Abstract
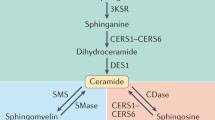
Ceramide accumulation is a hallmark in the manifestation of numerous obesity-related diseases, such as type 2 diabetes mellitus and atherosclerosis. Until the early 2000s, ceramides were viewed as a homogenous class of sphingolipids. However, it has now become clear that ceramides exert fundamentally different effects depending on the specific fatty acyl chain lengths, which are integrated into ceramides by a group of enzymes known as dihydroceramide synthases. In addition, alterations in ceramide synthesis, trafficking and metabolism in specific cellular compartments exert distinct consequences on metabolic homeostasis. Here, we examine the emerging concept of how the intracellular localization of ceramides with distinct acyl chain lengths can regulate glucose metabolism, thus emphasizing their potential as targets in the development of novel and specific therapies for obesity and obesity-associated diseases.
Key points
-
Increased levels of ceramides during obesity contribute to cellular dysfunction in key metabolic tissues, which results in insulin resistance.
-
The synthesis of ceramides with varying acyl chain lengths is regulated by six ceramide synthases (CerS1–CerS6), and their expression profiles differ throughout the body.
-
Genetic manipulation of individual ceramide synthases has identified a highly regulated specificity of distinct acyl chain ceramides in the pathogenesis of obesity, type 2 diabetes mellitus, hepatocellular carcinoma, myelination, hair loss and skin barrier function.
-
The location of ceramides with different acyl chains is important for cellular function and could contribute to metabolic tissue function.
-
Targeting ceramides with different acyl chains within different intracellular locations could help to provide new therapeutics for a range of obesity-related diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zimmet, P., Alberti, K. G. M. M. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414, 782–787 (2001).
Horowitz, J. F. et al. Effect of short-term fasting on lipid kinetics in lean and obese women. Am. J. Physiol. 276, E278–E284 (1999).
Bickerton, A. S. et al. Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia 51, 1466–1474 (2008).
Bonen, A. et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 18, 1144–1146 (2004).
Anstee, Q. M. & Goldin, R. D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 87, 1–16 (2006).
Aas, V., Kase, E. T., Solberg, R., Jensen, J. & Rustan, A. C. Chronic hyperglycaemia promotes lipogenesis and triacylglycerol accumulation in human skeletal muscle cells. Diabetologia 47, 1452–1461 (2004).
Zhou, Y. T. et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl Acad. Sci. USA 97, 1784–1789 (2000).
Assimacopoulos-Jeannet, F. Fat storage in pancreas and in insulin-sensitive tissues in pathogenesis of type 2 diabetes. Int. J. Obes. Relat. Metab. Disord. 28, S53–S57 (2004).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Holland, W. L. et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 (2007).
Raichur, S. et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014).
Hanada, K. et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803–809 (2003).
Mandon, E. C., Ehses, I., Rother, J., van Echten, G. & Sandhoff, K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J. Biol. Chem. 267, 11144–11148 (1992).
Pewzner-Jung, Y., Ben-Dor, S. & Futerman, A. H. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J. Biol. Chem. 281, 25001–25005 (2006).
Sassa, T., Hirayama, T. & Kihara, A. Enzyme activities of the ceramide synthases CERS2-6 are regulated by phosphorylation in the C-terminal region. J. Biol. Chem. 291, 7477–7487 (2016).
Laviad, E. L., Kelly, S., Merrill, A. H. Jr. & Futerman, A. H. Modulation of ceramide synthase activity via dimerization. J. Biol. Chem. 287, 21025–21033 (2012).
Mizutani, Y., Kihara, A. & Igarashi, Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390, 263–271 (2005).
Mizutani, Y., Kihara, A. & Igarashi, Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem. J. 398, 531–538 (2006).
Riebeling, C., Allegood, J. C., Wang, E., Merrill, A. H. Jr. & Futerman, A. H. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J. Biol. Chem. 278, 43452–43459 (2003).
Menuz, V. et al. Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science 324, 381–384 (2009).
Ebel, P. et al. Inactivation of ceramide synthase 6 in mice results in an altered sphingolipid metabolism and behavioral abnormalities. J. Biol. Chem. 288, 21433–21447 (2013).
