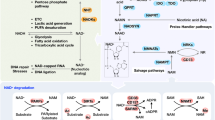
Abstract
Nicotinamide adenine dinucleotide (NAD+) is a coenzyme for redox reactions, making it central to energy metabolism. NAD+ is also an essential cofactor for non-redox NAD+-dependent enzymes, including sirtuins, CD38 and poly(ADP-ribose) polymerases. NAD+ can directly and indirectly influence many key cellular functions, including metabolic pathways, DNA repair, chromatin remodelling, cellular senescence and immune cell function. These cellular processes and functions are critical for maintaining tissue and metabolic homeostasis and for healthy ageing. Remarkably, ageing is accompanied by a gradual decline in tissue and cellular NAD+ levels in multiple model organisms, including rodents and humans. This decline in NAD+ levels is linked causally to numerous ageing-associated diseases, including cognitive decline, cancer, metabolic disease, sarcopenia and frailty. Many of these ageing-associated diseases can be slowed down and even reversed by restoring NAD+ levels. Therefore, targeting NAD+ metabolism has emerged as a potential therapeutic approach to ameliorate ageing-related disease, and extend the human healthspan and lifespan. However, much remains to be learnt about how NAD+ influences human health and ageing biology. This includes a deeper understanding of the molecular mechanisms that regulate NAD+ levels, how to effectively restore NAD+ levels during ageing, whether doing so is safe and whether NAD+ repletion will have beneficial effects in ageing humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sahar, S., Nin, V., Barbosa, M. T., Chini, E. N. & Sassone-Corsi, P. Altered behavioral and metabolic circadian rhythms in mice with disrupted NAD+ oscillation. Aging 3, 794–802 (2011).
Cambronne, X. A. & Kraus, W. L. Location, location, location: compartmentalization of NAD synthesis and functions in mammalian cells. Trends Biochem. Sci. 45, 858–873 (2020).
Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 350, 1208–1213 (2015).
Cambronne, X. A. et al. Biosensor reveals multiple sources for mitochondrial NAD+. Science 352, 1474–1477 (2016).
Liu, L. et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 27, 1067–1080.e5 (2018). This study demonstrates that NAD+ synthesis breakdown fluxes differ widely across tissues, suggesting a tissue-specific NAD+ metabolism.
Minhas, P. S. et al. Macrophage de novo NAD synthesis specifies immune function in aging and inflammation. Nat. Immunol. 20, 50–63 (2019).
Carrico, C., Meyer, J. G., He, W., Gibson, B. W. & Verdin, E. The mitochondrial acylome emerges: proteomics, regulation by sirtuins, and metabolic and disease implications. Cell Metab. 27, 497–512 (2018).
Masri, S. et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158, 659–672 (2014).
Cantó, C. et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213–219 (2010).
Ramsey, K. M. et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 (2009).
Nakahata, Y., Sahar, S., Astarita, G., Kaluzova, M. & Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 (2009). This study demonstrates that intracellular NAD+ levels are regulated by the core circadian regulator CLOCK–BMAL1 through the modulation of NAMPT expression.
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000). This study is the first to demonstrates that sirtuin function is dependent on NAD+.
Feldman, J. L., Baeza, J. & Denu, J. M. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J. Biol. Chem. 288, 31350–31356 (2013).
Jiang, H. et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496, 110–113 (2013).
He, W., Newman, J. C., Wang, M. Z., Ho, L. & Verdin, E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol. Metab. 23, 467–476 (2012).
Choudhary, C., Weinert, B. T., Nishida, Y., Verdin, E. & Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 (2014).
Haigis, M. C. et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126, 941–954 (2006).
Liszt, G., Ford, E., Kurtev, M. & Guarente, L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J. Biol. Chem. 280, 21313–21320 (2005).
Amat, R. et al. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α) gene in skeletal muscle through the PGC-1α Autoregulatory Loop and Interaction with MyoD. J. Biol. Chem. 284, 21872–21880 (2009).
Nemoto, S., Fergusson, M. M. & Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 280, 16456–16460 (2005).
Gurd, B. J. Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl. Physiol. Nutr. Metab. 36, 589–597 (2011).
Kang, H. T. & Hwang, E. S. Nicotinamide enhances mitochondria quality through autophagy activation in human cells. Aging Cell 8, 426–438 (2009).
Jang, S.-Y., Kang, H. T. & Hwang, E. S. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J. Biol. Chem. 287, 19304–19314 (2012).
Hottiger, M. O., Hassa, P. O., Lüscher, B., Schüler, H. & Koch-Nolte, F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem. Sci. 35, 208–219 (2010).
Bai, P. & Cantó, C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 16, 290–295 (2012).
Oliver, A. W. et al. Crystal structure of the catalytic fragment of murine poly(ADP-ribose) polymerase-2. Nucleic Acids Res. 32, 456–464 (2004).
Boehler, C. et al. Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc. Natl Acad. Sci. USA 108, 2783–2788 (2011).
Beck, C., Robert, I., Reina-San-Martin, B., Schreiber, V. & Dantzer, F. Poly(ADP-ribose) polymerases in double-strand break repair: focus on PARP1, PARP2 and PARP3. Exp. Cell. Res. 329, 18–25 (2014).
Huber, A., Bai, P., de Murcia, J. M. & de Murcia, G. PARP-1, PARP-2 and ATM in the DNA damage response: functional synergy in mouse development. DNA Repair. 3, 1103–1108 (2004).
Ray Chaudhuri, A. & Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 18, 610–621 (2017).
Bai, P. et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 (2011).
Pirinen, E. et al. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 19, 1034–1041 (2014).
Scheibye-Knudsen, M. et al. A high-fat diet and NAD+ activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 20, 840–855 (2014).
