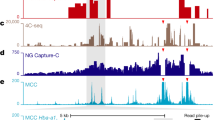
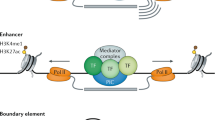
Abstract
In animals, the sequences for controlling gene expression do not concentrate just at the transcription start site of genes, but are frequently thousands to millions of base pairs distal to it. The interaction of these sequences with one another and their transcription start sites is regulated by factors that shape the three-dimensional (3D) organization of the genome within the nucleus. Over the past decade, indirect tools exploiting high-throughput DNA sequencing have helped to map this 3D organization, have identified multiple key regulators of its structure and, in the process, have substantially reshaped our view of how 3D genome architecture regulates transcription. Now, new tools for high-throughput super-resolution imaging of chromatin have directly visualized the 3D chromatin organization, settling some debates left unresolved by earlier indirect methods, challenging some earlier models of regulatory specificity and creating hypotheses about the role of chromatin structure in transcriptional regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Levine, M., Cattoglio, C. & Tjian, R. Looping back to leap forward: transcription enters a new era. Cell 157, 13–25 (2014).
Furlong, E. E. M. & Levine, M. Developmental enhancers and chromosome topology. Science 361, 1341–1345 (2018). This review discusses enhancers, transcriptional regulation and the role of 3D genome organization, focusing on models and hypotheses.
Robson, M. I., Ringel, A. R. & Mundlos, S. Regulatory landscaping: how enhancer-promoter communication is sculpted in 3D. Mol. Cell 74, 1110–1122 (2019).
de Laat, W. & Duboule, D. Topology of mammalian developmental enhancers and their regulatory landscapes. Nature 502, 499–506 (2013).
Marinić, M., Aktas, T., Ruf, S. & Spitz, F. An integrated holo-enhancer unit defines tissue and gene specificity of the Fgf8 regulatory landscape. Dev. Cell 24, 530–542 (2013).
Lorberbaum, D. S. et al. An ancient yet flexible cis-regulatory architecture allows localized Hedgehog tuning by patched/Ptch1. eLlife 5, e13550 (2016).
Ibrahim, D. M. & Mundlos, S. Three-dimensional chromatin in disease: what holds us together and what drives us apart? Curr. Opin. Cell Biol. 64, 1–9 (2020).
Long, H. K., Prescott, S. L. & Wysocka, J. Ever-changing landscapes: transcriptional enhancers in development and evolution. Cell 167, 1170–1187 (2016).
Santiago-Algarra, D., Dao, L. T. M., Pradel, L., España, A. & Spicuglia, S. Recent advances in high-throughput approaches to dissect enhancer function. F1000Res. 6, 939 (2017).
Agbleke, A. A. et al. Advances in chromatin and chromosome research: perspectives from multiple fields. Mol. Cell 79, 881–901 (2020).
Shaban, H. A., Barth, R. & Bystricky, K. Navigating the crowd: visualizing coordination between genome dynamics, structure, and transcription. Genome Biol. 21, 278 (2020).
Shaban, H. A. & Seeber, A. Monitoring the spatio-temporal organization and dynamics of the genome. Nucleic Acids Res. 48, 3423–3434 (2020).
Jerkovic´, I. & Cavalli, G. Understanding 3D genome organization by multidisciplinary methods. Nat. Rev. Mol. Cell Biol. 22, 511–528 (2021). This relatively comprehensive article reviews the primary methods used in studying chromatin structure and organization.
Lim, B. & Levine, M. S. Enhancer–promoter communication: hubs or loops? Curr. Opin. Genet. Dev. 67, 5–9 (2020).
Dekker, J. et al. The 4D nucleome project. Nature 549, 219–226 (2017).
Goel, V. Y. & Hansen, A. S. The macro and micro of chromosome conformation capture. Wiley Interdisc. Rev. Dev. Biol. 10, e395 (2021).
Mirny, L. & Dekker, J. Mechanisms of chromosome folding and nuclear organization: their interplay and open questions. Cold Spring Harb. Perspect. Biol. 14, a040147 (2021). This recent review summarizes major advances in the field (particularly from Hi-C and modelling), and highlights many open questions for the field.
