at medRxiv https://doi.org/10.1101/2020.02.05.20020107 (2020).
Xudong, X., Junzhu, C., Xingxiang, W., Furong, Z. & Yanrong, L. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 78, 2166–2171 (2006).
Dong, Y. et al. Epidemiology of COVID-19 among children in China. Pediatrics 145, 1–12 (2020).
Guan, W. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020).
The World Bank. World Development Indicators: Health risk factors and future challenges. The World Bank http://wdi.worldbank.org/table/2.17 (2020).
Wambier, C. G. et al. Androgen sensitivity gateway to COVID-19 disease severity. Drug. Dev. Res. 81, 771–776 (2020).
Lucas, J. M. et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 4, 1310–1325 (2014).
Channappanavar, R. et al. Sex-based differences in susceptibility to SARS-CoV infection. J. Immunol. 198, 4046–4053 (2017).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Agostino, P. et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann. N. Y. Acad. Sci. 876, 426–429 (1999).
Iyer, S. S. & Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 32, 23–63 (2012).
Capobianco, M. P. et al. Human Interleukin 2 (IL-2) promotion of immune regulation and clinical outcomes: a review. J. Cytokine Biol. 1, 1–4 (2016).
Musabak, U. et al. Gonadotropin treatment restores in vitro interleukin-1β and tumour necrosis factor-α production by stimulated peripheral blood mononuclear cells from patients with idiopathic hypogonadotropic hypogonadism. Clin. Exp. Immunol. 132, 265–270 (2003).
Malkin, C. J. et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 89, 3313–3318 (2004).
Han, X. et al. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J. Virol. 75, 3048–3052 (2001).
Robinson, D. P. et al. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol. Sex. Differ. 2, 1–11 (2011).
Wang, Z. & Xu, X. scRNA-seq profiling of human testes reveals the presence of ACE2 receptor, a target for SARS-CoV-2 infection, in spermatogonia, Leydig and Sertoli cells. Cells 9, 1–9 (2020).
Ma, L. et al. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. Preprint at medRxiv https://doi.org/10.1101/2020.03.21.20037267 (2020).
Ruan, Y. et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology 9, 99–106 (2021).
World Health Organization Department of Reproductive Health and Research. WHO laboratory manual for the examination and processing of human semen, fifth edition. WHO https://apps.who.int/iris/bitstream/handle/10665/44261/9789241547789_eng.pdf;jsessionid=F577E96867A29CC002AE25F470B4FFF3?sequence=1 (2010).
The Gates Foundation. What we do — Bill & Melinda Gates Foundation. The Gates Foundation https://www.gatesfoundation.org/What-We-Do (2010).
The Gates Foundation. Gender equality — Bill & Melinda Gates Foundation. The Gates Foundation https://www.gatesfoundation.org/What-We-Do/Global-Growth-and-Opportunity/Gender-Equality (2020).
Rovito, M. J. et al. A call for gender-inclusive global health strategies. Am. J. Mens Health 11, 1804–1808 (2017).
WHO. What is the global strategy? WHO https://www.who.int/life-course/partners/global-strategy/global-strategy-2016-2030/en/c (2016).
Christakis, N. A. & Allison, P. D. Mortality after the hospitalization of a spouse. N. Engl. J. Med. 354, 719–730 (2020).
Corden P. Financial implications of death of a partner (University of York, 2008).
Mohindra, K. S., Haddad, S. & Narayana, D. Debt, shame, and survival: becoming and living as widows in rural Kerala, India. BMC Int. Health Hum. Rights 12, 1–13 (2012).
Wyke, S. et al. Football Fans in Training (FFIT): a randomized controlled trial of a gender sensitised weight loss and healthy living programme delivered to men aged 35–65 by Scottish Premier League (SPL) football clubs. Public. Health Res. 3, 1–130 (2015).
Gray, C. M. et al. Long-term weight loss following a randomised controlled trial of a weight management programme for men delivered through professional football clubs: the Football Fans in Training follow-up study. Public. Health Res. 6, 1–114 (2018).
Russell, N., Harding, C., Chamberlain, C. & Johnston, L. Implementing a “Men’s Health Pitstop” in the Riverina, South-west New South Wales. Aust. J. Rural. Health 14, 129–131 (2006).
Wood, D. & Minhas, S. Science and society: the influence of charities in the field of men’s health. Nat. Rev. Urol. 12, 599–600 (2015).