Cantó, C., Sauve, A. A. & Bai, P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol. Asp. Med. 34, 1168–1201 (2013).
Gui, B. et al. Selective targeting of PARP-2 inhibits androgen receptor signaling and prostate cancer growth through disruption of FOXA1 function. Proc. Natl Acad. Sci. USA 116, 14573–14582 (2019).
Bai, P. et al. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 13, 450–460 (2011).
Liu, Q., Kriksunov, I. A., Hao, Q., Graeff, R. & Lee, H. C. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine NAAPD synthesis and hydrolysis activities. J. Biol. Chem. https://doi.org/10.2210/pdb2hct/pdb (2006).
Ernst, I. M. A., Fliegert, R. & Guse, A. H. Adenine dinucleotide second messengers and T-lymphocyte calcium signaling. Front. Immunol. 4, 259 (2013).
Yu, P. et al. Direct gating of the TRPM2 channel by cADPR via specific interactions with the ADPR binding pocket. Cell Rep. 27, 3684–3695.e4 (2019).
Mao, S. Architecture of the human TRPM2 channel. Science 362, 1372.12–1374 (2018).
Torti, M., Bertoni, A., Canobbio, I., Sinigaglia, F. & Balduini, C. Hydrolysis of NADP by platelet CD38 in the absence of synthesis and degradation of cyclic ADP-ribose 2′-phosphate. FEBS Lett. 455, 359–363 (1999).
Camacho-Pereira, J. et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139 (2016). This study demonstrates that CD38 expression increases in multiple tissues during aging and that CD38 is a major NADase involved in the ageing-related tissue decline of NAD+ levels.
Aomatsu, E. et al. Novel SCRG1/BST1 axis regulates self-renewal, migration, and osteogenic differentiation potential in mesenchymal stem cells. Sci. Rep. 4, 3652 (2014).
Preugschat, F. et al. A pre-steady state and steady state kinetic analysis of the N-ribosyl hydrolase activity of hCD157. Arch. Biochem. Biophys. 564, 156–163 (2014).
Covarrubias, A. J. et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2, 1265–1283 (2020).
Ortolan, E., Augeri, S., Fissolo, G., Musso, I. & Funaro, A. CD157: from immunoregulatory protein to potential therapeutic target. Immunol. Lett. 205, 59–64 (2019).
Reinherz, E. L., Kung, P. C., Goldstein, G., Levey, R. H. & Schlossman, S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc. Natl Acad. Sci. USA 77, 1588–1592 (1980).
Shubinsky, G. & Schlesinger, M. The CD38 lymphocyte differentiation marker: new insight into its ectoenzymatic activity and its role as a signal transducer. Immunity 7, 315–324 (1997).
Todd, R. F. 3rd, Roach, J. A. & Arnaout, M. A. The modulated expression of Mo5, a human myelomonocytic plasma membrane antigen. Blood 65, 964–973 (1985).
Quarona, V. et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin. Cytom. 84, 207–217 (2013).
Deaglio, S. et al. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J. Immunol. 160, 395–402 (1998).
Deaglio, S. et al. Human CD38 and its ligand CD31 define a unique lamina propria T lymphocyte signaling pathway. FASEB J. 15, 580–582 (2001).
Vallario, A. et al. Human myeloma cells express the CD38 ligand CD31. Br. J. Haematol. 105, 441–444 (1999).
Deaglio, S. et al. CD38/CD31 interactions activate genetic pathways leading to proliferation and migration in chronic lymphocytic leukemia cells. Mol. Med. 16, 87–91 (2010).
Partida-Sánchez, S. et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 7, 1209–1216 (2001).
Matalonga, J. et al. The nuclear receptor LXR limits bacterial infection of host macrophages through a mechanism that impacts cellular NAD metabolism. Cell Rep. 18, 1241–1255 (2017).
Hogan, K. A., Chini, C. C. S. & Chini, E. N. The multi-faceted ecto-enzyme CD38: roles in immunomodulation, cancer, aging, and metabolic diseases. Front. Immunol. 10, 1187 (2019).
Funaro, A. et al. CD157 is an important mediator of neutrophil adhesion and migration. Blood 104, 4269–4278 (2004).
Essuman, K. et al. The SARM1 Toll/interleukin-1 receptor domain possesses intrinsic NAD cleavage activity that promotes pathological axonal degeneration. Neuron 93, 1334–1343.e5 (2017). This study demonstrates that SARM1 has both NAD+ glycohydrolase and cyclase activity, clarifying the key role of this enzyme in NAD+ depletion during Wallerian degeneration.
Gerdts, J., Brace, E. J., Sasaki, Y., DiAntonio, A. & Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD destruction. Science 348, 453–457 (2015).
Wang, Q. et al. Sarm1/Myd88-5 regulates neuronal intrinsic immune response to traumatic axonal injuries. Cell Rep. 23, 716–724 (2018).
Lin, C.-W., Chen, C.-Y., Cheng, S.-J., Hu, H.-T. & Hsueh, Y.-P. Sarm1 deficiency impairs synaptic function and leads to behavioral deficits, which can be ameliorated by an mGluR allosteric modulator. Front. Cell. Neurosci. 8, 87 (2014).
Carty, M. et al. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 7, 1074–1081 (2006).
Panneerselvam, P. et al. T-cell death following immune activation is mediated by mitochondria-localized SARM. Cell Death Differ. 20, 478–489 (2013).
Zhao, Z. Y. et al. A cell-permeant mimetic of NMN activates SARM1 to produce cyclic ADP-ribose and induce non-apoptotic cell death. iScience 15, 452–466 (2019).
