Abstract
Flowering plants (angiosperms) are the most diverse of all land plants, becoming abundant in the Cretaceous and achieving dominance in the Cenozoic. However, the exact timing of their origin remains a controversial topic, with molecular clocks generally placing their origin much further back in time than the oldest unequivocal fossils. To resolve this discrepancy, we developed a Bayesian method to estimate the ages of angiosperm families on the basis of the fossil record (a newly compiled dataset of ~15,000 occurrences in 198 families) and their living diversity. Our results indicate that several families originated in the Jurassic, strongly rejecting a Cretaceous origin for the group. We report a marked increase in lineage accumulation from 125 to 72 million years ago, supporting Darwin’s hypothesis of a rapid Cretaceous angiosperm diversification. Our results demonstrate that a pre-Cretaceous origin of angiosperms is supported not only by molecular clock approaches but also by analyses of the fossil record that explicitly correct for incomplete sampling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data analysed in this study are available in Supplementary Table 3 and in a permanent Zenodo (zenodo.org) repository with doi: 10.5281/zenodo.4290423.
Code availability
We implemented the BBB method in Python v.3. The code and input files are available in Supplementary Table 3 and in a permanent Zenodo (zenodo.org) repository with doi: 10.5281/zenodo.4290423. The code and input files and any future updates of the program are additionally available as an open access repository: https://github.com/dsilvestro/rootBBB.
References
Clarke, J. T., Warnock, R. C. M. & Donoghue, P. C. J. Establishing a time-scale for plant evolution. New Phytol. 192, 266–301 (2011).
Friis, E. M., Pedersen, K. R. & Crane, P. R. Diversity in obscurity: fossil flowers and the early history of angiosperms. Phil. Trans. R. Soc. B 365, 369–382 (2010).
Coiro, M., Doyle, J. A. & Hilton, J. How deep is the conflict between molecular and fossil evidence on the age of angiosperms? New Phytol. 223, 83–99 (2019).
Donoghue, P. Evolution: the flowering of land plant evolution. Curr. Biol. 29, R738–R761 (2019).
Buggs, R. J. A. The deepening of Darwin’s abominable mystery. Nat. Ecol. Evol. 1, 0169 (2017).
Herendeen, P. S., Friis, E. M., Pedersen, K. R. & Crane, P. R. Palaeobotanical redux: revisiting the age of the angiosperms. Nat. Plants 3, 17015 (2017).
Darwin, C. More Letters of Charles Darwin Vol. 2 (John Murray, 1903).
Friedman, W. E. The meaning of Darwin’s ‘abominable mystery’.Am. J. Bot. 96, 5–21 (2009).
Marshall, C. R. Five paleobiological laws needed to understand the evolution of the living biota. Nat. Ecol. Evol. 1, 0165 (2017).
Slater, G. & Harmon, L. J. Unifying fossils and phylogenies for comparative analyses of diversification and trait evolution. Methods Ecol. Evol. 4, 699–702 (2013).
Didier, G., Royer-Carenzi, M. & Laurin, M. The reconstructed evolutionary process with the fossil record. J. Theor. Biol. 315, 26–37 (2012).
Silvestro, D., Warnock, R. C. M., Gavryushkina, A. & Stadler, T. Closing the gap between palaeontological and neontological speciation and extinction rate estimates. Nat. Commun. 9, 5237 (2018).
Stadler, T., Gavryushkina, A., Warnock, R. C. M., Drummond, A. J. & Heath, T. A. The fossilized birth–death model for the analysis of stratigraphic range data under different speciation concepts. J. Theor. Biol. 447, 41–55 (2018).
Budd, G. E. & Mann, R. P. The dynamics of stem and crown groups. Sci. Adv. 6, 1626 (2020).
Seward, A. C. The Jurassic flora II. Liassic and Oolitic floras of England. In Catalogue of the Mesozoic plants in the Department of Geology, British Museum (National History) (British Museum, 1904).
Cornet, B. Late Triassic angiosperm-like pollen from the Richmond Rift Basin of Virginia, U.S.A. Palaeontogr. Abt. B 213, 37–87 (1989).
Ren, D. Flower-associated Brachycera flies as fossil evidence for Jurassic angiosperm origins. Science 280, 85–88 (1998).