McCord, R. P., Kaplan, N. & Giorgetti, L. Chromosome conformation capture and beyond: toward an integrative view of chromosome structure and function. Mol. Cell 77, 688–708 (2020).
Dekker, J. & Mirny, L. A. The 3D genome as moderator of chromosomal communication. Cell 164, 1110–1121 (2016).
Banerji, J., Rusconi, S. & Schaffner, W. Expression of a B-globin gene is enhanced by remote SV40 DNA sequences. Cell 27, 299–308 (1981).
Banerji, J., Olson, L. & Schaffner, W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell 33, 729–740 (1983).
Driever, W., Thoma, G. & Nüsslein-Volhard, C. Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature 340, 363–367 (1989).
Stanojevic, D., Small, S. & Levine, M. Regulation of a segmentation stripe by overlapping activators and repressors in the Drosophila embryo. Science 254, 1385–1387 (1991).
Chung, J. H., Whiteley, M. & Felsenfeld, G. A 5′ element of the chicken β-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74, 505–514 (1993).
Udvardy, A., Maine, E. & Schedl, P. The 87A7 chromomere. Identification of novel chromatin structures flanking the heat shock locus that may define the boundaries of higher order domains. J. Mol. Biol. 185, 341–358 (1985).
Kellum, R. & Schedl, P. A position-effect assay for boundaries of higher order chromosomal domains. Cell 64, 941–950 (1991).
Dekker, J. & Misteli, T. Long-range chromatin interactions. Cold Spring Harb. Perspect. Biol. 7, a019356 (2015).
Han, J., Zhang, Z. & Wang, K. 3C and 3C-based techniques: the powerful tools for spatial genome organization deciphering. Mol. Cytogenet. 11, 21 (2018).
de Wit, E. & de Laat, W. A decade of 3C technologies: insights into nuclear organization. Genes Dev. 26, 11–24 (2012).
Sati, S. & Cavalli, G. Chromosome conformation capture technologies and their impact in understanding genome function. Chromosoma 126, 33–44 (2017).
Dekker, J., Rippe, K., Dekker, M. & Kleckner, N. Capturing chromosome conformation. Science 295, 1306–1311 (2002).
Deng, W. et al. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell 149, 1233–1244 (2012).
Palstra, R.-J. et al. The β-globin nuclear compartment in development and erythroid differentiation. Nat. Genet. 35, 190–194 (2003).
Kagey, M. H. et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature 467, 430–435 (2010).
Amano, T. et al. Chromosomal dynamics at the Shh locus: limb bud-specific differential regulation of competence and active transcription. Dev. Cell 16, 47–57 (2009).
Noordermeer, D. et al. The dynamic architecture of Hox gene clusters. Science 334, 222–225 (2011).
Montavon, T. et al. A regulatory archipelago controls hox genes transcription in digits. Cell 147, 1132–1145 (2011).
Schoenfelder, S. & Fraser, P. Long-range enhancer–promoter contacts in gene expression control. Nat. Rev. Genet. 20, 437–455 (2019).
Phillips-Cremins, J. E. et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell 4, 3–7 (2013).
Mumbach, M. R. et al. HiChIP: efficient and sensitive analysis of protein-directed genome architecture. Nat. Methods 13, 919–922 (2016).
Mumbach, M. R. et al. Enhancer connectome in primary human cells identifies target genes of disease-associated DNA elements. Nat. Genet. 49, 1602–1612 (2017).
Li, G. et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 (2012).
Fullwood, M. J. et al. An oestrogen-receptor-α-bound human chromatin interactome. Nature 462, 58–64 (2009).
Fang, R. et al. Mapping of long-range chromatin interactions by proximity ligation-assisted ChIP-seq. Cell Res. 26, 1345–1348 (2016).
Lieberman Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009).
Imakaev, M. et al. Iterative correction of Hi-C data reveals hallmarks of chromosome organization. Nat. Methods 9, 999–1003 (2012).