Gürtler, C. et al. SARM regulates CCL5 production in macrophages by promoting the recruitment of transcription factors and RNA polymerase II to the Ccl5 promoter. J. Immunol. 192, 4821–4832 (2014).
Uccellini, M. B. et al. Passenger mutations confound phenotypes of SARM1-deficient mice. Cell Rep. 31, 107498 (2020).
Lautrup, S., Sinclair, D. A., Mattson, M. P. & Fang, E. F. NAD in brain aging and neurodegenerative disorders. Cell Metab. 30, 630–655 (2019).
Hruby, A. & Hu, F. B. The epidemiology of obesity: a big picture. Pharmacoeconomics 33, 673–689 (2015).
Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 121, 21–33 (2009).
Salvestrini, V., Sell, C. & Lorenzini, A. Obesity may accelerate the aging process. Front. Endocrinol. 10, 266 (2019).
Katsyuba, E., Romani, M., Hofer, D. & Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2, 9–31 (2020).
Landry, J. et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl Acad. Sci. USA 97, 5807–5811 (2000).
Smith, J. S. et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl Acad. Sci. USA 97, 6658–6663 (2000).
Fukuwatari, T., Shibata, K., Ishihara, K., Fushiki, T. & Sugimoto, E. Elevation of blood NAD level after moderate exercise in young women and mice. J. Nutr. Sci. Vitaminol. 47, 177–179 (2001).
de Guia, R. M. et al. Aerobic and resistance exercise training reverses age-dependent decline in NAD salvage capacity in human skeletal muscle. Physiol. Rep. 7, e14139 (2019).
Cantó, C. et al. AMPK regulates energy expenditure by modulating NAD metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Mitchell, S. J. et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 23, 1093–1112 (2016).
Elamin, M., Ruskin, D. N., Masino, S. A. & Sacchetti, P. Ketogenic diet modulates NAD-dependent enzymes and reduces DNA damage in hippocampus. Front. Cell. Neurosci. 12, 263 (2018).
Levine, D. C. et al. NAD controls circadian reprogramming through PER2 nuclear translocation to counter aging. Mol. Cell 78, 835–849.e7 (2020).
Cantó, C. et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 (2012).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S.-I. Nicotinamide mononucleotide, a key NAD intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011). This study demonstrates that NAMPT expression can be dampened in inflammatory settings such as ageing and obesity.
Ear, P. H. et al. Maternal nicotinamide riboside enhances postpartum weight loss, juvenile offspring development, and neurogenesis of adult offspring. Cell Rep. 26, 969–983.e4 (2019).
Goodman, R. P. et al. Hepatic NADH reductive stress underlies common variation in metabolic traits. Nature https://doi.org/10.1038/s41586-020-2337-2 (2020).
Hirschey, M. D. et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 (2010).
Hirschey, M. D. et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 44, 177–190 (2011).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127, 1109–1122 (2006).
Pfluger, P. T., Herranz, D., Velasco-Miguel, S., Serrano, M. & Tschöp, M. H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl Acad. Sci. USA 105, 9793–9798 (2008).
Barbosa, M. T. P. et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 21, 3629–3639 (2007).
Szántó, M. & Bai, P. The role of ADP-ribose metabolism in metabolic regulation, adipose tissue differentiation, and metabolism. Genes Dev. 34, 321–340 (2020).
Tarragó, M. G. et al. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD decline. Cell Metab. 27, 1081–1095.e10 (2018). This study demonstrates that the pharmacological inhibition of CD38 reverses age-related NAD+ level decline, improving several physiological and metabolic features.
Stromsdorfer, K. L. et al. NAMPT-mediated NAD+ biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 16, 1851–1860 (2016).
Dollerup, O. L. et al. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J. Clin. Endocrinol. Metab. 104, 5703–5714 (2019).
Remie, C. M. E. et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am. J. Clin. Nutr. https://doi.org/10.1093/ajcn/nqaa072 (2020).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019).
Oishi, Y. & Manabe, I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Dis. 2, 16018 (2016).
van Beek, A. A., Van den Bossche, J., Mastroberardino, P. G., de Winther, M. P. J. & Leenen, P. J. M. Metabolic alterations in aging macrophages: ingredients for inflammaging? Trends Immunol. 40, 113–127 (2019).
Van Gool, F. et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat. Med. 15, 206–210 (2009). This study is one of the first to demonstrate that NAD+ levels can influence innate immune cell function.
Venter, G. et al. NAMPT-mediated salvage synthesis of NAD+ controls morphofunctional changes of macrophages. PLoS ONE 9, e97378 (2014).
Cameron, A. M. et al. Inflammatory macrophage dependence on NAD salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 20, 420–432 (2019).
Regdon, Z. et al. LPS protects macrophages from AIF-independent parthanatos by downregulation of PARP1 expression, induction of SOD2 expression, and a metabolic shift to aerobic glycolysis. Free Radic. Biol. Med. 131, 184–196 (2019).
Virág, L., Jaén, R. I., Regdon, Z., Boscá, L. & Prieto, P. Self-defense of macrophages against oxidative injury: fighting for their own survival. Redox Biol. 26, 101261 (2019).
Chini, C. C. S. et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat. Metab. 2, 1284–1304 (2020). This study, along with Covarrubias et al. (2020), demonstrated that senescent cell burden is linked to tissue NAD+ level decline via CD38+ immune cells.
Chini, C. et al. The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD decline. Biochem. Biophys. Res. Commun. 513, 486–493 (2019).
Youm, Y.-H. et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532 (2013).
Tabula Muris Consortium et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018). This is a compendium of single-cell transcriptomic data that comprises more than 100,000 cells from 20 mouse organs and tissues.
Pathria, P., Louis, T. L. & Varner, J. A. Targeting tumor-associated macrophages in cancer. Trends Immunol. 40, 310–327 (2019).
