Abstract
Recent advances in wastewater treatment processes have resulted in high removal efficiencies for various hazardous pollutants. Nevertheless, some technologies are more suitable for targeting specific contaminants than others. We comprehensively reviewed the recent advances in removing hazardous pollutants from industrial wastewater through membrane technologies, adsorption, Fenton-based processes, advanced oxidation processes (AOP), and hybrid systems such as electrically-enhanced membrane bioreactors (eMBRs), and integrated eMBR-adsorption system. Each technology’s key features are compared, and recent modifications to the conventional treatment approaches and limitations of advanced treatment systems are highlighted. The removal of emerging contaminants such as pharmaceuticals from wastewater is also discussed.
Similar content being viewed by others
Introduction
Industries are significant water consumers. On a global scale, they consume ~22% of the total water produced, whereas, in high-income counties, it can reach up to 60%. It is estimated that by 2050, manufacturing industries alone could increase their water usage by 400%1. Aqueous discard from the use of water in various industrial steps such as cooling tower, heating by the boiler, purification, etc. may contain numerous suspended or dissolved contaminants, and these effluents are referred to as industrial wastewater2. Industries such as chemical and petrochemical, paper and pulp, food processing, tannery, and other manufacturing industries constitute the primary sources of industrial wastewater3. These wastewaters usually have high organic strength (1-200 g/L), non-neutral pH, different temperatures, salinity, turbidity, and high heavy metal content4. Wastewater from leather manufacturing, food processing and preservation, textile processing, and petroleum refining may have high salt concentration5. Wastewater composition varies depending on the chemicals used in the upstream processes and the nature of treatment it has undergone; thus classifying industrial wastewater into specific categories is challenging2.
Ideally, industrial wastewater should undergo proper treatment and subsequent disposal into the environment or reuse for landscaping and housekeeping2. However, the regulatory control of industrial wastewater is region-specific, with many countries are having little or no effective legal frameworks supported by regulatory institutions6. According to UNESCO, 70% of industrial effluents in developing countries are dumped untreated7. However, as more countries are tightening their regulatory frameworks, industries are facing challenges in meeting the stringent water discharge and reuse requirements8.
Conventional treatments for removing heavy metals from wastewater include chemical precipitation, flotation, and ion exchange. However, these processes have several drawbacks, such as low removal efficiency, high energy consumption, and generation of toxic sludge, that limit their widespread application9. Recently, various alternative treatment methods have been investigated to improve the quality of the treated effluent. They include adsorption using low-cost materials, membrane separation, electro-technologies, and photocatalytic processes. Adsorption and membrane separation are widely used for treating wastewater contaminated with high heavy metals concentrations. Photocatalytic methods are effective in removing organic matter and recovering metals and hence are expected to be more prevalent in the future10. To the best of authors’ knowledge, no comprehensive and critical review in the literature discusses the latest innovations in the removal of hazardous pollutants from industrial wastewater. Therefore, this work is aimed at reviewing the recent advances in the removal of hazardous pollutants from industrial wastewaters. Membrane-based technologies, adsorption, Fenton-based process, advanced oxidation and photocatalytic processes, and hybrid systems are critically reviewed and discussed.
Membrane technologies
Microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO)
Membrane processes are increasingly being implemented for treating industrial and municipal wastewater because of their simplicity, modularity, and better energy efficiency11. Based on the pore size of the employed membrane, these processes can be broadly classified into microfiltration (MF), ultrafiltration (UF)8,12, nanofiltration (NF)8,13, and reverse osmosis (RO)13. MF, UF, and NF membranes are used for the removal of contaminants with a size range of 100–1000, 5–100, and 1–5 nm, respectively. There are several recent studies reporting treatment of industrial wastewater using membrane processes. For example, RO and NF were effective in removing Cu2+ and Cd2+ from synthetic wastewater14. RO achieved 98% and 99% removals for Cu2+ and Cd2+, respectively, whereas NF achieved ~90% for both Cu2+ and Cd2+. Another study reported 99.5% removal of Cu2+ and Ni2+ using RO15. Galambos et al. compared the chemical oxygen demand (COD) removal efficiency of RO and NF in treating the food industry wastewater; two samples of wastewater were used in this study with an initial COD of 9500 and 1160 ppm16. It was found that the permeate from RO has a COD of less than 125 mgO2 L−1 and hence can be discharged into natural waters. The study did not investigate the energy requirements associated with the treatment processes.
Polymer-supported ultrafiltration (PSU) has been investigated for treating synthetic wastewater containing heavy metals. Barakat and Schmidt added carboxyl methyl cellulose (CMC) as a water-soluble polymer for complexing the cationic forms of heavy metals such as Cu2+, Ni2+, and Cr3+ prior to filtration17. The results revealed that a high pH (≥7) enhanced the formation of CMC-heavy metal complexes, precipitation and size exclusive removal of metal hydroxides. In many cases, adjusting the solution pH is found to be an important step in heavy metal rejection as high pH can induce the precipitation of heavy metal hydroxides, which can then be filtered out through UF membranes. Juang and Shiau [20] investigated the removal of Cu2+ and Zn2+ from synthetic wastewater using chitosan-enhanced membrane filtration18. The presence of chitosan in the feed solution enhanced the metal removal by 6–10 times, which is attributed to the presence of the amino group in chitosan that served as a site for metal binding. Diethylaminoethyl cellulose19 and polyethyleneimine (PEI)20 are other examples of water-soluble metal-binding polymers used for heavy metal removal. Heavy metal removal can also be enhanced by the addition of minerals into the membrane reactor21. For example, the addition of 10 g L−1 vermiculite at pH=8 resulted in >99% removal of Pb2+, Ni2+, Cu2+ and Zn2+ 21. Consequently, the integrated process of sorption-UF with suitable pH adjustment (i.e. >7) can be applied as a pre-treatment stage to remove heavy metals from industrial wastewater, such as metal plating21,22.
