Abstract
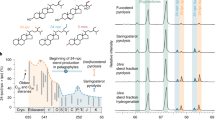
Sterane biomarkers preserved in ancient sedimentary rocks hold promise for tracking the diversification and ecological expansion of eukaryotes. The earliest proposed animal biomarkers from demosponges (Demospongiae) are recorded in a sequence around 100 Myr long of Neoproterozoic–Cambrian marine sedimentary strata from the Huqf Supergroup, South Oman Salt Basin. This C30 sterane biomarker, informally known as 24-isopropylcholestane (24-ipc), possesses the same carbon skeleton as sterols found in some modern-day demosponges. However, this evidence is controversial because 24-ipc is not exclusive to demosponges since 24-ipc sterols are found in trace amounts in some pelagophyte algae. Here, we report a new fossil sterane biomarker that co-occurs with 24-ipc in a suite of late Neoproterozoic–Cambrian sedimentary rocks and oils, which possesses a rare hydrocarbon skeleton that is uniquely found within extant demosponge taxa. This sterane is informally designated as 26-methylstigmastane (26-mes), reflecting the very unusual methylation at the terminus of the steroid side chain. It is the first animal-specific sterane marker detected in the geological record that can be unambiguously linked to precursor sterols only reported from extant demosponges. These new findings strongly suggest that demosponges, and hence multicellular animals, were prominent in some late Neoproterozoic marine environments at least extending back to the Cryogenian period.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Brocks, J. J. et al. The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548, 578–581 (2017).
Love, G. D. et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457, 718–721 (2009).
Simion, P. et al. Large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr. Biol. 27, 958–968 (2017).
McCaffrey, M. A. et al. Paleoenvironmental implications of novel C30 steranes in Precambrian to Cenozoic age petroleum and bitumen. Geochim. Cosmochim. Acta 58, 529–532 (1994).
Hofheinz, W. & Oesterhelt, G. 24-isopropylcholesterol and 22-dehydro-24-isopropylcholesterol, novel sterols from a sponge. Helv. Chim. Acta 62, 1307–1309 (1979).
Rooney, A. D., Strauss, J. V., Brandon, A. D. & Macdonald, F. A. A Cryogenian chronology: two long-lasting synchronous Neoproterozoic glaciations. Geology 43, 459–462 (2015).
Peters, K. E. et al. Recognition of an Infracambrian source rock based on biomarkers in the Baghewala-1 oil, India. AAPG Bull. 79, 1481–1494 (1995).
Grosjean, E. et al. Origin of petroleum in the Neoproterozoic–Cambrian South Oman Salt Basin. Org. Geochem. 40, 87–110 (2009).
Kelly, A. E., Love, G. D., Zumberge, J. E. & Summons, R. E. Hydrocarbon biomarkers of Neoproterozoic to Lower Cambrian oils from Eastern Siberia. Org. Geochem. 42, 640–654 (2011).
Antcliffe, J. B. Questioning the evidence of organic compounds called sponge biomarkers. Palaeontology 56, 917–925 (2013).
Siegl, A. et al. Single-cell genomics reveals the lifestyle of Poribacteria, a candidate phylum symbiotically associated with marine sponges. ISME J. 5, 61–70 (2011).
Love, G. D. & Summons, R. E. The record of Cryogenian sponges. A response to Antcliffe. Palaeontology 58, 1131–1136 (2015).
Gold, D. A. et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc. Natl Acad. Sci. USA 113, 2684–2689 (2016).
Grabenstatter, J. et al. Identification of 24-n-proplidenecholesterol in a member of the Foraminefera. Org. Geochem. 63, 145–151 (2013).
Erwin, D. H. et al. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097 (2011).
dos Reis, M. et al. Uncertainty in the timing of origin of animals and the limits of precision in molecular timescales. Curr. Biol. 25, 2939–2950 (2015).
Dohrmann, M. & Wörheide, G. Dating early animal evolution using phylogenomic data. Sci. Rep. 7, 3599 (2017).
Schuster, A. et al. Divergence times in demosponges (Porifera): first insights from new mitogenomes and the inclusion of fossils in a birth–death clock model. BMC Evol. Biol. 18, 114 (2018).