Cleal, C. J. & Rees, P. M. The Middle Jurassic flora from Stonesfield, Oxfordshire, UK. Palaeontology 46, 739–801 (2003).
Hochuli, P. A. & FeistBurkhardt, S. A boreal early cradle of angiosperms? Angiosperm-like pollen from the Middle Triassic of the Barents Sea (Norway). J. Micropalaeontol. 23, 97–104 (2004).
Hochuli, P. A. & FeistBurkhardt, S. Angiosperm-like pollen and Afropollis from the Middle Triassic (Anisian) of the Germanic Bascin (northern Switzerland). Front. Plant Sci. 4, e344 (2013).
Bell, C. D., Soltis, D. E. & Soltis, P. S. The age and diversification of the angiosperms re-visited. Am. J. Bot. 97, 1296–1303 (2010).
Li, H.-T. et al. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 5, 461–470 (2019).
Friis, E. M., Crane, P. R., Pedersen, K. R., Stampanoni, M. & Marone, F. Exceptional preservation of tiny embryos documents seed dormancy in early angiosperms. Nature 528, 551–554 (2018).
Doyle, J. A. Molecular and fossil evidence on the origin of angiosperms. Annu. Rev. Earth Planet. Sci. 40, 301–326 (2012).
Barba-Montoya, J., dosReis, M., Schneider, H., Donoghue, P. C. J. & Yang, Z. Constraining uncertainty in the timescale of angiosperm evolution and the veracity of a Cretaceous terrestrial revolution. New Phytol. 218, 819–834 (2018).
Strauss, D. & Sadler, P. M. Classical confidence-intervals and Bayesian probability estimates for ends of local taxon ranges. Math. Geol. 21, 411–421 (1989).
Marshall, C. R. Confidence-intervals on stratigraphic ranges. Paleobiology 16, 1–10 (1990).
Marshall, C. R. Confidence intervals on stratigraphic ranges with nonrandom distributions of fossil horizons. Paleobiology 23, 165–173 (1997).
Silvestro, D., Salamin, N., Antonelli, A. & Meyer, X. Improved estimation of macroevolutionary rates from fossil data using a Bayesian framework. Paleobiology 45, 546–570 (2019).
Warnock, R. C., Heath, T. A. & Stadler, T. Assessing the impact of incomplete species sampling on estimates of speciation and extinction rates. Paleobiology 46, 137–157 (2020).
Silvestro, D., Cascales-Miñana, B., Bacon, C. D. & Antonelli, A. Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytol. 207, 425–436 (2015).
Nowak, H., Schneebeli-Hermann, E. & Kustatscher, E. No mass extinction for land plants at the Permian–Triassic transition. Nat. Commun. 10, 384 (2019).
Hedman, M. M. Constraints on clade ages from fossil outgroups. Paleobiology 36, 16–31 (2010).
Lloyd, G. T., Bapst, D. W., Friedman, M. & Davis, K. E. Probabilistic divergence time estimation without branch lengths: dating the origins of dinosaurs, avian flight and crown birds. Biol. Lett. 12, 20160609 (2016).
Gavryushkina, A. et al. Bayesian total-evidence dating reveals the recent crown radiation of penguins. Syst. Biol. 66, 57–73 (2017).
Budd, G. E. & Mann, R. P. History is written by the victors: the effect of the push of the past on the fossil record. Evolution 72, 2276–2291 (2018).
Tanner, M. & Wing, H. The calculation of posterior distributions by data augmentation. J. Am. Stat. Assoc. 82, 528–540 (1987).
Holland, S. M. The non-uniformity of fossil preservation. Phil. Trans. R. Soc. B 371, 20150130 (2016).
Pimiento, C. et al. The Pliocene marine megafauna extinction and its impact on functional diversity. Nat. Ecol. Evol. 1, 1100–1106 (2017).
Brocklehurst, N., Upchurch, P., Mannion, P. D. & O’Connor, J. The completeness of the fossil record of Mesozoic birds: implications for early avian evolution. PLoS ONE 7, e39056 (2012).
Marshall, C. R. Using the fossil record to evaluate timetree timescales. Front. Genet. 10, 449 (2019).