Adsorbents can be used to increase the heavy metal removal efficiency in membrane processes. For instance, Malamis et al. (2010) used adsorbents such as bentonite, vermiculite, and zeolite (clinoptilolite) for removing Cu2+ from activated sludge permeate containing 317 mg L–1 Cu2+ 23. Adsorption using 10 and 20 g L–1 bentonite or vermiculite (pH: 5.5) followed by the ultrafiltration achieved a removal efficiency from 93.8–96.8 to 99.4% of Cu2+, respectively. The ultrafiltration alone was able to remove only 59.4–78.3% of Cu2+, indicating the superior performance of adsorbent-enhanced UF systems. The addition of zeolite and bentonite reduced the fouling, whereas vermiculite did not show any antifouling properties. As a follow-up study, Katsou et al. investigated the removal of Ni2+ via a combined adsorption and UF system24. In a batch UF unit with an initial Ni2+ concentration of 320 mg L–1 at a pH of 6, 65.3, and 80% removal efficiencies were obtained with 15 g L–1 each of bentonite and vermiculite respectively24. Membrane processes have also been used to treat saline industrial wastewaters containing high organic content. For instance, suspended solids (SS) and colloidal COD were reduced from seafood-processing wastewater by initial concentration via a UF system, followed by recycling of proteins to be used in fish meal production25. Turano et al. combined MF and centrifugation to remove up to 80% of the SS and 90% of the COD from olive oil mill effluent26.
It is worth noting that although RO is the most efficient in removing heavy metals from wastewater, it is not widely applied for this purpose when compared to other membrane processes such as MF, UF or NF, as it requires high energy for operation11. Also, the aerobic condition can promote the formation of metal oxides that can prematurely foul the RO membranes, necessitating frequent membrane replacements. However, if the wastewater also requires demineralization in addition to the removal of heavy metals, then RO might be a better technology to opt for.
Membrane bioreactors (MBRs)
Membrane bioreactors (MBRs) combine biological processes and membrane filtration to achieve better treated effluent quality by exploiting the dual benefits of membrane separation and activated sludge processes (ASPs). In the case of high strength industrial wastewater, operating parameters such as HRT, SRT, and MLSS should be optimized, and suitable pretreatment or neutralization should be provided to preserve the microbial community and prolong the membrane life2.
Metal sorption on activated sludge takes place as follows:
-
Cell wall and cell membrane contain various cations like Na+, Κ+, Mg2+, and Ca2+, and these ions will be exchanged with other dissolved metals in the MBR mixed liquor.
-
Interaction between metals and the sorption sites on cell surface induces complexation and/or microprecipitation.
The removal of metals in MBRs depends on a variety of parameters27,28 such as: (i) operating parameters like dissolved oxygen (DO) levels, mixed liquor SS (MLSS), and sludge retention time (SRT), (ii) physicochemical parameters such as metal type, species, and initial concentration, the type and concentration of ligands, the presence and concentration of competing cations, (iii) biochemical parameters such as the concentration and content of extracellular polymeric substances (EPS), ligands produced through bacterial activity, products from cell lysis, and bacterial species that favor metal uptake.
Aerobic MBR utilizes biological treatment with aerobic microorganisms that prosper in presence of oxygen. MBRs have been used for removing various pharmaceutical compounds with varying efficiencies. Although longer SRTs were usually associated with higher removal efficiencies, contradictory results were obtained for diclofenac and ethinylestradiol. It was concluded that antiepileptic compounds like carbamazepine were resistant to removal by MBR, whereas other compounds such as Bisphenol-A, the natural estrogens, ibuprofen, and bezafibrate were removed sufficiently. At a temperature of 10 °C, 80% removal of bisphenol-A was observed at SRT higher than 10 days. Also, the natural estrogens 17β-estradiol (E2), estrone (E1), and estriol (E3) were nearly removed completely. Similarly, the removal efficiency of more than 95% was observed for ibuprofen and bezafibrate29. GE Corp. has developed an effective MBR system named Zee-Weed MBR to treat wastewaters from the pharmaceuticals industry30. However, it should be noted that MBR processes, like any other biological process, is not specifically designed for removing pharmaceutical compounds. The level of biodegradation will thus depend on how recalcitrant these compounds are and whether they can be sorbed to activated sludge or remain in the liquid phase.
MBRs have been used for removing the oil, grease, and other organics from petrochemical-contaminated wastewater containing various compounds with typical 10,000–20,000 mg L–1 SS, 2000–4000 mg L–1 COD, and up to 1000 mg L–1 total Kjeldahl nitrogen (TKN)30. In another study, MBR reported 99.9% removal of fuel and lubricant oil at hydraulic retention time (HRT) of 13.3 h. It was observed that the quality of the treated effluent met industrial process water standards31. A study conducted on a full-scale MBR plant showed a 90% removal of COD and complete removal of grease, oil, and phenolics32, whereas, industrial site of Porto-Marghera has a huge petrochemical MBR plant, whose permeate is being discharged into Lagoon of Venice33.
Sometimes, industrial wastewater might contain compounds that are toxic to microorganisms, which necessitates pre-treatment before biological treatment3. Katsou et al. investigated a submerged MBR for treating heavy metals from a synthetic wastewater effluent21. The municipal wastewater was added with 3.9–14.7 mg L–1 Pb2+, 3.4–9.1 mg L–1 Cu2+, 3.2–12.1 mg L–1 Zn2+, and 4.3–14.7 mg L–1 Ni2+ and a hollow fiber membrane with a nominal pore size of 0.04 µm was employed. The MBR was operated at an HRT of 10.3 h and SRT of 15 days. A UF pre-treatment ensured the complete removal of total suspended solids (TSS). COD removal for the municipal wastewater was 95–97%, but it decreased to 83–91% upon the addition of heavy metals into the municipal wastewater. Comparing mixed liquor volatile suspended solids (MLVSS) before and after heavy metal addition showed that there was a 13% reduction in biomass due to the inhibition. Likewise, complete nitrification was observed in the case of municipal wastewater, but the addition of metals reduced it to 22–54%21.
When compared to traditional ASPs, MBR equipped with a UF membrane resulted in a 40–50% increase in the heavy metal removal efficiency34. Heavy metal removal mainly depends on the SRT, pH, and MLSS. High SRTs and MLSS, usually result in better heavy metal removal efficiency35. Metal ions are sorbed to the sludge particles attached to the UF membrane, which has resulted in the removal of Cu2+ (59.4–78.3%) and Ni2+ (23–50%) from industrial wastewater23. A submerged MBR with a hollow fiber membrane has also been found to significantly remove Cu2+ and Cr3+ from industrial wastewater36. Table 1 illustrates the literature data on MBR performance in industrial wastewater treatment for the removal of organics and nutrients from various industrial effluents.
There are several strategies for reducing the membrane fouling in MBRs. For instance, Deowan et al. coated a polyethersulfone (PES) UF membrane with an antifouling material and tested using synthetic textile dye wastewater13. The PBM MBR module showed around 10% higher dye removal when compared to commercial membranes and better fouling-resistance due to the antimicrobial properties of polymerizable cationic surfactant acryloyloxyundecyltriethylammonium bromide (AUTEAB).