Rao, S. S. P. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Nora, E. P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Sexton, T. et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148, 458–472 (2012).
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012).
Le Dily, F. et al. Distinct structural transitions of chromatin topological domains correlate with coordinated hormone-induced gene regulation. Genes Dev. 28, 2151–2162 (2014).
Symmons, O. et al. Functional and topological characteristics of mammalian regulatory domains. Genome Res. 24, 390–400 (2014).
Cavalheiro, G. R., Pollex, T. & Furlong, E. E. To loop or not to loop: what is the role of TADs in enhancer function and gene regulation? Curr. Opin. Genet. Dev. 67, 119–129 (2021).
Ibrahim, D. M. & Mundlos, S. The role of 3D chromatin domains in gene regulation: a multi-facetted view on genome organization. Curr. Opin. Genet. Dev. 61, 1–8 (2020).
Paliou, C. et al. Preformed chromatin topology assists transcriptional robustness of Shh during limb development. Proc. Natl Acad. Sci. USA 116, 12390–12399 (2019).
Williamson, I. et al. Developmentally regulated Shh expression is robust to TAD perturbations. Development 146, dev179523 (2019).
Williamson, I., Lettice, L. A., Hill, R. E. & Bickmore, W. A. Shh and ZRS enhancer colocalisation is specific to the zone of polarising activity. Development 143, 2994–3001 (2016).
Symmons, O. et al. The Shh topological domain facilitates the action of remote enhancers by reducing the effects of genomic distances. Dev. Cell 39, 529–543 (2016).
Lupiáñez, D. G. et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene–enhancer interactions. Cell 161, 1012–1025 (2015).
Kraft, K. et al. Serial genomic inversions induce tissue-specific architectural stripes, gene misexpression and congenital malformations. Nat. Cell Biol. 21, 305–310 (2019).
Despang, A. et al. Functional dissection of the Sox9–Kcnj2 locus identifies nonessential and instructive roles of TAD architecture. Nat. Genet. 51, 1263–1271 (2019).
Beagan, J. A. & Phillips-Cremins, J. E. On the existence and functionality of topologically associating domains. Nat. Genet. 52, 8–16 (2020).
Alexander, J. M. et al. Live-cell imaging reveals enhancer-dependent Sox2 transcription in the absence of enhancer proximity. eLife 8, e41769 (2019). This paper describes two-point live imaging in mouse embryonic stem cells with live readout of mRNA activity, and found no correlation of E-P proximity and promoter firing, in parallel work to Chen et al. (2018).
Huang, H. et al. CTCF mediates dosage- and sequence-context-dependent transcriptional insulation by forming local chromatin domains. Nat. Genet. 53, 1064–1074 (2021).
Flavahan, W. A. et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529, 110–114 (2016).
Phanstiel, D. H. et al. Static and dynamic DNA loops form AP-1-bound activation hubs during macrophage development. Mol. Cell 67, 1037–1048 (2017).
Bonev, B. et al. Multiscale 3D genome rewiring during mouse neural development. Cell 171, 557–572.e24 (2017).
Klein, D. C. & Hainer, S. J. Genomic methods in profiling DNA accessibility and factor localization. Chromosome Res. 28, 69–85 (2020).
Krietenstein, N. et al. Ultrastructural details of mammalian chromosome architecture. Mol. Cell 78, 554–565.e7 (2020).
Hsieh, T.-H. S. et al. Resolving the 3D landscape of transcription-linked mammalian chromatin folding. Mol. Cell 78, 539–553.e8 (2020).
Hsieh, T.-H. S. et al. Enhancer–promoter interactions and transcription are maintained upon acute loss of CTCF, cohesin, WAPL, and YY1. Preprint at bioRxiv https://doi.org/10.1101/2021.07.14.452365 (2021).
Aljahani, A. et al. Analysis of sub-kilobase chromatin topology reveals nano-scale regulatory interactions with variable dependence on cohesin and CTCF. Nat. Commun. 13, 2139 (2022).