Adriouch, S., Haag, F., Boyer, O., Seman, M. & Koch-Nolte, F. Extracellular NAD: a danger signal hindering regulatory T cells. Microbes Infect. 14, 1284–1292 (2012).
Hubert, S. et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J. Exp. Med. 207, 2561–2568 (2010).
Tullius, S. G. et al. NAD protects against EAE by regulating CD4 T-cell differentiation. Nat. Commun. 5, 5101 (2014).
Elkhal, A. et al. NAD regulates Treg cell fate and promotes allograft survival via a systemic IL-10 production that is CD4 CD25 Foxp3 T cells independent. Sci. Rep. 6, 22325 (2016).
Fagnoni, F. F. et al. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians. Immunology 88, 501–507 (1996).
Weng, N.-P., Akbar, A. N. & Goronzy, J. CD28- T cells: their role in the age-associated decline of immune function. Trends Immunol. 30, 306–312 (2009).
Jeng, M. Y. et al. Metabolic reprogramming of human CD8 memory T cells through loss of SIRT1. J. Exp. Med. 215, 51–62 (2018).
Chatterjee, S. et al. CD38-NAD+ axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. 27, 85–100.e8 (2018).
Lee, K.-A. et al. Characterization of age-associated exhausted CD8+ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell 15, 291–300 (2016).
Shimada, Y., Hayashi, M., Nagasaka, Y., Ohno-Iwashita, Y. & Inomata, M. Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp. Gerontol. 44, 517–522 (2009).
Xin Yu, J. et al. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat. Rev. Drug Discov. 19, 163–164 (2020).
Akinleye, A. & Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 12, 92 (2019).
Lages, C. S., Lewkowich, I., Sproles, A., Wills-Karp, M. & Chougnet, C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/ PD-L1 pathway. Aging Cell 9, 785–798 (2010).
Verma, V. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1CD38 cells and anti-PD-1 resistance. Nat. Immunol. 20, 1231–1243 (2019).
Chen, L. et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 8, 1156–1175 (2018).
Coppé, J.-P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Basisty, N. et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 (2020). This article presents a comprehensive proteomic database of soluble proteins and exosomal SASP factors originating from multiple senescence inducers and cell types.
Kirkland, J. L., Tchkonia, T., Zhu, Y., Niedernhofer, L. J. & Robbins, P. D. The clinical potential of senolytic drugs. J. Am. Geriatr. Soc. 65, 2297–2301 (2017).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Yoshino, J., Baur, J. A. & Imai, S.-I. NAD intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 27, 513–528 (2018).
Nacarelli, T. et al. NAD metabolism governs the proinflammatory senescence-associated secretome. Nat. Cell Biol. 21, 397–407 (2019). This article demonstrates that the NAD+ salvage pathway is upregulated during cellular senescence and regulates the expression of inflammatory SASP genes.
Desdín-Micó, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science https://doi.org/10.1126/science.aax0860 (2020).
Zhu, X.-H., Lu, M., Lee, B.-Y., Ugurbil, K. & Chen, W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl Acad. Sci. 112, 2876–2881 (2015).
Fang, E. F. et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD+/SIRT1 reduction. Cell 157, 882–896 (2014).
Fang, E. F. et al. NAD replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 24, 566–581 (2016).
Gong, B. et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging 34, 1581–1588 (2013).
Schöndorf, D. C. et al. The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep. 23, 2976–2988 (2018).
Birkmayer, J. G., Vrecko, C., Volc, D. & Birkmayer, W. Nicotinamide adenine dinucleotide (NADH)–a new therapeutic approach to Parkinson’s disease. Comparison of oral and parenteral application. Acta Neurol. Scand. Suppl. 146, 32–35 (1993).
Harlan, B. A. et al. Evaluation of the NAD biosynthetic pathway in ALS patients and effect of modulating NAD levels in hSOD1-linked ALS mouse models. Exp. Neurol. 327, 113219 (2020).
Salvadores, N., Sanhueza, M., Manque, P. & Court, F. A. Axonal degeneration during aging and its functional role in neurodegenerative disorders. Front. Neurosci. 11, 451 (2017).
Lingor, P., Koch, J. C., Tönges, L. & Bähr, M. Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res. 349, 289–311 (2012).
Gilley, J. & Coleman, M. P. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 8, e1000300 (2010).
Loreto, A. et al. Mitochondrial impairment activates the Wallerian pathway through depletion of NMNAT2 leading to SARM1-dependent axon degeneration. Neurobiol. Dis. 134, 104678 (2020).
Wang, J. et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J. Cell Biol. 170, 349–355 (2005).
Araki, T., Sasaki, Y. & Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013 (2004).
Sasaki, Y., Nakagawa, T., Mao, X., DiAntonio, A. & Milbrandt, J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD depletion. eLife 5, e19749 (2016).
Gilley, J., Adalbert, R., Yu, G. & Coleman, M. P. Rescue of peripheral and CNS axon defects in mice lacking NMNAT2. J. Neurosci. 33, 13410–13424 (2013).
Gilley, J., Orsomando, G., Nascimento-Ferreira, I. & Coleman, M. P. Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep. 10, 1974–1981 (2015).
Geisler, S. et al. Gene therapy targeting SARM1 blocks pathological axon degeneration in mice. J. Exp. Med. 216, 294–303 (2019).
Kitaoka, Y. et al. Axonal protection by Nmnat3 overexpression with involvement of autophagy in optic nerve degeneration. Cell Death Dis. 4, e860 (2013).
Yahata, N., Yuasa, S. & Araki, T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J. Neurosci. 29, 6276–6284 (2009).