Anaerobic membrane bioreactors (AnMBRs) are used to treat industrial wastewaters characterized by high organic matter. Anaerobic processes offer great sustainability benefits as they produce little sludge due to low biomass yield and consume less energy because of the absence of aeration. Moreover, anaerobic processes generate biogas that can be used as an alternative energy source3. Given the high organic loading of industrial wastewater, a comparably small amount of greenhouse gases is emitted to the atmosphere37. AnMBR system allows operation at high MLSS up to 15 g L–1 and can be run at long SRT of more than 20 days, and thus refractory and recalcitrant organics can be removed with high efficiency38. AnMBR can also retain specific microbial communities that can treat particular contaminants in wastewater39.
AnMBR has been employed for treating different types of wastewaters—from paper and pulp, food processing, winery, textile, pharmaceutical, oil, and petrochemical industries. For instance, the food processing industry produces wastewater that is non-toxic and readily biodegradable with high organic loading rate (OLR, 1000–85,000 mg COD L–1). AnMBR achieved a COD removal of >97% with OLRs higher than 12 kg COD m–3 d–1 4. It was reported that 15 full-scale AnMBR plants have been operating around the world for food industry treatment40.
Bioaugmentation involves supplementing the microbial community with particular microorganisms to improve biodegradation of recalcitrant molecules. The added microbes should be compatible and competitive with the existing microbial communities in the system to avoid any damaging effects. This method is more environment-friendly and affordable when compared to other extra physico-chemical processes. There are several studies on improving the degradation performance via bioaugmentation. For example, Saravanane and Sundararaman investigated the treatment of pharmaceutical wastewater with a high concentration of Cephalosporin derivative using an AnMBR with a submerged flat sheet membrane41. They found an enhanced removal efficiency upon bioaugmentation. In another study, Qu et al. bio-augmented an MBR with Sphingomonas xenophaga QYY strain for treating wastewater containing anthraquinone dyes intermediates to achieve an enhanced color and COD removals42. Thus, bioaugmentation or post-treatment may be needed with MBRs/AnMBRs to ensure the enhanced degradation of pollutants from industrial wastewaters.
Quorum sensing (QS) refers to the bacterial communication using chemical signals like N-acyl homoserine lactones (AHL), and subsequent regulation of gene expression in bacterial communities as a response to the population density43. Several studies showed a high correlation between AHL signals and biofilm growth44,45,46,47. When reaches a threshold level, QS activates the transcription of specific genes and induce the secretion of exocellular enzymes, extracellular polymeric substances (EPSs), soluble microbial products (SMP), antibiotics, virulence, and bioluminescence. Quorum quenching (QQ) refers to the interference with QS via inhibiting or degrading signals, and interfering or blocking signal receptors for mitigating biofouling in MBRs48,49. Thus the use of antimicrobial compounds can be minimized, and the risk of developing anti-microbial resistance can be avoided48. For instance, Kim et al. reduced the biofouling by exploiting the double-benefits of improved friction and QQ using porous alginate beads trapped with QQ bacteria50. The increased friction between loose beads and the static membranes helped to loosen the biofilm on the membranes, whereas QQ bacteria helped reduce the formation of EPS. Another study reported an improved antifouling by encapsulating QQ bacteria on a polymer membrane layer51.
In general, the use of MBRs is useful for treating industrial wastewater. However, further research is needed to optimize the process and overcome the limitations. For instance, membrane fouling due to the deposition and growth of microbes on membrane surface or pores causes a gradual increase in trans-membrane pressure (TMP) and a decline in water flux. This necessitates frequent cleaning and membrane replacements, leading to increased operation and maintenance costs52,53. Furthermore, the exact nature of the interactions between membrane surface and foulants cannot be easily identified. Also, most of the published research investigated the efficiency of the bench and pilot-scale systems. Limitations of pH, temperature, pressure, and some corrosive chemicals constrain the use of MBR, especially in large-scale operations2. Leakage of contaminants through the membranes, due to the gradual degradation of membranes poses another challenge. Although QQ is promising in ensuring the long-term operation of MBR with minimal fouling, further work should be conducted to evaluate MBR performance with QQ bacteria in treating industrial wastewater in the pilot and full-scale. The electricity consumption of MBRs is usually higher than most of treatment processes. Due to the high membrane aeration rates required to manage fouling and clogging, MBR energy consumption was three times higher even than that of conventional activated sludge (CAS) systems combined with advanced treatment techniques54. Recently, more efforts have been focusing on reducing the energy consumption associated with MBR. The electrical consumption was reported to be in the range 1.43 kWh m–3 to 4.23 kWh m–3 55, Energy consumption at 985 Japanese municipal WWTPs were analyzed and it was reported that CAS systems consumed between 0.3 kWh m–3 and 1.9 kWh m–3 56. A balanced comparison of MBR and CAS (or other) systems is only possible, however, when similar effluent quality is produced.
Adsorption and ion exchange
Adsorption refers to the transfer of gas or liquid molecules into a solid sorbent surface and holding them via physical and/or chemical intermolecular interactions10. Ion exchange refers to the transfer of ions between an electrolyte and a complex or between two electrolytes solutions. In many textbooks, ion exchange is grouped under adsorption, and henceforth in this article, the term adsorption also covers the ion exchange as well. Adsorbents can be natural (e.g., charcoal, clays, minerals such as bentonite and vermiculite, zeolites, and ores) or synthetic (produced from agricultural products and wastes, industrial or urban wastes, sewage sludge, metal oxides, and polymeric adsorbents)57,58,59. The adsorption has been effective in removing dyes, and organic pollutants and metals from various industrial wastewater effluents60. It is pivotal to determine the thermodynamic parameters such as standard free energy change (ΔG°), to predict the feasibility of the process. For instance, if ΔG°< 0, the process is both spontaneous and feasible and vice versa60. Recently, adsorption via activated carbon (AC), low-cost industrial products, and biosorption has been investigated and are discussed below.