Goel, V. Y., Huseyin, M. K. & Hansen, A. S. Region capture micro-C reveals coalescence of enhancers and promoters into nested microcompartments. Preprint at bioRxiv https://doi.org/10.1101/2022.07.12.499637 (2022).
Levo, M. et al. Transcriptional coupling of distant regulatory genes in living embryos. Nature 605, 754–760 (2022).
Batut, P. J. et al. Genome organization controls transcriptional dynamics during development. Science 375, 566–570 (2022).
Quinodoz, S. A. et al. Higher-order inter-chromosomal hubs shape 3D genome organization in the nucleus. Cell 174, 744–757.e24 (2018).
Beagrie, R. A. et al. Complex multi-enhancer contacts captured by genome architecture mapping. Nature 543, 519–524 (2017).
Winick-Ng, W. et al. Cell-type specialization is encoded by specific chromatin topologies. Nature 599, 684–691 (2021).
Bonev, B. & Cavalli, G. Organization and function of the 3D genome. Nat. Rev. Genet. 17, 661–678 (2016).
Barbieri, M. et al. Complexity of chromatin folding is captured by the strings and binders switch model. Proc. Natl Acad. Sci. USA 109, 16173–16178 (2012). This article introduced the ‘strings and binders’ model of chromatin folding, among the first models for TADs.
Jost, D., Carrivain, P., Cavalli, G. & Vaillant, C. Modeling epigenome folding: formation and dynamics of topologically associated chromatin domains. Nucleic Acids Res. 42, 9553–9561 (2014).
Shi, G., Liu, L., Hyeon, C. & Thirumalai, D. Interphase human chromosome exhibits out of equilibrium glassy dynamics. Nat. Commun. 9, 3161 (2018).
Fudenberg, G. et al. Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016). This article introduced the loop extrusion model to explain TADs, in parallel work with Sanborn et al. (2015).
Sanborn, A. L. et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl Acad. Sci. USA 112, E6456–E6465 (2015). In parallel work to Fudenberg et al. (2016), this article introduced a tension globule and loop extrusion model to explain TADs and provided one of the first tests of the border function of single, directed CTCF sites.
Nuebler, J., Fudenberg, G., Imakaev, M., Abdennur, N. & Mirny, L. A. Chromatin organization by an interplay of loop extrusion and compartmental segregation. Proc. Natl Acad. Sci. 115, E6697–E6706 (2018).
Cremer, T. & Cremer, M. Chromosome territories. Cold Spring Harb. Perspect. Biol. 2, 38 (2010).
Chang, L.-H., Ghosh, S. & Noordermeer, D. TADs and their borders: free movement or building a wall? J. Mol. Biol. 432, 643–652 (2019).
Szabo, Q. et al. TADs are 3D structural units of higher-order chromosome organization in Drosophila. Sci. Adv. 4, eaar8082 (2018).
Sikorska, N. & Sexton, T. Defining functionally relevant spatial chromatin domains: it is a TAD complicated. J. Mol. Biol. 432, 653–664 (2020).
Nagano, T. et al. Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nature 547, 61–67 (2017).
Flyamer, I. M. et al. Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature 544, 110–114 (2017).
Bonchuk, A. et al. Functional role of dimerization and CP190 interacting domains of CTCF protein in Drosophila melanogaster. BMC Biol. 13, 63 (2015).
Chetverina, D., Aoki, T., Erokhin, M., Georgiev, P. & Schedl, P. Making connections: Insulators organize eukaryotic chromosomes into independent cis-regulatory networks. Bioessays 36, 163–172 (2014).
Fujioka, M., Mistry, H., Schedl, P. & Jaynes, J. B. Determinants of chromosome architecture: insulator pairing in cis and in trans. PLoS Genet. 12, e1005889 (2016).
Ghavi-Helm, Y. et al. Enhancer loops appear stable during development and are associated with paused polymerase. Nature 512, 96–100 (2014).
Beagan, J. A. et al. Three-dimensional genome restructuring across timescales of activity-induced neuronal gene expression. Nat. Neurosci. 23, 707–717 (2020).
Ou, H. D. et al. ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 357, eaag0025 (2017).