Angiosperm Phylogeny Group et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 181, 1–20 (2016).
Magallón, S., Gòmez-Acevedo, S., Sánchez-Reyes, L. L. & Hernández-Hernández, T. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytol. 207, 437–453 (2015).
Müller, J. Significance of fossil pollen for angiosperm history. Ann. Mo. Bot. Gard. 71, 419–443 (1984).
Collinson, M. E., Boulter, M. C. & Holmes, P. L. The Fossil Record 2 (ed. Benton, M. J.) 809–841 (Chapman and Hall, 1993).
Doyle, J. A. & Endress, P. K. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: ANITA lines and relatives of Chloranthaceae. Int. J. Plant Sci. 175, 555–600 (2014).
Doyle, J. A. Recognising angiosperm clades in the Early Cretaceous fossil record. Hist. Biol. 27, 414–429 (2015).
Coiro, M., Martínez, L. C. A., Upchurch, G. R. & Doyle, J. A. Evidence for an extinct lineage of angiosperms from the Early Cretaceous of Patagonia and implications for the early radiation of flowering plants. New Phytol. 228, 344–360 (2020).
Beaulieu, J. M., O’Meara, B. C., Crane, P. & Donoghue, M. J. Heterogeneous rates of molecular evolution and diversification could explain the Triassic age estimate for angiosperms. Syst. Biol. 64, 869–878 (2015).
Louca, S. & Pennell, M. W. Extant timetrees are consistent with a myriad of diversification histories. Nature 580, 502–505 (2020).
Foote, M. On the probability of ancestors in the fossil record. Paleobiology 22, 141–151 (1996).
Drummond, A. J., Ho, S., Phillips, M. & Rambaut, A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4, e88 (2006).
Ronquist, F. et al. A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Syst. Biol. 61, 973–999 (2012).
van der Kooi, C. J. & Ollerton, J. The origins of flowering plants and pollinators. Science 368, 1306–1308 (2020).
Bateman, R. M. Hunting the Snark: the flawed search for mythical Jurassic angiosperms. J. Exp. Bot. 71, 22–35 (2020).
Smith, S. A., Beaulieu, J. M. & Donoghue, M. J. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc. Natl Acad. Sci. USA 107, 5897–5902 (2010).
Zhang, L. et al. The water lily genome and the early evolution of flowering plants. Nature 577, 79–84 (2020).
Warnock, R. C. M., Parham, J. F., Joyce, W. G., Tyler, R. L. & Donoghue, P. C. J. Calibration uncertainty in molecular dating analyses: there is no substitute for the prior evaluation of time priors. Proc. R. Soc. B 282, 20141013 (2015).
Ronquist, F., Lartillot, N. & Phillips, M. J. Closing the gap between rocks and clocks using total-evidence dating. Phil. Trans. R. Soc. B 371, 20150136 (2016).
Feild, T. S., Arens, N. C., Doyle, J. A., Dawson, T. E. & Donoghue, M. J. Dark and disturbed: a new image of early angiosperm ecology. Paleobiology 30, 82–107 (2004).
Ramírez-Barahona, S., Sauquet, H. & Magallón, S. The delayed and geographically heterogeneous diversification of flowering plant families. Nat. Ecol. Evol. 4, 1232–1238 (2020).
Sokoloff, D. D., Remizowa, M. V., El, E. S., Rudall, P. J. & Bateman, R. M. Supposed Jurassic angiosperms lack pentamery, an important angiosperm-specific feature. New Phytol. 228, 420–426 (2020).
Cascales-Miñana, B., Cleal, C. J. & Gerrienne, P. Is Darwin’s ‘abominable mystery’ still a mystery today? Cretac. Res. 61, 256–262 (2016).
Xing, Y. et al. Testing the biases in the rich Cenozoic angiosperm macrofossil record. Int. J. Plant Sci. 177, 371–388 (2016).
Friis, E. M., Crane, P. R. & Pedersen, K. R. Early Flowers and Angiosperm Evolution (Cambridge Univ. Press, 2011).
Manchester, S. R., Grímsson, F. & Zetter, R. Assessing the fossil record of asterids in the context of our current phylogenetic framework. Ann. Mo. Bot. Gard. 100, 329–363 (2015).