Adsorption on activated carbon (AC)
Activated carbon (AC) for industrial wastewater treatment is usually applied in two forms – powdered (PAC) and granular (GAC). Adsorption unit can either be used before biological treatment for removing toxic compounds or be placed after the physio-chemical treatment steps for ensuring the complete removal of micropollutants. AC can be used to remove organics such as pesticides, phenols, pharmaceuticals, organic halogens, non-biodegradable compounds, dyes, and inorganics such as Hg2+, Pb2+, Cd2+, Cu2+, and Ni2+,61,62. AC is also an efficient media for microbial growth, and biologically activated carbon (BAC) has been developed for the inactivation of biological pollutants within a short period. Attaching biomass to AC can remove contaminants by both adsorption and biodegradation62. However, AC has some disadvantages, such as expensive thermal/chemical regeneration methods and the loss of a significant fraction of adsorbent during regeneration63. Moreover, the adsorption mechanism on AC depends on various factors such as dispersive, electrostatic and chemical interactions; intrinsic properties of the solute and adsorbent and hence the interaction between the pollutants and AC is difficult to predict64. Although AC has a high adsorption capacity, it can maintain it only until the adsorption sites become exhausted with pollutants. Other absorbents such as polymeric absorbents are also used when recycling of valuable chemicals is desired58,62.
The adsorption of phenols by commercial PAC showed that the removal efficiency increases up to an optimum dosage, beyond which the improvement was negligible65. It can be observed that the GAC is usually used for removing natural organic matter, synthetic organic compounds, and heavy metals61. Zhang et al. used GAC for removing algal odorants like dimethyl trisulfide and ß-cyclocitral66. Adsorption isotherms were investigated for the process and found that Freundlich isotherm was fitting the best. The ΔG° for adsorption of ß-cyclocitral and dimethyl trisulfide were –4.24 and –3.61 kJ mol−1 at 298 K, respectively66.
Cyanide, for instance, was found to be better removed by biologically activated GAC compared to virgin GAC. Also, biodegradable anthraquinone dyes were removed more efficiently because of the elevated substrate concentration at the granular surface found in BAC systems62. Table 2 shows a summary of the adsorptive removal of heavy metals from industrial wastewater by AC.
Not only AC is an effective method to remove heavy metals, it has also been used for the removal of other pollutants. AC was used to remove crystal violet dye and had a maximum adsorption capacity of 84.11 mg/g and a removal efficiency of 85–90%67.
Adsorption on natural materials
Adsorption on natural materials such as zeolites has been gaining more interest. Adsorption of metal ions on the zeolite active sites produce inner and outer-sphere complexes. The interposition of at least one water molecule between the bound ion and the functional group of the adsorbent results in the formation of outer-sphere complexes. In contrast, inner-sphere complexes will be formed when there is no interposition of water molecule(s). A coordinate bond will be formed between the heavy metals and the surface functional groups68,69. For example, Clinoptilolite, a type of zeolite, has shown high selectivity to Pb2+, Cd2+, Zn2+, and Cu2+ 10. Also, it was found that polymeric materials can be used to increase the efficiency of natural clay to remove heavy metals by modifying the natural clay into a composite named clay-polymer composite70. For example, clay-poly(methoxyethyl)acrylamide (PMEA) composite has been synthesized to study its capacity to adsorb Pb2+ ions. Bentonite, another type of clay has also exhibited high removal (>99%) of heavy metals removals like Cu2+, Co2+, Ni2+, Zn2+, and Pb2+ ions. The adsorption affinities of the metal species were as in the below order: Co2+>Cu2+>Ni2+ = Zn2+>Pb2+ 71. Another study also reported the complete (100%) removal of Pb2+ from aqueous solutions using 20 g L–1 of bentonite72.
Natural phosphates (NP) constitute another category of raw adsorbents73. NPs are abundant, cheap, and non-hazardous to the environment, and can be used for heavy metals removal. It was reported that NP has a monolayer adsorption capacity of 26 mg g–1 for Cd2+ at a pH of 5.074. Another study reported a room temperature adsorption capacity of 200 mg Pb2+/g of rock phosphate (low-grade) when the initial aqueous Pb2+ concentration was 50 mg L–1. Adsorption of Pb2+ on PO43- followed a pseudo-first-order rate and Langmuir isotherm75. Nano-hydroxyapatite (nHA) is a less-soluble, abundant and stable phosphate that has a high sorption capacity for heavy metals. It has been used in the adsorptive removal of Pb2+ and Cd2+ from polluted soils73. Applying nHA can reduce water-soluble Pb2+ by 72% and Cd2+ by 90%, bioaccessible Pb2+ by 12.5–27.5% and Cd2+ by 17.7–34.6%73. It was also reported that nHA had a maximum adsorption capacity of 2500 mg of Sn2+ g–1. The process was endothermic and followed Langmuir isotherm76.
NPs have also been used to remove emerging and persistent organic contaminants. The adsorption of a reactive dye, Reactive Yellow 84, by hydroxyapatite (HAP) has been found to follow Langmuir isotherm with a monolayer adsorption capacity of 50.3 mg g–1 at a pH of 5.0. Adsorption of this dye is endothermic (enthalpy of adsorption is 2.2 kJ mol–1) and has a low temperature dependency77. Nanocrystalline HAP has been used to remove nitrobenzene78. The authors achieved a 52.4% removal with an adsorbent dosage of 5 g L–1, whereas the removal was increased up to 95% when the dosage was 25 g L–1 78. However, the adsorption capacity of nitrobenzene on nanocrystalline HAP is relatively low compared to adsorption on AC (100–300 mg g–1). This was attributed to the smaller surface area of nanocrystalline HAP (42.3 m2 g−1) when compared to AC (1000 m2 g–1)78. The effectiveness of mesoporous silica-alumina (MSA) on the removal of hydrocarbons from industrial wastewater has been investigated79. The dehydroxylated silicon content is the main factor in the adsorption process. Water is adsorbed on the MSA surface by interacting with silanol groups; aromatic hydrocarbons such as benzene and toluene interact with electron-poor acid sites. However, the presence of areas rich in hydrophilic Al enclosed in an active silica matrix can create spots that can deter the efficient removal of hydrophobic hydrocarbons.
Adsorption on industrial by-products
Industrial by-products such as carbonaceous wastes, agricultural by-products, mineral-derived sources, etc. can be used as low-cost adsorbents for industrial wastewater treatment80. For instance, steelmaking slag comprised of hydroxides of iron and calcium is used as a low-cost adsorbent81,82,83,84. Fe(OH)3 and Fe(OH)2 present in the slag provide adsorption sites for ions such as As3+, and Cr3+ whereas Ca(OH)2 increases the solution pH and enhances the heavy metal precipitation83. The removal of Cd2+ by steel industry slag has also been investigated. The optimum adsorbent concentration was found to be 10 g L–1 for treating 100 mg L–1 of Cd2+ solution at pH 4.0, and the removal rate could reach up to 99.1%. The removal is attributed to the chemisorption, including chemical precipitation and coordination reactions84.