Baker P. Review of the national men’s health policy and action plan 2008–2013 final report for the health service executive. MHFI https://www.mhfi.org/policyreview2015.pdf (2015).
Evans D. S., Walshe K., Gillen P. Farmers have heart project evaluation. Lenus https://www.lenus.ie/bitstream/handle/10147/84216/farmershaveheartsrepfJan21.pdf?sequence=1&isAllowed=y (2009).
Richardson, N. Building momentum, gaining traction: Ireland’s National Men’s Health Policy 5 years on. N. Male Stud. 2, 93–103 (2013).
Clarke N., Sharp L., O’Leary E. & Richardson N. A report on the excess burden of cancer among men in the Republic of Ireland (National Cancer Registry Ireland, 2013).
Richardson N., Clarke N., Fowler C. Men’s health in Ireland. An executive summary report on the All-Ireland Young Men and Suicide Project. MHFI www.mhfi.org (2013).
Murray, M., Campbell, C., Kendall, L., Whitt-Glover, M. C. & Watson, K. S. Perspectives from Project Brotherhood: facilitating engagement of African American men in research. Prog. Community Health Partnersh. 13, 137–142 (2019).
WHO. Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference. WHO https://www.who.int/governance/eb/who_constitution_en.pdf (1946).
Schwartz, B. G. & Kloner, R. A. Cardiovascular implications of erectile dysfunction. Circulation 123, 609–611 (2011).
Vlachopoulos, C. V., Terentes-Printzios, D. G., Ioakeimidis, N. K., Aznaouridis, K. A. & Stefanadis, C. I. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction. Circ. Cardiovasc. Qual. Outcomes 6, 99–109 (2013).
Johannes, C. B. et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J. Urol. 163, 460–463 (2000).
Salonia, A. et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur. Urol. 56, 1025–1032 (2009).
Leisegang, K., Udodong, A., Bouic, P. J. D. & Henkel, R. R. Effect of the metabolic syndrome on male reproductive function: a case-controlled pilot study. Andrologia 46, 167–176 (2014).
Eisenberg, M. L. et al. Semen quality, infertility and mortality in the USA. Hum. Reprod. 29, 1567–1574 (2014).
Oh, J. Y., Barrett-Connor, E., Wedick, N. M. & Wingard, D. L. Endogenous sex hormones and the development of type 2 diabetes in older men and women: The Rancho Bernardo Study. Diabetes Care 25, 55–60 (2002).
Jones, T. H. et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 34, 828–837 (2011).
Kupelian, V. et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston area community health survey. J. Urol. 189, 107–114 (2009).
Pesonen, J. S. et al. The impact of nocturia on mortality: a systematic review and meta-analysis. J. Urol. 203, 486–495 (2020).
EAU Guidelines Committee. EAU Guidelines on Sexual and Reproductive Health. Uroweb https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Sexual-and-Reproductive-Health-2020.pdf (2020).
Pastuszak, A. W. et al. Erectile dysfunction as a marker for cardiovascular disease diagnosis and intervention: s cost analysis. J. Sex. Med. 12, 975–984 (2015).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Tsilidis, K. K. et al. Burden of cancer in a large consortium of prospective cohorts in Europe. J. Natl Cancer Inst. 108, 1–7 (2016).
Eisenberg, M. L., Betts, P., Herder, D., Lamb, D. J. & Lipshultz, L. I. Increased risk of cancer among azoospermic men. Fertil. Steril. 100, 681–685 (2013).
Jacobsen, R. et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. Br. Med. J. 321, 789–792 (2000).
Skakkebæk, N. E., Rajpert-De Meyts, E. & Main, K. M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 (2001).
Office for National Statistics. Suicides in the UK: 2018 registrations. ONS https://www.ons.gov.uk/releases/suicidesintheuk2018registrations (2001).
Wasserman, D., Cheng, Q. & Jiang, G.-X. Global suicide rates among young people aged 15–19. World Psychiatry 4, 114–120 (2005).
Hawton, K. et al. Psychosocial interventions for self-harm in adults. Cochrane Database Syst. Rev. 5, 1–237 (2016).
Clayton, A. H., El Haddad, S., Iluonakhamhe, J. P., Ponce Martinez, C. & Schuck, A. E. Sexual dysfunction associated with major depressive disorder and antidepressant treatment. Expert. Opin. Drug. Saf. 13, 1361–1374 (2014).