Pellegatta, M. & Taveggia, C. The complex work of proteases and secretases in wallerian degeneration: beyond neuregulin-1. Front. Cell. Neurosci. 13, 93 (2019).
Conforti, L., Gilley, J. & Coleman, M. P. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat. Rev. Neurosci. 15, 394–409 (2014).
Williams, P. A. et al. Nicotinamide and WLD act together to prevent neurodegeneration in glaucoma. Front. Neurosci. 11, 232 (2017).
Brown, K. D. et al. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 20, 1059–1068 (2014).
Stefano, M. D. et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 22, 731–742 (2015).
Voorhees, J. R. et al. (-)-P7C3-S243 protects a rat model of Alzheimer’s disease from neuropsychiatric deficits and neurodegeneration without altering amyloid deposition or reactive glia. Biol. Psychiatry 84, 488–498 (2018).
Tesla, R. et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 109, 17016–17021 (2012).
Blacher, E. et al. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann. Neurol. 78, 88–103 (2015).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 599 (2018).
Long, A. N. et al. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 15, 19 (2015).
Long, A. et al. CD38 knockout mice show significant protection against ischemic brain damage despite high level poly-ADP-ribosylation. Neurochem. Res. 42, 283–293 (2017).
Mayo, L. et al. Dual role of CD38 in microglial activation and activation-induced cell death. J. Immunol. 181, 92–103 (2008).
Banerjee, S. et al. CD38/cyclic ADP-ribose regulates astrocyte calcium signaling: implications for neuroinflammation and HIV-1-associated dementia. J. Neuroimmune Pharmacol. 3, 154–164 (2008).
Van Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019).
Jin, D. et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45 (2007).
Higashida, H. et al. An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC Neurosci. 18, 35 (2017).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69, S4–S9 (2014).
Zhang, P. et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 22, 719–728 (2019).
Kauppinen, T. M. et al. Poly(ADP-ribose)polymerase-1 modulates microglial responses to amyloid β. J. Neuroinflammation 8, 152 (2011).
Bayrakdar, E. T. et al. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Aβ(1–42)-induced rat model of Alzheimer’s disease. Free. Radic. Res. 48, 146–158 (2014).
Wu, X.-L., Wang, P., Liu, Y.-H. & Xue, Y.-X. Effects of poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide on blood–brain barrier and dopaminergic neurons of rats with lipopolysaccharide-induced Parkinson’s disease. J. Mol. Neurosci. 53, 1–9 (2014).
Mandir, A. S. et al. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc. Natl Acad. Sci. USA 96, 5774–5779 (1999).
Kim, T. W. et al. ADP-ribose) polymerase 1 and AMP-activated protein kinase mediate progressive dopaminergic neuronal degeneration in a mouse model of Parkinson’s disease. Cell Death Dis. 4, e919 (2013).
Hou, Y. et al. NAD supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl Acad. Sci. USA 115, E1876–E1885 (2018).
Yao, Z., Yang, W., Gao, Z. & Jia, P. Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci. Lett. 647, 133–140 (2017).
Wang, X., Hu, X., Yang, Y., Takata, T. & Sakurai, T. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 1643, 1–9 (2016).
Chi, Y. & Sauve, A. A. Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care 16, 657–661 (2013).
Sorrentino, V. et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552, 187–193 (2017).
Fang, E. F. et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 22, 401–412 (2019).
Lehmann, S., Loh, S. H. Y. & Miguel Martins, L. Enhancing NAD salvage metabolism is neuroprotective in a PINK1 model of Parkinson’s disease. Biol. Open 6, 141–147 (2017).
Jia, H. et al. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J. Neurosci. Res. 86, 2083–2090 (2008).
Okabe, K., Yaku, K., Tobe, K. & Nakagawa, T. Implications of altered NAD metabolism in metabolic disorders. J. Biomed. Sci. 26, 34 (2019).
Connell, N. J., Houtkooper, R. H. & Schrauwen, P. NAD metabolism as a target for metabolic health: have we found the silver bullet? Diabetologia 62, 888–899 (2019).
Martens, C. R. et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD in healthy middle-aged and older adults. Nat. Commun. 9, 1286 (2018).
Dollerup, O. L. et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 108, 343–353 (2018).
de la Rubia, J. E. et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled human pilot study. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 115–122 (2019).
Elhassan, Y. S. et al. Nicotinamide riboside augments the aged human skeletal muscle NAD metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 28, 1717–1728.e6 (2019).
Belenky, P. et al. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell 129, 473–484 (2007).
Mouchiroud, L. et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 (2013).
Schmeisser, K. et al. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat. Chem. Biol. 9, 693–700 (2013).
Gallo, C. M., Smith, D. L. Jr & Smith, J. S. Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol. Cell. Biol. 24, 1301–1312 (2004).
Saldeen, J., Tillmar, L., Karlsson, E. & Welsh, N. Nicotinamide- and caspase-mediated inhibition of poly(ADP-ribose) polymerase are associated with p53-independent cell cycle (G2) arrest and apoptosis. Mol. Cell. Biochem. 243, 113–122 (2003).
Avalos, J. L., Bever, K. M. & Wolberger, C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD+ cosubstrate specificity of a Sir2 enzyme. Mol. Cell 17, 855–868 (2005).
Hwang, E. S. & Song, S. B. Possible adverse effects of high-dose nicotinamide: mechanisms and safety assessment. Biomolecules 10, 387 (2020).
Fang, E. F. et al. NAD augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat. Commun. 10, 5284 (2019).
Ryu, D. et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci. Transl Med. 8, 361ra139 (2016).
Zhang, H. et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443 (2016).
Mills, K. F. et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 24, 795–806 (2016).
Mitchell, S. J. et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metab. 27, 667–676.e4 (2018).