Pewzner-Jung, Y. et al. A critical role for ceramide synthase 2 in liver homeostasis: II. Insights into molecular changes leading to hepatopathy. J. Biol. Chem. 285, 10911–10923 (2010).
Pewzner-Jung, Y. et al. A critical role for ceramide synthase 2 in liver homeostasis: I. Alterations in lipid metabolic pathways. J. Biol. Chem. 285, 10902–10910 (2010).
Gosejacob, D. et al. Ceramide synthase 5 is essential to maintain C16:0-ceramide pools and contributes to the development of diet-induced obesity. J. Biol. Chem. 291, 6989–7003 (2016).
Zhao, L. et al. A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLOS Genet. 7, e1002063 (2011).
Turpin-Nolan, S. M. et al. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 26, 1–10.e7 (2019).
Meikle, P. J. & Summers, S. A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 13, 79–91 (2017).
Bauer, R. et al. Schlank, a member of the ceramide synthase family controls growth and body fat in Drosophila. EMBO J. 28, 3706–3716 (2009).
Venkataraman, K. et al. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 277, 35642–35649 (2002).
Tserng, K. Y. & Griffin, R. Quantitation and molecular species determination of diacylglycerols, phosphatidylcholines, ceramides, and sphingomyelins with gas chromatography. Anal. Biochem. 323, 84–93 (2003).
Ardail, D. et al. Occurrence of ceramides and neutral glycolipids with unusual long-chain base composition in purified rat liver mitochondria. FEBS Lett. 488, 160–164 (2001).
White-Gilbertson, S. et al. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene 28, 1132–1141 (2009).
Sridevi, P. et al. Stress-induced ER to Golgi translocation of ceramide synthase 1 is dependent on proteasomal processing. Exp. Cell Res. 316, 78–91 (2010).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Ozcan, L. & Tabas, I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu. Rev. Med. 63, 317–328 (2012).
Ning, J. et al. Constitutive role for IRE1α-XBP1 signaling pathway in the insulin-mediated hepatic lipogenic program. Endocrinology 152, 2247–2255 (2011).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Lipson, K. L. et al. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 4, 245–254 (2006).
Deldicque, L. et al. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 299, E695–E705 (2010).
Soeda, J. et al. Maternal obesity alters endoplasmic reticulum homeostasis in offspring pancreas. J. Physiol. Biochem. 72, 281–291 (2016).
Seo, J. et al. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes 58, 2565–2573 (2009).
Usui, M. et al. Atf6α-null mice are glucose intolerant due to pancreatic β-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metabolism 61, 1118–1128 (2012).
Turpin, S. M., Lancaster, G. I., Darby, I., Febbraio, M. A. & Watt, M. J. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am. J. Physiol. Endocrinol. Metab. 291, E1341–E1350 (2006).
Holland, W. L. et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17, 790–797 (2013).
Novgorodov, S. A. et al. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J. Biol. Chem. 286, 4644–4658 (2011).
Yu, J. et al. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J. Biol. Chem. 282, 25940–25949 (2007).
Contreras, C. et al. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. 9, 366–377 (2014).
Liu, Z. et al. Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca2+ homeostasis in human adenoid cystic carcinoma cells. Cell Biosci. 4, 71 (2014).
Wei, Y., Wang, D., Topczewski, F. & Pagliassotti, M. J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291, E275–E281 (2006).
Montgomery, M. K. et al. Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: a beneficial role for very long-chain sphingolipid species. Biochim. Biophys. Acta 1861, 1828–1839 (2016).
Senkal, C. E., Ponnusamy, S., Bielawski, J., Hannun, Y. A. & Ogretmen, B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 24, 296–308 (2010).
Choi, S. et al. Myristate-induced endoplasmic reticulum stress requires ceramide synthases 5/6 and generation of C14-ceramide in intestinal epithelial cells. FASEB J. 32, 5724–5736 (2018).
González-García, I. et al. Estradiol regulates energy balance by ameliorating hypothalamic ceramide-induced ER stress. Cell Rep. 25, 413–423.e5 (2018).
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Senkal, C. E. et al. Alteration of ceramide synthase 6/C16-ceramide induces activating transcription factor 6-mediated endoplasmic reticulum (ER) stress and apoptosis via perturbation of cellular Ca2+ and ER/Golgi membrane network. J. Biol. Chem. 286, 42446–42458 (2011).