King, K. E. Patient satisfaction in a one-stop haematuria clinic and urology outpatients: a comparison of clinics. Int. J. Urol. Nurs. 10, 127–136 (2016).
Gorman, M., Coelho, J., Gujral, S. & McKay, A. One-stop clinic utilization in plastic surgery: our local experience and the results of a UK-Wide National Survey. Plast. Surg. Int. 2015, 1–9 (2015).
Naspro, R., Da & Pozzo, L. F. Urology in the time of corona. Nat. Rev. Urol. 17, 251–253 (2020).
Ribal, M. J. et al. European Association of Urology Guidelines Office Rapid Reaction Group: an organisation-wide collaborative effort to adapt the European Association of Urology Guidelines Recommendations to the Coronavirus Disease 2019 era. Eur. Urol. 78, 21–28 (2020).
Teo, C. H., Ng, C. J., Ho, C. C. K. & Tan, H. M. A consensus on men’s health status and policy in Asia: a Delphi survey. Public Health 129, 60–67 (2015).
Rovito, M. & Leone, J. Faith and masculinity: a discussion on raising awareness and promoting cancer screening among Latino men. Calif. J. Health Promot. 10, 70–77 (2012).
Resnicow, K. et al. Body and soul: a dietary intervention conducted through African-American churches. Am. J. Prev. Med. 27, 97–105 (2004).
Resnicow, K. et al. A motivational interviewing intervention to increase fruit and vegetable intake through Black churches: results of the eat for life trial. Am. J. Public Health 91, 1686–1693 (2001).
Sugimoto, C. R., Ahn, Y. Y., Smith, E., Macaluso, B. & Larivière, V. Factors affecting sex-related reporting in medical research: a cross-disciplinary bibliometric analysis. Lancet 393, 550–559 (2019).
Milner A., Shields M., King T. The influence of masculine norms and mental health on health literacy among men: evidence from the Ten to Men Study. Am. J. Mens Health 13, 1557988319873532 (2019)
Short, S. E. & Mollborn, S. Social determinants and health behaviors: conceptual frames and empirical advances. Curr. Opin. Psychol. 5, 78–84 (2015).
Teo, C. H., Ng, C. J., Lo, S. K., Lim, C. D. & White, A. A mobile web app to improve health screening uptake in men (Screenmen): utility and usability evaluation study. JMIR mHealth uHealth 7, e10216 (2019).
Novara, G. et al. Telehealth in urology: a systematic review of the literature. how much can telemedicine be useful during and after the COVID-19 pandemic? Eur. Urol. 78, 786–811 (2020).
Chandra, R. et al. Cellular mosaicism for X-linked polymorphisms and IRAK1 expression presents a distinct phenotype and improves survival following sepsis. J. Leukoc. Biol. 95, 497–507 (2014).
Acknowledgements
T.T. is funded by an Imperial Private Services Fellowship. C.N.J. is funded by a NIHR Post-Doctoral Fellowship.
Author information
Authors and Affiliations
Contributions
T.T. and C.C.K. researched data for the article. All authors contributed substantially to discussion of the content. T.T. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Additional information
Competing interests
Both C.N.J. and S.M. hold investigator-led grants from LogixX Pharma Ltd. C.N.J. has received a speaker fee for The Society for Endocrinology Debate sponsored by Besins Healthcare. The other authors declare no competing interests.
Peer review information
Nature Reviews Urology thanks D. Griffith, H. M. Tan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Global Health 5050: https://globalhealth5050.org/covid19/
WHO guidelines for clinical management of COVID-19: https://www.who.int/publications/i/item/clinical-management-of-covid-19
Glossary
- Intersectional uniqueness paradigm
-
Assumes that a person’s social identities are intertwined and experienced simultaneously; thus, experiences associated with multiple identities cannot be separated or considered additive.
Rights and permissions
About this article
Cite this article
Tharakan, T., Khoo, C.C., Giwercman, A. et al. Are sex disparities in COVID-19 a predictable outcome of failing men’s health provision?. Nat Rev Urol 19, 47–63 (2022). https://doi.org/10.1038/s41585-021-00535-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00535-4
This article is cited by
-
Sexual orientation, gender identity and COVID: a complicated picture
Nature Reviews Urology (2022)
-
The COVID-19 pandemic — what have urologists learned?
Nature Reviews Urology (2022)