Christenhusz, M. J. M. & Byng, J. W. The number of known plants species in the world and its annual increase. Phytotaxa 261, 201–217 (2016).
Acknowledgements
We thank R. C. M. Warnock, T. Stadler’s lab and E. Carlisle for feedback on the methods and models presented here. We also thank P. R. Crane for constructive feedback on the manuscript. D.S. received funding from the Swiss National Science Foundation (grant no. PCEFP3_187012) and from the Swedish Research Council (grant no. 2019-04739). A.A. acknowledges financial support from the Swedish Research Council (grant no. 2019-05191), the Swedish Foundation for Strategic Research (grant no. FFL15-0196), the Knut and Alice Wallenberg Foundation (grant no. KAW 2014.0216) and the Royal Botanic Gardens, Kew. Y.X. received funding from the National Natural Science Foundation of China (grant nos 31770226 and U1802242) and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB31000000).
Author information
Authors and Affiliations
Contributions
D.S., C.D.B. and Y.X. conceived the study. W.D., Q.Z. and Y.X. compiled the fossil data. D.S. developed and implemented the methods and analysed the data. D.S. wrote the manuscript with contributions from C.D.B., W.D., Q.Z., P.C.J.D., A.A. and Y.X.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Ecology & Evolution thanks Pamela Soltis, David Cerny and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Relative errors of the estimated Brownian bridge log variances plotted against the relative error of the estimated time of origin based on 200 simulations.
While log variances tended to be slightly underestimated (mostly negative relative errors) they do not have a biasing effect on the estimated times of origin, which show an unbiased error around zero (see also Fig. 2, main text).
Extended Data Fig. 2 Parameter estimates from 200 simulated datasets obtained under MCMC and an approximated MCMC.
In the approximated MCMC, a fraction of the iterations involve no parameter updates (that is qT, a, T, and σ2 do not change), but a new set of conditional Brownian bridges are drawn and accepted as samples from the approximate posterior. This procedure was found to improve the convergence of the MCMC, while having negligible effect on the estimated time of origin a, and sampling rates b, rate trend c, and log variance d,.
Extended Data Fig. 3 Analysis of 200 simulated datasets with random varying sampling rates through time using a BBB model with constant sampling rate (a = 0).
The times of origin were accurately estimated (a); circles and bars indicate posterior estimates and 95% credible intervals. The relative errors on the time of origin were smaller in datasets with richer simulated fossil record (b). The size of the 95% credible intervals around the times of origin decreased with increasing numb er of fossils (c). The log variances were slightly underestimated (d), while the estimated sampling rates (e; the X-axis is log10-transformed) cannot be plotted against true values because the underlying simulations were based on time-heterogeneous sampling with di?erent rates in each time bin. However, we plot for comparison the distribution from which sampling rates were sampled, randomly for each time bin (f; the X-axis is log 10 -transformed).
Extended Data Fig. 4 Analysis of 200 simulated datasets with sampling rates moderately increasing through time using a BBB model with time-varying sampling rates.
The times of origin were underestimated in some cases (a); circles and bars indicate posterior estimates and 95% credible intervals. The relative errors on the time of origin were smaller in datasets with richer simulated fossil record (b). The size of the 95% credible intervals around the times of origin decreased with increasing numb er of fossils (c). The log variances were slightly underestimated (d), while the estimated sampling rates at the time of origin and rate trends (e and f, respectively; the X-axis is log10-transformed) cannot be plotted against true values because they do not have a direct equivalent in the underlying simulations. The distribution from which sampling rates were sampled for each time bin is shown for reference in Extended Data Fig. 3f.
Extended Data Fig. 5 Analysis of 200 simulated datasets with sampling rates strongly increasing through time using a BBB model with time-varying sampling rates.
The times of origin were frequently underestimated (a); circles and bars indicate posterior estimates and 95% credible intervals. The relative errors on the time of origin were smaller in datasets with richer simulated fossil record (b). The size of the 95% credible intervals around the times of origin decreased with increasing numb er of fossils (c). The log variances were slightly underestimated (d), while the estimated sampling rates at the time of origin and rate trends (e and f, respectively; the X-axis is log10-transformed) cannot be plotted against true values because they do not have a direct equivalent in the underlying simulations. The distribution from which sampling rates were sampled for each time bin is shown for reference in Extended Data Fig. 3f.