Sigal, Y. M., Zhou, R. & Zhuang, X. Visualizing and discovering cellular structures with super-resolution microscopy. Science 361, 880–887 (2018).
Huang, B., Babcock, H. & Zhuang, X. Breaking the diffraction barrier: super-resolution imaging of cells. Cell 143, 1047–1058 (2010).
Schermelleh, L., Heintzmann, R. & Leonhardt, H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 190, 165–175 (2010).
Boettiger, A. & Murphy, S. Advances in chromatin imaging at kilobase-scale resolution. Trends Genet. 36, 273–287 (2020).
Boettiger, A. N. et al. Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature 529, 418–422 (2016).
Szabo, Q. et al. Regulation of single-cell genome organization into TADs and chromatin nanodomains. Nat. Genet. 52, 1151–1157 (2020).
Giorgetti, L. et al. Predictive polymer modeling reveals coupled fluctuations in chromosome conformation and transcription. Cell 157, 950–963 (2014).
Cattoni, D. I. et al. Single-cell absolute contact probability detection reveals chromosomes are organized by multiple low-frequency yet specific interactions. Nat. Commun. 8, 1753 (2017).
Fudenberg, G. & Imakaev, M. FISH-ing for captured contacts: towards reconciling FISH and 3C. Nat. Methods 14, 673–678 (2017). This article provides an excellent comparison of FISH and Hi-C measurements, using both theory and experiment.
Wang, S. et al. Spatial organization of chromatin domains and compartments in single chromosomes. Science 353, 598–602 (2016). This article introduced chromosome tracing (1-Mb resolution), enabling validation of Hi-C compartments and analysis of compartments at the single-cell level.
Mateo, L. J. et al. Visualizing DNA folding and RNA in embryos at single-cell resolution. Nature 568, 49–54 (2019). This paper introduced the ORCA chromosome tracing approach (up to 2-kb resolution), combined with RNA labelling to map cell-type specific changes in chromatin structure during embryonic development.
Cardozo Gizzi, A. M. et al. Microscopy-based chromosome conformation capture enables simultaneous visualization of genome organization and transcription in intact organisms. Mol. Cell 74, 212–222.e5 (2019). This paper introduced the Hi-M chromosome tracing in whole embryos, combined with antibody detection of RNA.
Liu, M. et al. Multiplexed imaging of nucleome architectures in single cells of mammalian tissue. Nat. Commun. 11, 2907 (2020). This article introduced the MINA chromosome tracing approach in mouse embryonic tissue, combined with RNA and protein imaging.
Su, J.-H., Zheng, P., Kinrot, S. S., Bintu, B. & Zhuang, X. Genome-scale imaging of the 3D organization and transcriptional activity of chromatin. Cell 182, 1641–1659.e26 (2020). In parallel work to Takei et al. (2021), this paper introduced the multiplexed barcoding DNA MERFISH method, locating over 1000 unique genomic loci throughout the genome in combination with genome-scale detection of nascent transcription and major protein markers of nuclear organization.
Takei, Y. et al. Integrated spatial genomics reveals global architecture of single nuclei. Nature 590, 344–350 (2021). In parallel work to Su et al. (2020), this article introduced the multiplexed barcoding SeqFISH+ method, locating 3,600 unique genomic loci, combined with labelling nascent RNA and protein.
Takei, Y. et al. Single-cell nuclear architecture across cell types in the mouse brain. Science 374, 586–594 (2021).
Cardozo Gizzi, A. M. et al. Direct and simultaneous observation of transcription and chromosome architecture in single cells with Hi-M. Nat. Protoc. 15, 840–876 (2020).
Liu, M. et al. Chromatin tracing and multiplexed imaging of nucleome architectures (MINA) and RNAs in single mammalian cells and tissue. Nat. Protoc. 16, 2667–2697 (2021).
Mateo, L. J., Sinnott-Armstrong, N. & Boettiger, A. N. Tracing DNA paths and RNA profiles in cultured cells and tissues with ORCA. Nat. Protoc. 16, 1647-1713 (2021).
Moffitt, J. R. et al. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc. Natl Acad. Sci. USA 113, 11046–11051 (2016).