Fukasawa, M., Nishijima, M. & Hanada, K. Genetic evidence for ATP-dependent endoplasmic reticulum-to-Golgi apparatus trafficking of ceramide for sphingomyelin synthesis in Chinese hamster ovary cells. J. Cell Biol. 144, 673–685 (1999).
Yamaji, T. & Hanada, K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic 16, 101–122 (2015).
Kumagai, K. et al. CERT mediates intermembrane transfer of various molecular species of ceramides. J. Biol. Chem. 280, 6488–6495 (2005).
Kudo, N. et al. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc. Natl Acad. Sci. USA 105, 488–493 (2008).
Gjoni, E. et al. Glucolipotoxicity impairs ceramide flow from the endoplasmic reticulum to the Golgi apparatus in INS-1 β-cells. PLOS One 9, e110875 (2014).
Boslem, E. et al. A lipidomic screen of palmitate-treated MIN6 β-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem. J. 435, 267–276 (2011).
Wang, X. et al. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J. Cell Biol. 184, 143–158 (2009).
Liu, L.-K., Choudhary, V., Toulmay, A. & Prinz, W. A. An inducible ER–Golgi tether facilitates ceramide transport to alleviate lipotoxicity. J. Cell Biol. 216, 131 (2017).
Galadari, S., Rahman, A., Pallichankandy, S., Galadari, A. & Thayyullathil, F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 12, 98 (2013).
Perry, R. J. & Ridgway, N. D. Molecular mechanisms and regulation of ceramide transport. Biochim. Biophys. Acta 1734, 220–234 (2005).
Hsueh, Y. W., Giles, R., Kitson, N. & Thewalt, J. The effect of ceramide on phosphatidylcholine membranes: a deuterium NMR study. Biophys. J. 82, 3089–3095 (2002).
Huang, H. W., Goldberg, E. M. & Zidovetzki, R. Ceramides modulate protein kinase C activity and perturb the structure of phosphatidylcholine/phosphatidylserine bilayers. Biophys. J. 77, 1489–1497 (1999).
Silva, L., de Almeida, R. F., Fedorov, A., Matos, A. P. & Prieto, M. Ceramide-platform formation and -induced biophysical changes in a fluid phospholipid membrane. Mol. Membr. Biol. 23, 137–148 (2006).
Grassme, H. et al. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 276, 20589–20596 (2001).
Grassme, H., Cremesti, A., Kolesnick, R. & Gulbins, E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene 22, 5457–5470 (2003).
Grassme, H., Riethmuller, J. & Gulbins, E. Biological aspects of ceramide-enriched membrane domains. Prog. Lipid Res. 46, 161–170 (2007).
Blouin, C. M. et al. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 59, 600–610 (2010).
Park, J. W. et al. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology 57, 525–532 (2013).
Mahfouz, R. et al. Characterising the inhibitory actions of ceramide upon insulin signaling in different skeletal muscle cell models: a mechanistic insight. PLOS One 9, e101865 (2014).
Boon, J. et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62, 401–410 (2013).
McInnes, J. Mitochondrial-associated metabolic disorders: foundations, pathologies and recent progress. Nutr. Metab. 10, 63 (2013).
Bionda, C., Portoukalian, J., Schmitt, D., Rodriguez-Lafrasse, C. & Ardail, D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem. J. 382, 527–533 (2004).
Mesicek, J. et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 22, 1300–1307 (2010).
Hammerschmidt, P. et al. CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 177, 1536–1552 (2019).
Zigdon, H. et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 288, 4947–4956 (2013).
Di Paola, M., Cocco, T. & Lorusso, M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry 39, 6660–6668 (2000).
Gudz, T. I., Tserng, K. Y. & Hoppel, C. L. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 272, 24154–24158 (1997).
Sentelle, R. D. et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 8, 831–838 (2012).
Sims, K. et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem. 285, 38568–38579 (2010).
Scarlatti, F. et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J. Biol. Chem. 279, 18384–18391 (2004).
Jiang, W. & Ogretmen, B. Autophagy paradox and ceramide. Biochim. Biophys. Acta 1841, 783–792 (2014).
Restelli, L. M. et al. Neuronal mitochondrial dysfunction activates the integrated stress response to induce fibroblast growth factor 21. Cell Rep. 24, 1407–1414 (2018).