Extended Data Fig. 6 Analysis of 200 simulated datasets with sampling rates moderately increasing through time using a BBB model with constant sampling rate.
The times of origin were frequently underestimated (a); circles and bars indicate posterior estimates and 95% credible intervals. The relative errors on the time of origin were smaller in datasets with richer simulated fossil record (b). The size of the 95% credible intervals around the times of origin decreased with increasing number of fossils (c). The log variances were slightly underestimated (d), while the estimated sampling rate (e; the X-axis is log10-transformed) cannot be plotted against true values because it does not have a direct equivalent in the underlying simulations. The distribution from which sampling rates were sampled for each time bin is shown for reference in Extended Data Fig. 3f.
Extended Data Fig. 7 Analysis of 200 simulated datasets with sampling rates strongly increasing through time using a BBB model with constant sampling rate.
The times of origin were consistently underestimated (a); circles and bars indicate posterior estimates and 95% CI. The relative errors on the time of origin were smaller in datasets with richer simulated fossil record (b). The size of the 95% credible intervals around the times of origin decreased with increasing number of fossils (c). The log variances were slightly underestimated (d), while the estimated sampling rate (e; the X-axis is log10-transformed) cannot be plotted against true values because it does not have a direct equivalent in the underlying simulations. The distribution from which sampling rates were sampled for each time bin is shown for reference in Extended Data Fig. 3f.
Extended Data Fig. 8 Family-level origination times inferred using bin sizes equal to 1, 2.5, and 5 Myr.
The estimated times of origin and credible intervals were highly consistent across different settings.
Extended Data Fig. 9 Parameters estimated across angiosperm families.
a, Size of the 95% credible intervals for the estimated time of origin of angiosperm families plotted against the number of fossils available: the relationship reflects the observations based on simulated data. Increasing number of fossils results in substantially smaller credible intervals. b, Distributions of estimated variances of the Brownian bridge (σ2; log-scale), c, sampling rates at the time of origin (qT; log-scale), and d, sampling temporal trend (a; log-scale) as inferred across angiosperm families.
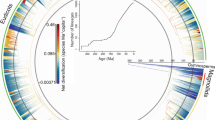
Extended Data Fig. 10 Estimated origination times across angiosperm families.
a, Posterior samples of the oldest time of origin across all families obtained after combining the estimated ages of each. The red line indicates the boundary between the Jurassic and the Cretaceous. Only 0.2% of the samples fall within the Cretaceous providing strong statistical evidence for an earlier origin of crown angiosperm. b, Root age estimates of extant families of angiosperm with 95% credible intervals (left) as inferred from meso- and macrofossils only, excluding pollen data and cumulative family diversity (right) based on those estimates (Y-axis is log10 transformed). The analyses we run under a BBB model with time-increasing sampling rates. c, Root age estimates of extant families of angiosperm with 95% credible intervals (left) as inferred from a BBB model with sampling rate set to be constant (parameter a = 0) and cumulative family diversity (right) based on those estimates (Y-axis is log10-transformed).
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Table 3
Fossil occurrences included in the analyses with taxonomic classifications, age ranges and references.
Rights and permissions
About this article
Cite this article
Silvestro, D., Bacon, C.D., Ding, W. et al. Fossil data support a pre-Cretaceous origin of flowering plants. Nat Ecol Evol 5, 449–457 (2021). https://doi.org/10.1038/s41559-020-01387-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-01387-8
This article is cited by
-
Plastid phylogenomics and fossil evidence provide new insights into the evolutionary complexity of the ‘woody clade’ in Saxifragales
BMC Plant Biology (2024)
-
Micro-CT results exhibit ovules enclosed in the ovaries of Nanjinganthus
Scientific Reports (2023)
-
A new remarkable Early Cretaceous nelumbonaceous fossil bridges the gap between herbaceous aquatic and woody protealeans
Scientific Reports (2023)
-
Intra-gastric phytoliths provide evidence for folivory in basal avialans of the Early Cretaceous Jehol Biota
Nature Communications (2023)
-
Landscape dynamics and the Phanerozoic diversification of the biosphere
Nature (2023)