Gomes, A. P. et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638 (2013).
Sims, C. A. et al. Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock. JCI Insight 3, e120182 (2018).
Uddin, G. M., Youngson, N. A., Sinclair, D. A. & Morris, M. J. Head to head comparison of short-term treatment with the NAD+ precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice. Front. Pharmacol. 7, 258 (2016).
Guan, Y. et al. Nicotinamide mononucleotide, an NAD precursor, rescues age-associated susceptibility to AKI in a sirtuin 1–dependent manner. J. Am. Soc. Nephrol. 28, 2337–2352 (2017).
Lee, C. F. et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 134, 883–894 (2016).
Lin, J. B. et al. NAMPT-mediated NAD+ biosynthesis is essential for vision in mice. Cell Rep. 17, 69–85 (2016).
Martin, A. S. et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight 2, e93885 (2017).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 (2016).
Tarantini, S. et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 24, 101192 (2019).
Das, A. et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell 173, 74–89.e20 (2018).
Giroud-Gerbetant, J. et al. A reduced form of nicotinamide riboside defines a new path for NAD biosynthesis and acts as an orally bioavailable NAD precursor. Mol. Metab. 30, 192–202 (2019).
Yang, Y., Zhang, N., Zhang, G. & Sauve, A. A. NRH salvage and conversion to NAD requires NRH kinase activity by adenosine kinase. Nat. Metab. 2, 364–379 (2020).
Zhou, T. et al. Structure of human nicotinamide/nicotinic acid mononucleotide adenylyltransferase. Basis for the dual substrate specificity and activation of the oncolytic agent tiazofurin. J. Biol. Chem. 277, 13148–13154 (2002).
Wang, G. et al. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 158, 1324–1334 (2014).
Gardell, S. J. et al. Boosting NAD with a small molecule that activates NAMPT. Nat. Commun. 10, 3241 (2019).
Katsyuba, E. et al. De novo NAD synthesis enhances mitochondrial function and improves health. Nature 563, 354–359 (2018).
Diaz-Ruiz, A. et al. Benefits of caloric restriction in longevity and chemical-induced tumorigenesis are transmitted independent of NQO1. J. Gerontol. A Biol. Sci. Med. Sci. 74, 155–162 (2019).
Kim, H.-J. et al. Augmentation of cellular NAD by NQO1 enzymatic action improves age-related hearing impairment. Aging Cell 18, e13016 (2019).
Lee, J.-S. et al. Beta-lapachone, a modulator of NAD metabolism, prevents health declines in aged mice. PLoS ONE 7, e47122 (2012).
Morales, J. et al. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. Eukaryot. Gene Expr. 24, 15–28 (2014).
Xia, Q. et al. PARP-1 inhibition rescues short lifespan in hyperglycemic C. elegans and improves GLP-1 secretion in human cells. Aging Dis. 9, 17 (2018).
Brown, J. S., Kaye, S. B. & Yap, T. A. PARP inhibitors: the race is on. Br. J. Cancer 114, 713–715 (2016).
Alano, C. C. et al. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J. Neurosci. 30, 2967–2978 (2010).
Almeida, G. S. et al. PARP inhibitor rucaparib induces changes in NAD levels in cells and liver tissues as assessed by MRS. NMR Biomed. https://doi.org/10.1002/nbm.3736 (2017).
Mathews, M. T. & Berk, B. C. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler. Thromb. Vasc. Biol. 28, 711–717 (2008).
Escande, C. et al. Flavonoid apigenin is an inhibitor of the NAD+ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62, 1084–1093 (2013).
Ogura, Y., Kitada, M., Xu, J., Monno, I. & Koya, D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging 12, 11325–11336 (2020).
Boslett, J., Hemann, C., Zhao, Y. J., Lee, H.-C. & Zweier, J. L. Luteolinidin protects the postischemic heart through CD38 inhibition with preservation of NAD(P)(H). J. Pharmacol. Exp. Ther. 361, 99–108 (2017).
Taliou, A., Zintzaras, E., Lykouras, L. & Francis, K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Ther. 35, 592–602 (2013).
Haffner, C. D. et al. Discovery, synthesis, and biological evaluation of thiazoloquin(az)olin(on)es as potent CD38 inhibitors. J. Med. Chem. 58, 3548–3571 (2015).
Boslett, J., Reddy, N., Alzarie, Y. A. & Zweier, J. L. Inhibition of CD38 with the thiazoloquin(az)olin(on)e 78c protects the heart against postischemic injury. J. Pharmacol. Exp. Ther. 369, 55–64 (2019).
Chini, E. N., Chini, C. C. S., Espindola Netto, J. M., de Oliveira, G. C. & van Schooten, W. The pharmacology of CD38/NADase: an emerging target in cancer and diseases of aging. Trends Pharmacol. Sci. 39, 424–436 (2018).
Liu, D. et al. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol. Aging 34, 1564–1580 (2013).
Park, J. H., Long, A., Owens, K. & Kristian, T. Nicotinamide mononucleotide inhibits post-ischemic NAD+ degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol. Dis. 95, 102–110 (2016).
Wei, C.-C. et al. Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway. Sci. Rep. 7, 717 (2017).
Wei, C.-C. et al. NAD replenishment with nicotinamide mononucleotide protects blood-brain barrier integrity and attenuates delayed tissue plasminogen activator-induced haemorrhagic transformation after cerebral ischaemia. Br. J. Pharmacol. 174, 3823–3836 (2017).
North, B. J. et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 33, 1438–1453 (2014).
Frederick, D. W. et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 24, 269–282 (2016).
Gariani, K. et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology 63, 1190–1204 (2016).