Rouhanifard, S. H. et al. ClampFISH detects individual nucleic acid molecules using click chemistry-based amplification. Nat. Biotechnol. 37, 84–89 (2019).
Bintu, B. et al. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science 362, eaau1783 (2018). This article reported improved resolution of chromosome tracing (30-kb), enabling validation of TADs and showing TADs to be statistical features emerging from averaging heterogeneous, TAD-like folds at the single-cell level.
Nir, G. et al. Walking along chromosomes with super-resolution imaging, contact maps, and integrative modeling. PLoS Genet. 14, e1007872 (2018).
Hafner, A., Park, M., Berger, S. E., Nora, E. P. & Boettiger, A. N. Loop stacking organizes genome folding from TADs to chromosomes. Preprint at bioRxiv https://doi.org/10.1101/2022.07.13.499982 (2022).
Finn, E. H. et al. Extensive heterogeneity and intrinsic variation in spatial genome organization. Cell 176, 1502–1515.e10 (2019).
Maeda, R. K. & Karch, F. The open for business model of the bithorax complex in Drosophila. Chromosoma 124, 293–307 (2015).
Nicodemi, M. & Prisco, A. Thermodynamic pathways to genome spatial organization in the cell nucleus. Biophys. J. 96, 2168–2177 (2009).
Mirny, L. A. The fractal globule as a model of chromatin architecture in the cell. Chromosome Res. 19, 37–51 (2011).
Nicodemi, M. & Bianco, S. Chromosomes phase transition to function. Biophys. J. 119, 724–725 (2020).
Mirny, L. A., Imakaev, M. & Abdennur, N. Two major mechanisms of chromosome organization. Curr. Opin. Cell Biol. 58, 142–152 (2019).
Szabo, Q., Bantignies, F. & Cavalli, G. Principles of genome folding into topologically associating domains. Sci. Adv. 5, eaaw1668 (2019).
Bickmore, W. A. V. The spatial organization of the human genome. Annu. Rev. Genomics Hum. Genet. 14, 67–84 (2013).
Bickmore, W. A. & Van Steensel, B. Genome architecture: domain organization of interphase chromosomes. Cell 152, 1270–1284 (2013).
Yildirim, A., Boninsegna, L., Zhan, Y. & Alber, F. Uncovering the principles of genome folding by 3D chromatin modeling. Cold Spring Harb. Perspect. Biol. 14, a039693 (2022).
Conte, M. et al. Polymer physics indicates chromatin folding variability across single-cells results from state degeneracy in phase separation. Nat. Commun. 11, 3289 (2020).
Espinola, S. M. et al. Cis-regulatory chromatin loops arise before TADs and gene activation, and are independent of cell fate during early Drosophila development. Nat. Genet. 53, 477–486 (2021). This paper reported that ‘loop-dots’ at the docs gene cluster form before TADs and are stable independently of transcription state.
Finn, E. H. & Misteli, T. Molecular basis and biological function of variability in spatial genome organization. Science 365, eaaw9498 (2019).
Khanna, N., Zhang, Y., Lucas, J. S., Dudko, O. K. & Murre, C. Chromosome dynamics near the sol–gel phase transition dictate the timing of remote genomic interactions. Nat. Commun. 10, 2771 (2019).
Chen, H. et al. Dynamic interplay between enhancer-promoter topology and gene activity. Nat. Genet. 50, 1296–1303 (2018). This paper describes two-point live imaging in D. melanogaster embryos with live readout of mRNA activity, and demonstrates strong correlation of E-P proximity and promoter firing for a synthetic transgene, within 4–8 minutes, in parallel work to Alexander et al. (2019).
Gabriele, M. et al. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science 376, 496–501 (2022). This article describes live-cell imaging that shows that chromatin loops are highly dynamic and short-lived, in parallel work to Mach et al. (2022).
Mach, P. et al. Live-cell imaging and physical modeling reveal control of chromosome folding dynamics by cohesin and CTCF. Preprint at bioRxiv https://doi.org/10.1101/2022.03.03.482826 (2022). This paper describes live-cell imaging that shows that chromatin loops are highly dynamic and short-lived, in parallel work to Gabriele et al. (2022).