Other examples of low-cost industrial by-product adsorbents are fly ashes85,86,87,88,89, waste Fe, hydrous TiO2, and other waste products which can be fine-tuned chemically to enhance pollutant removal10. Fly ash can be chemically modified with NaOH and CH3(CH2)15N(Br)(CH3)3 for Cd2+ and Cu2+ removal89. The mechanisms of adsorption on industrial by-products differ from one material to another. Adsorption on hydrothermally modified fly ash, for instance, is based on electrostatic attraction. Other mechanisms depend on ion exchange and the structure of surfaces11. In general, using low-cost adsorbents (such as the ones obtained as by-products or from natural resources) has introduced an alternative for industrial wastewater treatment systems. The comparative advantages of low-cost adsorbents are their relatively low prices and abundance since they are natural materials or by-products from agricultural and industrial activities. Some of these adsorbents exhibit a high selectivity for specific contaminants. Required wastewater-pretreatment and fine-tuning the adsorbent materials are some limitations. Moreover, in some cases, the heavy metals in the slag may leach out and cause secondary contamination, and hence using those industrial by-products as adsorbents has to be done carefully83.
Biosorption
Biosorption involves concentrating pollutants, particularly heavy metals, by binding them with inactive microbial biomass mainly via adsorption and chelations10,90,91,92. Although this is attributed to the metabolism-independent binding of heavy metals to the cell walls, the actual mechanisms are yet to be understood10,90. Several mechanisms, such as chemisorption (ion exchange and chelation), complexation, and physical adsorption, are proposed90. For example, a study on the removal mechanism of Cu2+, Ni2+, Zn2+, Pb2+ and Cr3+ by Penicillium chrysogenum attributed ion exchange as the principal removal mechanism93. The uptake of Pb2+ by R. glutinis is also attributed to the transfer of ions and biomass released phosphate induced precipitation. Also, the uptake of Pb2+ by Aspergillus parasiticus cell wall takes place by ion exchange and complexation processes94. Cu2+ ions are also bioadsobed by Fucus serratus by ion exchange. When Ca2+ ions are released from the surface of the biomass, a bond between Cu2+ and functional groups forms94,95. Also, when Ni2+ is bioadsorbed by Lathyrus sativus, dative bonds will be formed between Ni2+ and nitrogen in the ammonia functional group of the biomass species96. Chelation and ion-exchange are proposed to be the two main mechanisms of chemisorption of Cu2+ by walnut and hazelnut shells96. Many other biomass sources such as peanut and hazelnut shells 10,97, green alga98, orange peel99, Rhizopus sp. biomass100, jackfruit10, maize cob or husk10, and their chemical modification or thermal conversion to AC have been used during biosorption10.
Weak Van der Waals forces between the heavy metal ions and adsorbent surfaces constitute the primary removal mechanism in physisorption. An example of physical adsorption is Cd2+ adsorption by olive cake, which achieved a 66% removal at 28 °C and pH of 6101. Another study on the removal of Zn2+, Pb2+, Fe2+, and Cu2+ using dried red seaweed Kappaphycus sp. also indicated the removal of metals vis physisorption102. Malamis et al. (2011) applied activated sludge coupled with UF to enhance the removal of Ni2+, Cu2+, Pb2+ and Ni2+ 103. The highest removal of Pb2+ was found at pH=4 and Zn2+, Ni2+, and Cu2+ at pH = 6. Table 3 illustrates data obtained from the literature of several biosorption cases.
Biosorption has been reported to remove pollutants other than heavy metals. For instance, modified lemon leaf was used to remove cationic dye, and was found to have an adsorption maximum capacity of 36.10 mg g–1 and yielded 70% removal efficiency104. Another example was the utilization of modified biogas residue to remove nitrate and phosphate. The maximum adsorption capacity was reported to be 64.12 mg g–1 and 34.40 mg g–1 for nitrate and phosphate, respectively105.
Several modified biopolymers have been developed for heavy metal removal applications, which include natural rubber, Lyocell fiber, and chitosan-based adsorbents11. Biopolymers are widely used in industries due to their availability, environmental safety, and ability to reduce heavy metals to parts per billion10. Such materials do have certain drawbacks, however. For example, the biopolymer chitson in its natural form has low specific selectivity for heavy metals and low adsorption capacity for complex polluted wastewater106. Nevertheless, good sorption capacity for transition metals has been demonstrated for chitosan with a high content of hydroxyl and amine groups, but little to no sorption capacity for alkaine or alkaine earth elements106.
Heavy metal removal by biopolymers can be enhanced by modifying its chemical and physical properties10,11,107. For instance, chemical and physical methods can be used on chitosan to improve its removal efficiency. Chitsoan can be modified physically by preparing the polymer in different forms. Other forms of chitosan include water-soluble and water-insoluble chitosan, such as flakes nanoparticles and beads11,106. Modified chitosan beads were suggested for the diffusion of various metal ions and specifically Cu2+ ions through spherical chitosan-tripolyphosphate (TPP) chelating resins, which are prepared using an in-liquid ionotropic crosslinking method108. Additionally, the study of Liu et al. [149] suggests that non-porous glass beads can be used to create hybrid materials by immobilizing chitosan on their surface109. Chemically modified chitosan is also beneficial for wide heavy metal sorption applications. The most highlighted modifications of chitosan are the grafting chitosan and the cross-linked chitosan11. Polysaccharide-based-materials have also been developed as modified biopolymer adsorbent, which is derived from chitin, chitosan, and starch for the removal of heavy metals from wastewater10.
Future research should aim at developing new and low-cost adsorption materials with high treatment efficiencies as for most adsorption processes, the cost of the adsorbent constitutes up to 70% of the total cost110. Also, most of the studies focus on determining the maximum adsorption capacity of an adsorbent, which assumes a fixed-bed adsorption system. This might not be the case in industry, and more studies should aim at investigating the maximum capacity in real-life processes. The performance of fixed-bed adsorbers is different when compared to agitated batch adsorbers for instance. Another very important aspect that is often not focused on is desorption and regeneration. Since the world is more concerned with sustainability and environment nowadays, more studies should focus on adsorbents regeneration and reuse.
