Abstract
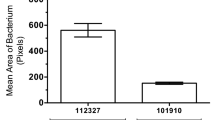
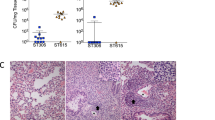
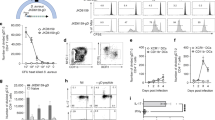
Bacterial septicaemia is a major cause of mortality, but its pathogenesis remains poorly understood. In experimental pneumococcal murine intravenous infection, an initial reduction of bacteria in the blood is followed hours later by a fatal septicaemia. These events represent a population bottleneck driven by efficient clearance of pneumococci by splenic macrophages and neutrophils, but as we show in this study, accompanied by occasional intracellular replication of bacteria that are taken up by a subset of CD169+ splenic macrophages. In this model, proliferation of these sequestered bacteria provides a reservoir for dissemination of pneumococci into the bloodstream, as demonstrated by its prevention using an anti-CD169 monoclonal antibody treatment. Intracellular replication of pneumococci within CD169+ splenic macrophages was also observed in an ex vivo porcine spleen, where the microanatomy is comparable with humans. We also showed that macrolides, which effectively penetrate macrophages, prevented septicaemia, whereas beta-lactams, with inefficient intracellular penetration, failed to prevent dissemination to the blood. Our findings define a shift in our understanding of the pneumococcus from an exclusively extracellular pathogen to one with an intracellular phase. These findings open the door to the development of treatments that target this early, previously unrecognized intracellular phase of bacterial sepsis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gratz, N., Loh, L. N. & Tuomanen, E. in Streptococcus Pneumoniae (eds Hammerschmidt, S. & Orihuela, C.) 433-451 (Academic Press, London, 2015).
van der Poll, T. Future of sepsis therapies. Crit. Care 20, 106 (2016).
Pneumococcal Vaccines: WHO Position Paper WER Vol. 87, 14 (WHO, 2012).
Musher, D. M. & Thorner, A. R. Community-acquired pneumonia. New Engl. J. Med. 371, 1619–1628 (2014).
Lim, W. S., Smith, D. L., Wise, M. P. & Welham, S. A. British Thoracic Society community acquired pneumonia guideline and the NICE pneumonia guideline: how they fit together. Thorax https://doi.org/10.1136/thoraxjnl-2015-206881 (2015).
Simell, B. et al. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccin. 11, 841–855 (2012).
Rogers, D. E. Host mechanisms which act to remove bacteria from the blood stream. Bacteriol. Rev. 24, 50–66 (1960).
Gerlini, A. et al. The role of host and microbial factors in the pathogenesis of pneumococcal bacteraemia arising from a single bacterial cell bottleneck. PLoS Pathog. 10, e1004026 (2014).
Brown, E. J., Hosea, S. W. & Frank, M. M. The role of the spleen in experimental pneumococcal bacteremia. J. Clin. Investig. 67, 975–982 (1981).
Deniset, J. F., Surewaard, B. G., Lee, W.-Y. & Kubes, P. Splenic Ly6Ghigh mature and Ly6Gint immature neutrophils contribute to eradication of S. pneumoniae. J. Exp. Med. 214, 1333–1350 (2017).
Kono, M. et al. Single cell bottlenecks in the pathogenesis of Streptococcus pneumoniae. PLoS Pathog. 12, e1005887 (2016).
Horan, M. & Colebatch, J. H. Relation between splenectomy and subsequent infection: a clinical study. Arch. Dis. Child. 37, 398–414 (1962).
Theilacker, C. et al. Overwhelming postsplenectomy infection: a prospective multicenter cohort study. Clin. Infect. Dis. 62, 871–878 (2016).
Shinefield, H. R., Steinberg, C. R. & Kaye, D. Effect of splenectomy on the susceptibility of mice inoculated with diplococcus pneumoniae. J. Exp. Med. 123, 777–794 (1966).
Martinez-Pomares, L. et al. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J. Exp. Med. 184, 1927–1937 (1996).
Oetke, C., Vinson, M. C., Jones, C. & Crocker, P. R. Sialoadhesin-deficient mice exhibit subtle changes in B- and T-cell populations and reduced immunoglobulin M levels. Mol. Cell. Biol. 26, 1549–1557 (2006).
Kjos, M. et al. Bright fluorescent Streptococcus pneumoniae for live-cell imaging of host-pathogen interactions. J. Bacteriol. 197, 807–818 (2015).
Aichele, P. et al. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J. Immunol. 171, 1148–1155 (2003).
Jones, C., Virji, M. & Crocker, P. R. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 49, 1213–1225 (2003).