Lee, H. J., Hong, Y.-S., Jun, W. & Yang, S. J. Nicotinamide riboside ameliorates hepatic metaflammation by modulating NLRP3 inflammasome in a rodent model of type 2 diabetes. J. Med. Food 18, 1207–1213 (2015).
Mukherjee, S. et al. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology 65, 616–630 (2017).
Hamity, M. V. et al. Nicotinamide riboside, a form of vitamin B3 and NAD+ precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain 158, 962–972 (2017).
Trammell, S. A. J. et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci. Rep. 6, 26933 (2016).
Fang, Q. et al. HSP90 regulates DNA repair via the interaction between XRCC1 and DNA polymerase β. Nat. Commun. 5, 5513 (2014).
Ratajczak, J. et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. 7, 13103 (2016).
Grozio, A. et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 1, 47–57 (2019). This article describes the discovery and characterization of a novel NMN transporter in mammals.
Berger, F., Lau, C., Dahlmann, M. & Ziegler, M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 280, 36334–36341 (2005).
Zhang, X. et al. Structural characterization of a human cytosolic NMN/NaMN adenylyltransferase and implication in human NAD biosynthesis. J. Biol. Chem. 278, 13503–13511 (2003).
Koch-Nolte, F., Fischer, S., Haag, F. & Ziegler, M. Compartmentation of NAD+-dependent signalling. FEBS Lett. 585, 1651–1656 (2011).
Yamamoto, M. et al. Nmnat3 is dispensable in mitochondrial NAD level maintenance in vivo. PLoS ONE 11, e0147037 (2016).
Bruzzone, S. et al. Catastrophic NAD+ depletion in activated T lymphocytes through Nampt inhibition reduces demyelination and disability in EAE. PLoS ONE 4, e7897 (2009).
Yang, H. et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107 (2007).
Pillai, J. B., Isbatan, A., Imai, S.-I. & Gupta, M. P. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J. Biol. Chem. 280, 43121–43130 (2005).
Luo, X. & Kraus, W. L. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 26, 417–432 (2012).
Revollo, J. R., Grimm, A. A. & Imai, S.-I. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 (2004).
Wang, T. et al. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat. Struct. Mol. Biol. 13, 661–662 (2006).
Yoon, M. J. et al. SIRT1-mediated eNAMPT Secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab. 21, 706–717 (2015).
Sociali, G. et al. SIRT6 deacetylase activity regulates NAMPT activity and NAD(P)(H) pools in cancer cells. FASEB J. 33, 3704–3717 (2019).
Yoshida, M. et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 30, 329–342.e5 (2019). This article demonstrates the presence of the catalytically active extracellular NAMPT in exosomes and a novel paracrine mechanism of NAD+ biosynthesis.
Samal, B. et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 14, 1431–1437 (1994).
Garten, A. et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 11, 535–546 (2015).
Kieswich, J. et al. Monomeric eNAMPT in the development of experimental diabetes in mice: a potential target for type 2 diabetes treatment. Diabetologia 59, 2477–2486 (2016).
Hara, N., Yamada, K., Shibata, T., Osago, H. & Tsuchiya, M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS ONE 6, e22781 (2011).
Pissios, P. Nicotinamide N-methyltransferase: more than a vitamin B3 clearance enzyme. Trends Endocrinol. Metab. 28, 340–353 (2017).
Kraus, D. et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 508, 258–262 (2014).
Komatsu, M. et al. NNMT activation can contribute to the development of fatty liver disease by modulating the NAD metabolism. Sci. Rep. 8, 8637 (2018).
Rudolphi, B. et al. Body weight predicts nicotinamide N-methyltransferase activity in mouse fat. Endocr. Res. 43, 55–63 (2018).
Hong, S. et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat. Med. 21, 887–894 (2015).
Gokarn, R. et al. Long-term dietary macronutrients and hepatic gene expression in aging mice. J. Gerontol. A Biol. Sci. Med. Sci. 73, 1618–1625 (2018).
Neelakantan, H. et al. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem. Pharmacol. 163, 481–492 (2019).
Hoxhaj, G. et al. Direct stimulation of NADP synthesis through Akt-mediated phosphorylation of NAD kinase. Science 363, 1088–1092 (2019).
Baar, E. L., Carbajal, K. A., Ong, I. M. & Lamming, D. W. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell 15, 155–166 (2016).
Acknowledgements
This Review was supported by NIH grant R24DK085610 (E.V.) and Buck Institute for Research on Aging intramural funds (E.V.). A.J.C. is a recipient of a University of California President’s Postdoctoral Fellowship at the University of California, San Francisco, and is also supported by an NIH T32 training grant (3T32AG000266-19S1).
Author information
Authors and Affiliations
Contributions
All authors contributed to the research for and discussion, writing and review of the manuscript. A.J.C., R.P. and A.G. designed the figures and tables.
Corresponding author
Ethics declarations
Competing interests
E.V. is a scientific co-founder of Napa Therapeutics and serves on the scientific advisory board of Seneque. E.V., A.J.C. and R.P. receive research support from Napa Therapeutics. E.V. and A.G. receive research support from BaReCia. A.G serves as Chief Scientific Officer for Seneque USA and is one of the inventors on a patent (PCT/US18/46233) for the SLC12A8 nicotinamide mononucleotide transporter, whose applicant is Washington University in St. Louis and which has been licensed by Teijin Limited (Japan).
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks J. Auwerx and M. Hirschey and T. Nakagawa for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Redox reactions
-
Oxidation–reduction chemical reactions that involve a transfer of electrons between two species.
- Hydride
-
Formally, the anion of hydrogen (H−). The term is commonly used to describe a binary compound that hydrogen forms with other electropositive elements.