Advanced oxidation processes (AOPs)
Advanced oxidation processes (AOPs) are chemical treatments involving the generation of hydroxyl radicals (OH•) that can efficiently oxidize recalcitrant pollutants111. OH• are characterized by their high standard oxidation potential (up to 2.80 V) and their high reaction rate in comparison to common oxidants like chlorine, oxygen, ozone, H2O2, or potassium permanganate. Hence, high rate constants can be achieved during the reaction of OH• with both inorganic and organic solutes112. AOPs, in general, employ the efficacies of different oxidants to degrade hazardous pollutants by converting them from their reduced forms to their final harmless oxidized forms. This conversion mineralizes and degrades the contaminants to harmless substances for overcoming the environmental impacts due to the disposal of the primary pollutants to the aquatic ecosystem. Although these systems use different oxidants, they all tend to share the same radical production113. These processes have a high potential to purify water from pollutants that are hard to be removed by biological methods114. AOPs include two main stages: The formation of strong OH•/oxidants and the interaction of these radicals with the targeted pollutants to convert them to carbon and water in the best-case scenario. When two OH• interact, H2O2 is formed, as shown in below equation:
The comparative advantages of AOPs are (i) high disinfection strength: Several AOPs are used because of their great disinfection properties115, and (ii) standalone destruction of organic contaminants. If methyl tert-butyl ether, for example, was removed by stripping, additional processes such as catalytic oxidation would be needed for effective treatment. However, AOPs destroy the organic contamination directly without the need for other chemical processes116.
However, the generation of undesirable oxidation by-products affect AOPs. If these oxidation by-products have slow reaction rates, there would be a delay in mineralization, leading to unwanted accumulation. Also, inorganic substances are formed during some AOPs, such as bromide conversion to bromate during ozonation. These inorganic compounds interfere with AOPs and inhibit the oxidation reactions. These compounds scavenge the OH•, which are meant to remove and destroy the concerned contaminants116. Therefore, there is need to address the issue of radical scavengers and the likelihood of producing unwanted intermediate derivatives from the oxidized forms of pollutants. pH influences the acid-base equilibrium involving OH• formation and the radical’s concentration.
Two critical parameters should be taken into considerations while designing and constructing an efficient AOP system. Firstly, the dosage of chemicals as it will increase the cost and may give the possibility of by-product formation. Secondly, reactor configuration and contact time, which is often determined when implementing a pilot study rather than a lab-scale, should also be considered116. The quality of industrial wastewater and other operating conditions also affect the efficiency of degradation of concerned pollutants. It is known that most of the organic substances would react instantly with the introduced radicals116. Turbidity also acts as an influencer to the system performance because the more turbid the industrial wastewater, the lower is the penetration of UV source to the water. Additionally, Fe2+ and Cu2+ or other heavy metals in wastewater may also cause the formation of Fe or Cu organic complexes thus results in fouling for the system116. The recently employed AOPs are the Fenton-based processes, electrokinetic treatment, and degradation with metal oxides.
Fenton-based processes
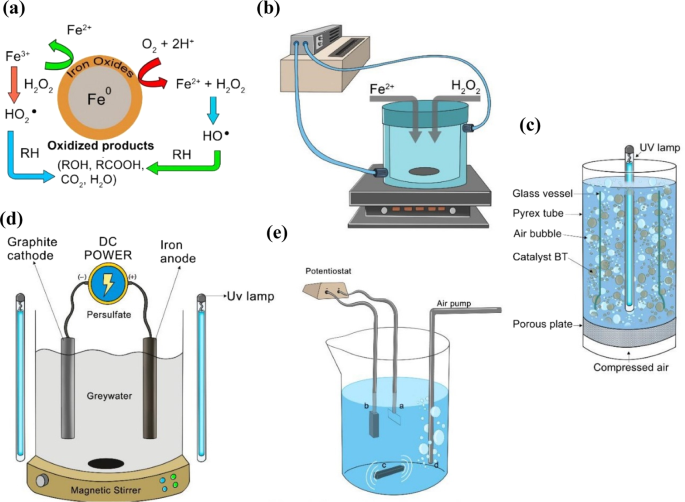
Fenton processes are catalytic processes that generate hydroxyl radicals (OH•) from H2O2 upon the addition of Fe2+ 112. OH• are produced from the oxidation of Fe2+ to Fe3+ as in below equation 111:
However, the Fenton process produces Fe sludge waste because Fe3+ precipitates as FeO(OH)112. Thus, the typical Fenton process can be improved by coupling it with electricity to have an electro-Fenton process, or with UV or solar light to have a photo-Fenton process, to reduce Fe3+ back to Fe2+ and reduce the amount of waste sludge111. The UV produces more OH• by photolysis and activates photo-decarboxylation of ferric carboxylates117. Table 4 summarizes the application of Fenton treatment for the removal of various types of contaminants. Recent advances in the Fenton-based processes such as Fenton, photo-Fenton, and electro-Fenton are elaborated in the next subsections.
FeSO4 is an adequate salt for ferrous generation, and it showed a TOC removal efficiency of 94% after 2 h118. FeSO4 can be generated by adding Fe catalyst to H2SO4. Fenton process via FeSO4 was shown to be very useful and efficient in terms of oxidation and degradation of TOC118. Organophosphorus pesticides removal from wastewater has been evaluated using the Fenton process under various reaction conditions at room pressure and temperature. The optimum condition was determined by several parameters such as pH, stirring time, and dosage of FeSO4 and H2O2. COD removal efficiencies for an actual triazophs wastewater treatment plant and a bench-scale experiments were 85.4% and 96.3%, respectively. Complete oxidation of phosphorus and nitrogen content was observed through which eutrophication is minimized119. The efficiency of treating wastewater obtained from a synthetic fiber factory that uses acrylic polymer has also been investigated. The more H2O2 was added, the more the effectiveness of degrading the unwanted pollutants. However, an increase in the COD content of the treated effluent was observed at a concentration above 500 mg L–1 of H2O2. Also, pH levels seemed to impact the removal efficiency of color and COD. The pH was carefully selected for the removal of color since a decrease in pH (<6) led to the destabilization and aggregation of particles120.
Apart from FeSO4, Fe3O4 has also been examined as a reagent that can be combined with H2O2 in a Fenton process for the degradation of phenols121,122. Many characteristics were taken into consideration, but the one that played a significant role was Fe2+ and Fe3+ ratio, which was determined by the chemical analysis. Fenton reaction started slow but accelerated eventually. Phenol degradation was achieved efficiently in Fe3O4 that has a higher structural content of Fe2+. The use of magnetite showed positive features such as safe levels of Fe2+ content in wastewater/water effluent, a magnetic behavior that separates the reagent easily from the treated feed, and easy absorption of UVA radiation, which enhanced the reaction.
Recently, Fenton pilot-scale experiment has been implemented to degrade synthesized C20H18NO4+ 123. The main parameters were optimized using response surface methodology technique. Acidic industrial wastewater sample was collected from a chemical factory, and subsequently, pH was adjusted prior to the oxidation process. Values given by the statistical method were highly efficient and relatively close to the obtained experimental results. The berberine removal efficiency was found to be 35.6% at a pH of 3.5.