Heikema, A. P. et al. Enhanced, sialoadhesin-dependent uptake of Guillain-Barré syndrome-associated Campylobacter jejuni strains by human macrophages. Infect. Immun. 81, 2095–2103 (2013).
Chang, Y.-C. et al. Role of macrophage sialoadhesin in host defense against the sialylated pathogen group B streptococcus.J. Mol. Med. 92, 951–959 (2014).
Uchiyama, S. et al. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J. Exp. Med. 206, 1845–1852 (2009).
Vanderheijden, N. et al. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 77, 8207–8215 (2003).
Maurin, M. & Raoult, D. in Antimicrobial Agents and Intracellular Pathogens (ed. Raoult, D.) 21–37 (CRC Press Inc, Boca Raton, 1993).
Steiniger, B., Barth, P. & Hellinger, A. The perifollicular and marginal zones of the human splenic white pulp: do fibroblasts guide lymphocyte immigration? Am. J. Pathol. 159, 501–512 (2001).
Steiniger, B. S. Human spleen microanatomy: why mice do not suffice. Immunology 145, 334–346 (2015).
Fairbairn, L., Kapetanovic, R., Sester, D. P. & Hume, D. A. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J. Leukoc. Biol. 89, 855–871 (2011).
Meurens, F., Summerfield, A., Nauwynck, H., Saif, L. & Gerdts, V. The pig: a model for human infectious diseases. Trends Microbiol. 20, 50–57 (2012).
Ezquerra, A. et al. Porcine myelomonocytic markers and cell populations. Dev. Comp. Immunol. 33, 284–298 (2009).
Alvarez, B. et al. Phenotypic and functional heterogeneity of CD169+and CD163+macrophages from porcine lymph nodes and spleen. Dev. Comp. Immunol. 44, 44–49 (2014).
de Greeff, A. et al. Pneumococcal colonization and invasive disease studied in a porcine model. BMC Microbiol. 16, 102 (2016).
Chung, W. Y. et al. Steps for the autologous ex vivo perfused porcine liver-kidney experiment. J. Vis. Exp 82, e50567 (2013).
Moxon, E. R. & Murphy, P. A. Haemophilus influenzae bacteremia and meningitis resulting from survival of a single organism. Proc. Natl Acad. Sci. USA 75, 1534–1536 (1978).
Lehar, S. M. et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 527, 323–328 (2015).
Heikema, A. P. et al. Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect. Immun. 78, 3237–3246 (2010).
Klaas, M. et al. Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni . J. Immunol. 189, 2414–2422 (2012).
De Schryver, M. et al. Monoclonal antibody binding to the macrophage-specific receptor sialoadhesin alters the phagocytic properties of human and mouse macrophages. Cell. Immunol. 312, 51–60 (2017).
Bewley, M. A. et al. Pneumolysin activates macrophage lysosomal membrane permeabilization and executes apoptosis by distinct mechanisms without membrane pore formation. mBio 5, e01710-14 (2014).
Dockrell, D. H., Lee, M., Lynch, D. H. & Read, R. C. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 184, 713–722 (2001).
Gordon, S. B., Irving, G. R. B., Lawson, R. A., Lee, M. E. & Read, R. C. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect. Immun. 68, 2286–2293 (2000).
Davis, K. M., Nakamura, S. & Weiser, J. N. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J. Clin. Invest. 121, 3666–3676 (2011).
Honke, N. et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat. Immunol. 13, 51–57 (2012).
Van Breedam, W., Verbeeck, M., Christiaens, I., Van Gorp, H. & Nauwynck, H. J. Porcine, murine and human sialoadhesin (Sn/Siglec-1/CD169): portals for porcine reproductive and respiratory syndrome virus entry into target cells. J. General. Virol. 94, 1955–1960 (2013).
Backer, R. et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc. Natl Acad. Sci. USA 107, 216–221 (2010).
Veninga, H. et al. Antigen targeting reveals splenic CD169+ macrophages as promoters of germinal center B-cell responses. Eur. J. Immunol. 45, 747–757 (2015).
Mastroeni, P., Grant, A., Restif, O. & Maskell, D. A dynamic view of the spread and intracellular distribution of Salmonella enterica. Nat. Rev. Micro. 7, 73–80 (2009).
Levin, B. R. & Antia, R. Why we don’t get sick: the within-host population dynamics of bacterial infections. Science 292, 1112–1115 (2001).
Shaw, S., Smith, A. L., Anderson, P. & Smith, D. H. The paradox of Hemophilus infuenzae type B bacteremia in the presence of serum bactericidal activity. J. Clin. Invest. 58, 1019–1029 (1976).