Love, G. D. et al. An optimised catalytic hydropyrolysis method for the rapid screening of microbial cultures for lipid biomarkers. Org. Geochem. 36, 63–82 (2005).
Theobald, N. & Djerassi, C. Determination of the absolute configuration of stelliferasterol and strongylosterol—two marine sterols with ‘extended’ side chains. Tetrahedron Lett. 45, 4369–4372 (1978).
Theobald, N., Wells, R. J. & Djerassi, C. Minor and trace sterols in marine invertebrates. 8. Isolation, structure elucidation, and partial synthesis of two novel sterols—stelliferasterol and isostelliferasterol. J. Am. Chem. Soc. 100, 7677–7684 (1978).
Kennedy, J. A. Resolving the ‘Jaspis stellifera’ complex. Mem. Queensl. Mus. 45, 453–476 (2000).
Bortolloto, M., Braekman, J. C., Daloze, D. & Tursch, D. Chemical studies of marine invertebrates. XXXXVI. Strongylosterol, a novel C-30 sterol from the sponge Strongylophora durissima Dendy. Bull. Soc. Chim. Belg. 87, 539–543 (1978).
Vacelet, J. et al. Morphological, chemical and biochemical characterization of a new species of sponge without skeleton (Porifera, Demospongiae) from the Mediterranean Sea. Zoosytema 22, 313–326 (2000).
Volkman, J. K. Sterols in microorganisms. Appl. Microbiol. Biotechnol. 60, 495–506 (2003).
Kodner, R. B., Pearson, A., Summons, R. E. & Knoll, A. H. Sterols in red and green algae: quantification, phylogeny, and relevance for the interpretation of geologic steranes. Geobiology 6, 411–420 (2008).
Blumenberg, M., Thiel, V., Pape, T. & Michaelis, W. The steroids of hexactinellid sponges. Naturwissenschaften 89, 415–419 (2002).
Hagemann, A., Voigt, O., Wörheide, G. & Thiel, V. The sterols of calcareous sponges (Calcarea, Porifera). Chem. Phys. Lipids 156, 26–32 (2008).
Brocks, J. J. et al. Early sponges and toxic protists: possible sources of cryostane, an age diagnostic biomarker antedating Sturtian Snowball Earth. Geobiology 14, 129–149 (2015).
Adam, P., Schaeffer, P. & Brocks, J. J. Synthesis of 26-methylcholestane and identification of cryostane in mid-Neoproterozoic sediments. Org. Geochem. 115, 246–249 (2018).
Botting, J. P. & Muir, L. A. Early sponge evolution: a review and phylogenetic framework. Palaeoworld 27, 1–29 (2018).
Muscente, A. D., Marc Michel, F., Dale, J. G. & Xiao, S. Assessing the veracity of Precambrian ‘sponge’ fossils using in situ nanoscale analytical techniques. Precam. Res. 263, 142–156 (2015).
Berelson, W. M. et al. Anaerobic diagenesis of silica and carbon in continental margin sediments: discrete zones of TCO2 production. Geochim. Cosmochim. Acta 69, 4611–4629 (2005).
Whelan, N. V. et al. Ctenophore relationships and their placement as the sister group to all other animals. Nat. Ecol. Evol. 1, 1737–1746 (2017).
Rohrssen, M., Love, G. D., Fischer, W., Finnegan, S. & Fike, D. A. Lipid biomarkers record fundamental changes in the microbial community structure of tropical seas during the Late Ordovician Hirnantian glaciation. Geology 41, 127–130 (2013).
Rohrssen, M., Gill, B. C. & Love, G. D. Scarcity of the C30 sterane biomarker, 24-n-propylcholestane, in Lower Paleozoic marine paleoenvironments. Org. Geochem. 80, 1–7 (2015).
Haddad, E. E. et al. Lipid biomarker stratigraphic records through the Late Devonian Frasnian/Famennian boundary: comparison of high-and low-latitude epicontinental marine settings. Org. Geochem. 98, 38–53 (2016).
Stoilov, I. L., Thompson, J. E., Cho, J. H. & Djerassi, C. Biosynthetic studies of marine lipids. 9. Stereochemical aspects and hydrogen migrations in the biosynthesis of the triply alkylated side chain of the sponge sterol strongylosterol. J. Am. Chem. Soc. 108, 8235–8241 (1986).