The photo-Fenton process has improved the TOC removal efficiency when compared with the Fenton process due to the enhancement of TOC degradation rate by photons124. Photo-Fenton process at neutral pH with UV254 is a promising technique in which it degrades all pollutants in a limited time. For this process, Fe is not necessarily needed if the treated water contains at least 1.5 mg L–1 of Fe2+ or Fe3+ 125. This technique is appropriate for large-scale systems and can be used to replace the available conventional solutions such as ozonation. However, a relatively low amount of pollutant degradation would be observed when photo-Fenton is implemented using regular sunlight.
Photo-Fenton technology has also proved to be efficient for the removal of pesticides from the water with high salinities126. Additionally, photo-Fenton technology has also been reported as efficient for the removal of pesticides from the water with high salinities. Oxidation rates were much faster from the beginning when the water was more saline. For water with lower salinity, oxidation rates started to increase only after about 60 min of treatment. The effect of conductivity on the mineralization of organic content of saline water was mainly caused by interference by chloride. The photo-Fenton process has also been used for dye removal. Low levels of H2O2 in the process may result in the formation of more toxic products. In some cases, however, the pollutant may be degraded without the creation of any toxic by-products depending on the solubility of the contaminant in water. Therefore, the solubility of pollutant in water has a significant role in the photo-Fenton process. Photo-Fenton is also reported as useful for the full degradation of 4 colorants in wastewater streams from the food and cosmetics industries127.
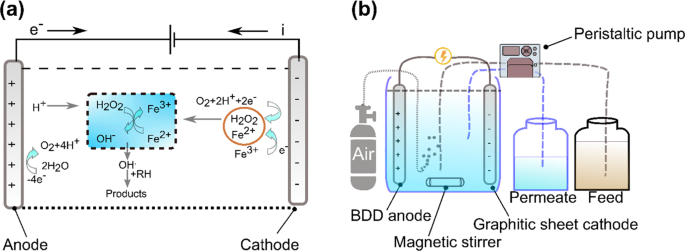
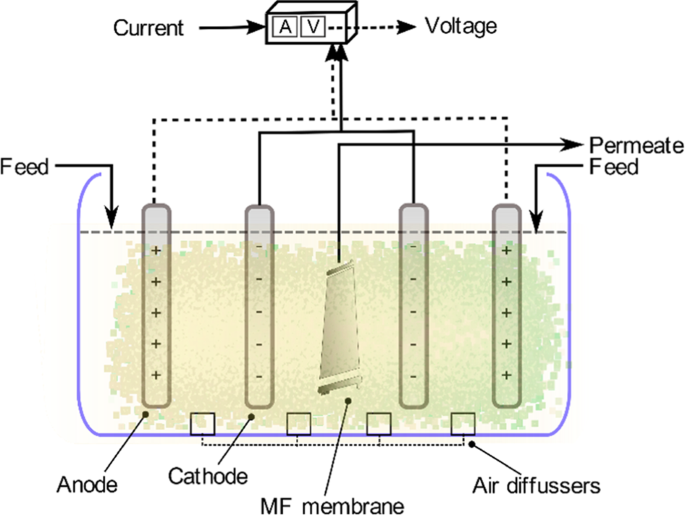
Electro-Fenton is an emerging process where H2O2 is electro-generated at the cathode made with carbon materials. This method is safe because H2O2 is produced in-situ, and the risk of handling H2O2 is reduced. It is also a faster process in degrading many pollutants because of the constant generation of Fe catalyst at the cathode128. A simple illustration of the electro-Fenton mechanism is shown in Fig. 1a. Removal of Alizarin Red has been successfully implemented recently using the electro-Fenton technique with a graphite-felt electrode where the cathode was fed with air to reproduce H2O2. Ferrous ions played the central role in the removal since the oxidant alone has a limited capability. Through this method, Alizarin Red was oxidized to a colorless acid and then to carbon dioxide129. 100% color removal has also been achieved through the electro-Fenton process130. By using the GAC electrode as the cathode and air in the electro-Fenton process, stability, and efficiency in removing methyl orange has been achieved130. It was, however, observed that the electro-generation of H2O2 was limited at longer times due to the oxidation to oxygen at the anode, but still, the limited value is much more significant when compared to methods without air bubbling approach. It was also mentioned that oxygen did not contribute to the reduction process since the rate of increase of H2O2 generation followed a linear relationship with the imposed current for the first hour130. The use of AC cathode was also studied previously in Taiwan by131, where the pollutant adsorption on the cathode was measured, and the highest COD removal reached around 75%.
Electro-Fenton process has also been applied for the degradation of surfactants by using graphite-felt cathode119. The critical parameter was to apply different electrolytes in the presence of Fe catalyst. The decay kinetics of the surfactant was unaffected by its initial dosage, highly dependent on the applied current, Fe catalyst concentration, and pH; and followed a pseudo-first-order reaction. Another recent application of the graphite cathode electro-Fenton process is the removal of antibiotic residue from industrial wastewater. A highly bioactive antibiotic (tetracycline) was mineralized to carbon dioxide through this method123. Meanwhile, the recent development and use of nano-enhanced carbon electrodes in the electro-Fenton process can ensure enhanced stability and pollutant degradation rate. Graphite cathodes have been compared with carbon nanotube (CNT) sponge in an electro-Fenton process114. The CNT sponge was used as the cathode of the electro-Fenton process under high electrical power. The CNT sponge showed enhanced stability and a good degradation rate that was estimated to be ten times higher than the one obtained by graphite cathode. Recently, the efficiency of carbon-felt electrode has also been compared with those of alternative non-carbon materials as anode132. The materials, such as boron-doped diamond (BDD), Ti with Pt coating, and Ti with TiO2 coating, were used as electrodes to remove carbamazepine from wastewater. The BDD anode showed the fastest oxidation and mineralization efficiency; and exhibited much better catalytic ability than the others125. Also, the BDD anode showed a better performance in mineralizing Atrazine into C3H3N3O3 as the ultimate end product. This study compared its results with the previous experiment conducted on C8H14ClN5 removal with classical Pt anode. Although the classical Pt anode was faster in decaying Atrazine, it was slower in mineralizing it. BDD anode has the potential to produce high amounts of OH•. These formed OH• are physically adsorbed to the anode surface, which enhanced pollutant uptake. The study concluded a full removal of the insecticide at an initial pH of 2. The efficiency of the BDD anode agreed with previous results132. A continuous electro-Fenton process with graphite sheet cathode and BDD anode is illustrated in Fig. 1b.