Grant, A. J. et al. Modelling within-host spatiotemporal dynamics of invasive bacterial disease. PLoS Biol. 6, e74 (2008).
Surewaard, B. G. J. et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J. Exp. Med. 213, 1141–1151 (2016).
Avery, O. T., MacLeod, C. M. & McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79, 137–158 (1944).
Iannelli, F., Pearce, B. J. & Pozzi, G. The type 2 capsule locus of Streptococcus pneumoniae. J. Bacteriol. 181, 2652–2654 (1999).
Manco, S. et al. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect. Immun. 74, 4014–4020 (2006).
Tettelin, H. et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293, 498–506 (2001).
Pearce, B. J., Iannelli, F. & Pozzi, G. Construction of new unencapsulated (rough) strains of Streptococcus pneumoniae. Res. Microbiol. 153, 243–247 (2002).
Morton, D. & Griffiths, P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 116, 431–436 (1985).
Kadioglu, A. et al. Sex-based differences in susceptibility to respiratory and systemic pneumococcal disease in mice. J. Infect. Dis. 204, 1971–1979 (2011).
Oggioni, M. R. et al. Antibacterial activity of a competence-stimulating peptide in experimental sepsis caused by Streptococcus pneumoniae. Antimicrob. Agents Chemother. 48, 4725–4732 (2004).
Oggioni, M. R. et al. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol. Microbiol. 61, 1196–1210 (2006).
Lee, E.-J., Pontes, M. H. & Groisman, E. A. A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium’s own F1Fo ATP synthase. Cell 154, 146–156 (2013).
Taylor, P. R. et al. Development of a specific system for targeting protein to metallophilic macrophages. Proc. Natl Acad. Sci. USA 101, 1963–1968 (2004).
Acknowledgements
G.E. was funded through an academic collaboration agreement between the University of Oxford and University of Leicester and in part by MRC grant MR/M003078/1. The authors thank J.W. Veening for providing GFP and RFP pneumococci, F. Focarelli for construction of the non-encapsulated GFP-expressing strain, M. De Ste Croix for mutant construction, S. Glenn for help with the infection experiments, the Electron Microscopy Facility, the University of Leicester for technical support and R. Kumar and J. Isherwood for help with the perfusion of the porcine organs at explant, the staff of Joseph Morris Butchers Ltd, Michael F Wood Butchers and the staff of Leicester Preclinical Research Facility for support.
Author information
Authors and Affiliations
Contributions
G.E. performed almost all experiments and wrote the manuscript. V.E.F. led and performed the animal infections. W.Y.C. led and performed the porcine spleen perfusion experiments. J.J.W. contributed to microscopy and animal infection work, and contributed to the writing of the manuscript. C.D.B. contributed to the design of the experimental work. K.S. led the microscopy work. S.T. performed the infections of the CD169 KO mice. P.R.C. discussed the work and supervised the infections in the KO mice, and contributed to the writing of the manuscript. A.D. designed and led the porcine perfusion work, and contributed to the writing of the manuscript. L.M.-P. designed the immunological work and overall setup of experimentation, and contributed to the writing of the manuscript. P.W.A. participated in the overall design and setup of the experimentation, and contributed to the writing of the manuscript. E.R.M. initiated and participated in the overall design and setup of the experimentation, and contributed to the writing of the manuscript. M.R.O. led the design and setup of the project, and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–5, Supplementary Tables 1–3
Rights and permissions
About this article
Cite this article
Ercoli, G., Fernandes, V.E., Chung, W.Y. et al. Intracellular replication of Streptococcus pneumoniae inside splenic macrophages serves as a reservoir for septicaemia. Nat Microbiol 3, 600–610 (2018). https://doi.org/10.1038/s41564-018-0147-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0147-1
This article is cited by
-
A combined adjuvant and ferritin nanocage based mucosal vaccine against Streptococcus pneumoniae induces protective immune responses in a murine model
Nature Communications (2025)
-
Host AAA-ATPase VCP/p97 lyses ubiquitinated intracellular bacteria as an innate antimicrobial defence
Nature Microbiology (2025)
-
Supramolecular discrimination and diagnosis-guided treatment of intracellular bacteria
Nature Communications (2025)
-
Acute organ injury and long-term sequelae of severe pneumococcal infections
Pneumonia (2023)
-
How mycobacterium tuberculosis infection could lead to the increasing risks of chronic fatigue syndrome and the potential immunological effects: a population-based retrospective cohort study
Journal of Translational Medicine (2022)