Rao, S. S. P. et al. Cohesin loss eliminates all loop domains. Cell 171, 305–320.e24 (2017).
Abramo, K. et al. A chromosome folding intermediate at the condensin-to-cohesin transition during telophase. Nat. Cell Biol. 21, 1393–1402 (2019).
Zhang, H. et al. Chromatin structure dynamics during the mitosis-to-G1 phase transition. Nature 576, 158–162 (2019).
Oudelaar, A. M. et al. Single-allele chromatin interactions identify regulatory hubs in dynamic compartmentalized domains. Nat. Genet. 50, 1744–1751 (2018).
Oudelaar, A. M. et al. A revised model for promoter competition based on multi-way chromatin interactions at the α-globin locus. Nat. Commun. 10, 5412 (2019).
Allahyar, A. et al. Enhancer hubs and loop collisions identified from single-allele topologies. Nat. Genet. 50, 1151–1160 (2018).
Tavares-Cadete, F., Norouzi, D., Dekker, B., Liu, Y. & Dekker, J. Multi-contact 3C reveals that the human genome during interphase is largely not entangled. Nat. Struct. Mol. Biol. 27, 1105–1114 (2020).
Cheutin, T. & Cavalli, G. The multiscale effects of polycomb mechanisms on 3D chromatin folding. Crit. Rev. Biochem. Mol. Biol. 54, 399–417 (2019).
Zenk, F. et al. HP1 drives de novo 3D genome reorganization in early Drosophila embryos. Nature 593, 289–293 (2021).
Weber, C. M. et al. mSWI/SNF promotes Polycomb repression both directly and through genome-wide redistribution. Nat. Struct. Mol. Biol. 28, 501–511 (2021).
Kim, J. & Kingston, R. E. The CBX family of proteins in transcriptional repression and memory. J. Biosci. 45, 16 (2020).
Narlikar, G. J. Phase-separation in chromatin organization. J. Biosci. 45, 5 (2020).
Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 (2018).
Strom, A. R. & Brangwynne, C. P. The liquid nucleome–phase transitions in the nucleus at a glance. J. Cell Sci. 132, jcs235093 (2019).
Finn, E. H. & Misteli, T. A genome disconnect. Nat. Genet. 51, 1205–1206 (2019).
Xiao, J. Y., Hafner, A. & Boettiger, A. N. How subtle changes in 3D structure can create large changes in transcription. eLife 10, e64320 (2021).
Zuin, J. et al. Nonlinear control of transcription through enhancer–promoter interactions. Nature 604, 571–577 (2022).
Rajpurkar, A. R., Mateo, L. J., Murphy, S. E. & Boettiger, A. N. Deep learning connects DNA traces to transcription to reveal predictive features beyond enhancer–promoter contact. Nat. Commun. 12, 3423 (2021).
Mir, M., Bickmore, W., Furlong, E. E. M. & Narlikar, G. Chromatin topology, condensates and gene regulation: shifting paradigms or just a phase? Development 146, dev182766 (2019).
Payne, A. C. et al. In situ genome sequencing resolves DNA sequence and structure in intact biological samples. Science 371, eaay3446 (2021).
Xie, L. et al. 3D ATAC-PALM: super-resolution imaging of the accessible genome. Nat. Methods 17, 430–436 (2020).
Lu, T., Ang, C. E. & Zhuang, X. Spatially resolved epigenomic profiling of single cells in complex tissues. Preprint at bioRxiv https://doi.org/10.1101/2022.02.17.480825 (2022).
Nguyen, H. Q. et al. 3D mapping and accelerated super-resolution imaging of the human genome using in situ sequencing. Nat. Methods 17, 822–832 (2020).
Jia, B. B., Jussila, A., Kern, C., Zhu, Q. & Ren, B. A spatial genome aligner for multiplexed DNA-FISH. Preprint at bioRxiv https://doi.org/10.1101/2022.03.25.485845 (2022).