- Kynurenic acid
-
A quinoline-2-carboxylic acid with a hydroxy group at C-4 (4-hydroxyquinoline-2-carboxylic acid). It is a product of the metabolism of l-tryptophan.
- Picolinic acid
-
A pyridinemonocarboxylic acid in which the carboxy group is located at position 2 (pyridine-2-carboxylic acid). It is an intermediate in the metabolism of l-tryptophan.
- Extracellular vesicles
-
Lipid bilayer-delimited particles of endosomal and plasma membrane origin that are released by cells in the extracellular milieu.
- Insulin resistance
-
An impaired response to exogenous or endogenous insulin to increase glucose uptake and utilization, resulting in elevated levels of glucose in the blood.
- Xeroderma pigmentosum
-
A rare autosomal recessive genetic disorder characterized by a defect in the DNA repair system (primarily in the nucleotide excision repair system) that causes increased sensitivity to the DNA-damaging effects of ultraviolet radiation.
- Progeroid diseases
-
A group of rare genetic disorders characterized by clinical features typical of physiological ageing and mostly due to defects in the DNA repair system or in lamin A.
- Ataxia telangiectasia
-
A rare autosomal recessive genetic disorder caused by defects in the ATM gene, which is involved in cell division and DNA repair. It is characterized by neurodegeneration, immunodeficiency, increased radiation sensitivity and cancer susceptibility.
- Cockayne syndrome
-
A rare autosomal recessive genetic disorder caused by defects in the ERCC6 or ERCC8 gene, which is involved in DNA repair. It is characterized by severe photosensitivity, neurodegeneration and premature ageing.
- Hormesis
-
A biphasic dose response to an agent characterized by a stimulatory or beneficial effect at low dose and an inhibitory or toxic effect at high dose.
- AKT
-
A serine/threonine-specific protein kinase that participates in multiple signalling pathways related to metabolism, cell survival, motility, transcription and cell cycle progression.
- K m
-
Michaelis–Menten constant representing the substrate concentration at which the reaction is half the maximum velocity (Vmax) in the Michaelis–Menten enzymatic kinetic model.
- V max
-
The maximum reaction rate achieved by the system at saturated substrate concentration in the Michaelis–Menten enzymatic kinetic model.
- Ectoenzymes
-
Enzymes located on the outer surface of a cell’s membrane with their catalytic site available to the exterior environment of the cell.
- Transmembrane protein with a type II orientation
-
Integral cell membrane protein with the amino terminus on the cytoplasmic side and the carboxy terminus on the extracellular side of the membrane. The transmembrane domain is located close to the amino terminus. Type III transmembrane proteins show an opposite orientation.
- Glycophosphatidylinositol-anchored protein
-
Soluble protein attached by a glycolipid anchor (glycophosphatidylinositol) to the cell membrane.
- Paneth cells
-
Highly specialized epithelial cells located in the small intestine secreting antimicrobial peptides and proteins.
- Inflammageing
-
Defined as the low-grade chronic inflammation and immune cell dysregulation/exhaustion that occurs gradually during the ageing process. Inflammageing is emerging as a key causal factor for many age-related diseases.
- NLRP3 inflammasome
-
A multiprotein complex made up of the proteins ASC, NLRP3 and caspase 1 that is activated in response to pathogens or sterile cell and tissue damage. When activated, the complex leads to the cleavage of the proforms of the cytokines IL-1β and IL-18, which themselves become activated and secreted to further amplify inflammation and immune responses.
- Virtual memory T cells
-
A subpopulation of CD8+ T cells that have a memory-like phenotype (semidifferentiated) but are never been exposed to a foreign antigen (antigen naive).
- Regulatory T cells
-
A subpopulation of CD4+ T cells that modulate the immune system, suppressing the immune response and maintaining tolerance to self-antigens.
- Immune checkpoint
-
Molecules present on the surface of different cell types (that is, T cells, antigen-presenting cells and cancer cells) that regulate the immune response via inhibitory or activating immune signalling pathways.
- Senolytics
-
Agents that target and eliminate senescent cells.
- Wallerian degeneration
-
An active process of degeneration that occurs after any lesion or interruption of axons of neurons that ultimately leads to cell death.
- Microglia
-
Type of glial cell located throughout the brain and spinal cord that functions as a resident macrophage and is the first and main form of active immune defence in the central nervous system.
- Astrocytes
-
Highly specialized star-shaped glial cells located in the brain and spinal cord and involved in several processes, including support of the blood–brain barrier, provision of nutrients to neurons, repair and scarring following injury, and facilitation of neurotransmission
- α-Amino-β-carboxymuconate ε-semialdehyde decarboxylase
-
In de novo NAD+ biosynthesis, it is the enzyme that catalyses the decarboxylation of α-amino-β-carboxymuconate ε-semialdehyde to α-aminomuconate ε-semialdehyde.
- Flavonoid
-
A member of a class of polyphenolic secondary metabolites found in plants.
- AMPK
-
5′-AMP-activated protein kinase, an enzyme that on changes in the ATP:AMP ratio phosphorylates downstream targets to redirect metabolism towards increased catabolism and decreased anabolism.
- mTOR–p70S6K
-
Signalling pathway that involves the serine/threonine protein kinase mTOR and the mitogen-activated serine/threonine protein kinase p70S6K and regulates protein synthesis and cell proliferation, differentiation and survival.
- ERK
-
Extracellular signal-regulated kinase involved in cell growth by controlling many proteins required in translation regulation.
Rights and permissions
About this article
Cite this article
Covarrubias, A.J., Perrone, R., Grozio, A. et al. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 22, 119–141 (2021). https://doi.org/10.1038/s41580-020-00313-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-020-00313-x