Cho, J. H., Thompson, J. E., Stoilov, I. L. & Djerassi, C. Biosynthetic studies of marine lipids. 14. 24(28)-dehydroaplysterol and other sponge sterols from Jaspis stellifera. J. Org. Chem. 53, 3466–3469 (1988).
Pehr, K. et al. Ediacara biota thrived in oligotrophic and bacterially dominated marine environments across Baltica. Nat. Commun. 9, 1807 (2018).
Acknowledgements
Funding support for this work came from the NASA Astrobiology Institute teams Alternative Earths (NNA15BB03A) and Foundations of Complex Life (NNA13AA90A), NASA Exobiology program (grant number 80NSSC18K1085), NSF Frontiers in Earth System Dynamics programme (grant number 1338810), Agouron Institute, and European Union’s Horizon 2020 research and innovation programme through the SponGES project (grant agreement number 679849). Many thanks go to the sponge collectors, including T. Pérez, H. Tore Rapp, A. Plotkin, J.-S. Hong, Y. M. Huang, S. Rohde, S. Nichols, J. V. Lopez, B. Calcinai, G. Gatti, B. Ciperling, J. Boavida, J.-P. Fonseca, L. Magro and F. Azzini. The fieldwork on the Mohns and Knipovich Ridges aboard the RV G. O. Sars in 2014 was supported by the Research Council of Norway through the Centre for Geobiology, UiB (contract number 179560). A Vazella pourtalesii specimen was provided by E. Kenchington through funding from the Marine Conservation Target fund of the Department of Fisheries and Oceans Canada. Other specimens were provided by E. Kenchington as Canadian lead for the NEREIDA (NAFO Potential Vulnerable Marine Ecosystems-Impacts of Deep-sea Fisheries) project led by Spain and Canada. We thank K. Ubhayasekera (Department of Chemistry, Uppsala University) for GC-MS analyses, and S. Rajendran and T. Aljazar (Department of Medicinal Chemistry, Uppsala University) for help with the isolation of sponge sterols. We thank Petroleum Development Oman for Neoproterozoic–Cambrian rock samples from the South Oman Salt Basin for ancient biomarker analysis. We are grateful to D. Rocher for GC-QQQ-MS analysis and GeoMark Research (Houston, TX) for providing oil samples from Eastern Siberia.
Author information
Authors and Affiliations
Contributions
J.A.Z. and G.D.L. planned the investigation and wrote the manuscript with input from P.C. and E.A.S. J.A.Z. processed and interpreted the lipid biomarker data with help from G.D.L., E.G., R.E.S. and M.R. P.C., E.A.S., R.E.S., S.G., M.R., E.G. and J.P.G. provided comments on drafts of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures 1–6, tables 2–4; 6, chart
Supplementary Table 1
Selected biomarker ratios and yields obtained from free saturate fractions of sediment cores and cuttings from South Oman Salt Basin
Supplementary Table 5
C30 regular (4-desmethyl) sterane patterns from catalytic hydropyrolysis (HyPy) of the modern sponges used in this study
Rights and permissions
About this article
Cite this article
Zumberge, J.A., Love, G.D., Cárdenas, P. et al. Demosponge steroid biomarker 26-methylstigmastane provides evidence for Neoproterozoic animals. Nat Ecol Evol 2, 1709–1714 (2018). https://doi.org/10.1038/s41559-018-0676-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0676-2
This article is cited by
-
Silica-associated proteins from hexactinellid sponges support an alternative evolutionary scenario for biomineralization in Porifera
Nature Communications (2024)
-
Molecular fossils in reefal carbonates and sponges of the deep fore reef of Mayotte and Mohéli, Comoro Islands, western Indian Ocean
Facies (2024)
-
Sterol methyltransferases in uncultured bacteria complicate eukaryotic biomarker interpretations
Nature Communications (2023)
-
A Diverse Microfossil Assemblage from the Ediacaran–Cambrian Deep-Water Chert of the Liuchapo Formation in Guizhou Province, South China
Journal of Earth Science (2023)
-
Ice-free tropical waterbelt for Snowball Earth events questioned by uncertain clouds
Nature Geoscience (2022)