Lucki, N. C. & Sewer, M. B. Nuclear sphingolipid metabolism. Annu. Rev. Physiol. 74, 131–151 (2012).
Voelzmann, A. et al. Nuclear Drosophila CerS Schlank regulates lipid homeostasis via the homeodomain, independent of the lag1p motif. FEBS Lett. 590, 971–981 (2016).
Tsugane, K., Tamiya-Koizumi, K., Nagino, M., Nimura, Y. & Yoshida, S. A possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J. Hepatol. 31, 8–17 (1999).
Chocian, G. et al. High fat diet induces ceramide and sphingomyelin formation in rat’s liver nuclei. Mol. Cell Biochem. 340, 125–131 (2010).
Chatelut, M. et al. Natural ceramide is unable to escape the lysosome, in contrast to a fluorescent analogue. FEBS Lett. 426, 102–106 (1998).
Ballabio, A. & Gieselmann, V. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta 1793, 684–696 (2009).
Okino, N. et al. The reverse activity of human acid ceramidase. J. Biol. Chem. 278, 29948–29953 (2003).
Li, C. M. et al. Insertional mutagenesis of the mouse acid ceramidase gene leads to early embryonic lethality in homozygotes and progressive lipid storage disease in heterozygotes. Genomics 79, 218–224 (2002).
Xia, J. Y. et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22, 266–278 (2015).
Holland, W. L. et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 (2011).
Chavez, J. A., Holland, W. L., Bar, J., Sandhoff, K. & Summers, S. A. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 280, 20148–20153 (2005).
Vasiliauskaite-Brooks, I. et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544, 120–123 (2017).
Fujimoto, T. & Parton, R. G. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 3, a004838 (2011).
Senkal, C. E. et al. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 25, 686–697 (2017).
Mason, R. R. et al. PLIN5 deletion remodels intracellular lipid composition and causes insulin resistance in muscle. Mol. Metab. 3, 652–663 (2014).
Trevino, M. B. et al. Liver perilipin 5 expression worsens hepatosteatosis but not insulin resistance in high fat-fed mice. Mol. Endocrinol. 29, 1414–1425 (2015).
Bartholomew, S. R. et al. Distinct cellular pools of perilipin 5 point to roles in lipid trafficking. Biochim. Biophys. Acta 1821, 268–278 (2012).
Haberkant, P. et al. Bifunctional sphingosine for cell-based analysis of protein-sphingolipid interactions. ACS Chem. Biol. 11, 222–230 (2016).
Acknowledgements
S.M.T.-N. was supported by the Alexander von Humboldt Foundation fellowship and CECAD Senior Postdoctoral Research Grant. J.C.B. was supported by the Leibniz Preis (BR1492/7-1), the Cologne Excellence Cluster on Cellular Stress Responses in Ageing Associated Diseases (CECAD; funded by the DFG within the Excellence Initiative by German Federal and State Governments), by the Federal Government (BMBF) through collaboration in Deutsche Zentrum für Diabetesforschung e.V. (DZD, FKZ 82DZD00502) and the Cologne Center for Molecular Medicine Cologne (CMMC).
Author information
Authors and Affiliations
Contributions
S.M.T.-N. and J.C.B. researched data for the article, provided substantial contributions to discussions of the content, reviewed and/or edited the manuscript before submission and contributed equally to the writing of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks M. Oresic, P. Meikle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turpin-Nolan, S.M., Brüning, J.C. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol 16, 224–233 (2020). https://doi.org/10.1038/s41574-020-0320-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-020-0320-5
This article is cited by
-
Circulating ceramide levels and ratios in Emirati youth under 18 years: associations with cardiometabolic risk factors
Lipids in Health and Disease (2024)
-
Deficiency of the lipid flippase ATP10A causes diet-induced dyslipidemia in female mice
Scientific Reports (2024)
-
Ceramide sensing by human SPT-ORMDL complex for establishing sphingolipid homeostasis
Nature Communications (2023)
-
Prosaposin maintains lipid homeostasis in dopamine neurons and counteracts experimental parkinsonism in rodents
Nature Communications (2023)
-
CerS6-dependent ceramide synthesis in hypothalamic neurons promotes ER/mitochondrial stress and impairs glucose homeostasis in obese mice
Nature Communications (2023)