Atkinson, T. J. & Halfon, M. S. Regulation of gene expression in the genomic context. Comput. Struct. Biotechnol. J. 9, e201401001 (2014).
Haberle, V. & Stark, A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 19, 621–637 (2018).
Juven-Gershon, T. & Kadonaga, J. T. Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev. Biol. 339, 225–229 (2010).
Halfon, M. S. Studying transcriptional enhancers: the founder fallacy, validation creep, and other biases. Trends Genet. 35, 93–103 (2019).
Catarino, R. R., Neumayr, C. & Stark, A. Promoting transcription over long distances. Nat. Genet. 49, 972–973 (2017).
Deng, W., Shi, X., Tjian, R., Lionnet, T. & Singer, R. H. CASFISH: CRISPR/Cas9-mediated in situ labeling of genomic loci in fixed cells. Proc. Natl Acad. Sci. USA 112, 11870–11875 (2015).
Wang, Y. et al. Genome oligopaint via local denaturation fluorescence in situ hybridization. Mol. Cell https://doi.org/10.1016/j.molcel.2021.02.011 (2021).
Brown, J. M. et al. A tissue-specific self-interacting chromatin domain forms independently of enhancer–promoter interactions. Nat. Commun. 9, 3849 (2018).
Beckwith, K. S. et al. Visualization of loop extrusion by DNA nanoscale tracing in single human cells. Preprint at bioRxiv https://doi.org/10.1101/2021.04.12.439407 (2021).
Karr, J. P., Ferrie, J. J., Tjian, R. & Darzacq, X. The transcription factor activity gradient (TAG) model: contemplating a contact-independent mechanism for enhancer–promoter communication. Genes Dev. 36, 7–16 (2022).
Barinov, L., Ryabichko, S., Bialek, W. & Gregor, T. Transcription-dependent spatial organization of a gene locus. Preprint at arXiv https://doi.org/10.48550/arXiv.2012.15819 (2020).
Acknowledgements
The authors thank the members of the Boettiger laboratory for critical discussions and C. Walker for detailed feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
Both authors researched, discussed, wrote and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks M. Nollmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Topologically associating domains
-
(TADs). These are defined on population-level contact-frequency maps as domains of higher interaction frequency within a region than between regions.
- Mediator complex
-
A multisubunit protein complex that is a general regulator of transcription by RNA polymerase II.
- Cohesin complex
-
A multisubunit protein complex that mediates sister-chromatid cohesion in mitosis and is essential for topologically associating domain (TAD) formation.
- CTCF
-
The CCCTC binding factor (CTCF) is a zinc-finger transcription factor that is enriched at the boundaries of TADs.
- Tethers
-
These are cis-regulatory elements that function to bring together distal DNA elements.
- Phase separation
-
The process by which a mixture (such as oil and water) spontaneously separates into distinct phases, with molecules of one type separating from molecules of a second type.
- Enthalpic
-
Interactions driven by the binding energy between molecules, such as homotypic interactions among chromatin states.
- Loop extrusion
-
A model of how CTCF and cohesin are thought to form topologically associating domains (TADs), whereby cohesin is loaded onto the DNA and extrudes a loop until it is blocked by CTCF bound at the base of the loop.
- Chromosome territories
-
Specific, largely non-overlapping areas in the nucleus that each chromosome occupies.
- Diffraction limit
-
The distance limit below which two objects cannot be distinguished by conventional light microscopy. It depends on the wavelength of light and the objective used.
- Fiducial markers
-
Markers used to correct for drift that may occur during an experiment. These can be fluorescent beads or labels on the DNA that remain constant throughout the imaging experiment.
- Entropic
-
Changes that increase the number of accessible microstates in the system and do not require input energy.
- Convolutional neural networks
-
(CNNs). Algorithms designed to learn from the data to uncover connections. CNNs are frequently used in image recognition and have been increasingly used to uncover relationships in biological data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hafner, A., Boettiger, A. The spatial organization of transcriptional control. Nat Rev Genet 24, 53–68 (2023). https://doi.org/10.1038/s41576-022-00526-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-022-00526-0