Furthermore, Tylosin antibiotic was degraded after 15 min of applying electro-Fenton, and it followed pseudo-first-order124. The degradation of Tylosin antibiotic showed a possibility to combine biological treatment with electro-Fenton because the biodegradability showed an improvement where the BOD5/COD ratio increased from zero to 0.6 after 6 h of electrolysis. After 6 h, the application of electro-Fenton alone gave more energy efficiency than the combination. It was suggested that the mineralization should be optimized by balancing the energy used in case both processes are operating together. In addition, electro-Fenton has been integrated with absorption in order to enhance the kinetics of color removal. The use of Fe-loaded AC as an absorbent, together with electro-Fenton has shown promising results in winery wastewater treatment133. Fe-loaded AC implementation alone exhibited 23% color removal after 24 h. However, almost total color removal was achieved at the same time when electro-Fenton was integrated with Fe-loaded AC absorption. The adsorbent was used as a catalyst to optimize the process kinetics so that higher degradation rates can be reached. The adsorbent also showed a better physical capability than Mn and Fe alginate beads. Several conventional electro-Fenton process studies emphasized that the process can ensure a total removal or high removal efficiency at a pH of 3128. However, a recent optimization study using Box-Behnken design and response surface methodology showed higher efficiency when the pH was increased up to 5128. It was illustrated that if the pH was below 5, then H2O2 cannot be decomposed to the oxidant radical by Fe2+. At pH below 5, hydrogen would gain one electron, and this would lead to a reduction in the rate of reaction between the Fe2+ and H2O2. Consequently, a reduction in the degradation of pollutants would be observed. The results obtained in several recent studies on Fenton-based processes in terms of the removal of highly hazardous pollutants are shown in Table 5. An overview illustration of various Fenton processes is presented in Fig. 2
Electrokinetic treatment
Electrokinetic treatments such as electrocoagulation (EC), electrochemical oxidation (EO), and electroflotation (EF), electroosmosis, and electrophoresis help in the degradation of various pollutants via electrochemical mechanisms. They are specifically beneficial for the treatment of industrial wastewater, such as textile wastewater. Some of the contaminants that have been removed successfully by electrokinetic treatments include decolorizing reactive dye solutions and phenolic compounds, and heavy metals134.
Electrocoagulation (EC) is an electrokinetic process that utilizes the electrical field to form aggregated particles. EC generates coagulates via the decomposition of electrodes. Ion generation occurs at the anode, while hydrogen gas is produced at the cathode, allowing electofloculation to take place since the hydrogen released helps in keeping the flocculated particles float135. Colloidal solids and particles, metals, and soluble inorganic pollutants are some of the materials that EC can remove from aqueous media by neutralizing their charges using the charged polymeric metal hydroxide species. Neutralizing suspended solids charges causes the contaminates to agglomerate and then separate from the aqueous phase.
EC has been widely used for treating industrial wastewaters with metallic content136. The efficiency of EC depends on the type of wastewater. For example, COD removal by EC in textile wastewater has been found to be from 40 to 70%, 96% for soluble oils, and 80% for paper waste137. One of the key factors to consider when using EC for the treatment of industrial wastewater is the electrode material. The effect of three different types of electrode materials, namely Al, stainless steel, and Fe on EC’s efficiency for the removal SO42- from a refinery’s wastewater has been reported138. The electrodes used in the study have the same area, contact time, and CD. It was observed that Al electrodes have the highest efficiency, in which, the sulfide reduction was 2.5 times higher than stainless steel and Fe electrodes. This is due to the reaction between Al(OH)3 and SO42- in the cell, causing sulfate salts to participate at the bottom of the cell. SO42- can also get trapped in porous precipitate and increase extraction efficiency. Additionally, the solubility of Al2(SO4)3 is less than that of ferrous sulfates (400 kg m−3 vs. 600 kg m−3). Therefore, the precipitation of Al2(SO4)3 can occur at a faster rate. The electrode with the lowest efficiency among the three materials is stainless steel, due to its resistance to corrosion138. However, a high concentration of calcium or magnesium ions can increase stainless steel effectivity drastically135. This demonstrates that the type of pollutant should be taken into consideration when choosing an electrode. Wang and Chou [209] reported that using Al as cathode and anode, because of its low hydrogen overvoltage, gives the highest turbidity removal and COD in the treatment of tanning wastewater. At the same time, Fe or steel can be more effective in treating the same wastewater, depending on the targeted pollutant139. Another study showed that Al electrodes yield better effluent quality for leather tanning industrial wastewater treatment in comparison with other electrodes140. Therefore, in general, Al pairs of electrodes might be more suitable for the removal of organic matter. In contrast, Fe electrodes might provide a higher removal of inorganic pollutants, such as Cr, Ca, and Zn141. For instance, Fe electrode is not effective for COD removal, since soluble and miscible organic compounds such as glucose, isopropyl alcohol, phenol, sucrose do not react with Fe2+ and Fe3+. Only a small amount of these organic compounds can be incidentally removed by sorption on the floc. COD value might increase when compounds (usually acids) react with Fe2+ to form soluble products, which remain in the solution. Additionally, COD can be partially removed when sodium oxalate, other similar salts, and certain acids are present in the wastewater. In addition to protons, EC generates Fe ions which hydrolyze to form Fe(OH)2 and Fe(OH)3. OH− ions are not attracted to sodium ions since Fe ions are more acidic, which causes a very low percentage of CH3COO− and similar ions are removed. Acids, such as C6H8O7, C7H6O3, C4H6O6 and C2H2O4 acids, react with Fe2+ and Fe3+ to form soluble and insoluble, respectively. COD can only be effectively removed if the present organic compounds can react with Fe ions to form insoluble compounds, as exhibited by hydroxoaluminum ions142. Therefore, when Al and Fe electrodes are compared, Al electrodes show higher COD removal efficiencies, especially at lower pH values. This is because Al has only one oxidation state, namely Al3+, which allows a complete reaction of the organic compound to form an insoluble compound142.
EC can also be used to treat oil-in-water emulsions143,144,145,146,147. The coagulants produced in-situ cause the break-up of the emulsion by reducing the surface charge of the droplets. This causes the coalescence of oil droplets, which is then followed by separation either by settling or by dissolved air-flotation. The primary removal mechanism is bridging flocculation or the attachment of absorbing macromolecules to several droplets simultaneously148,149. In bridging flocculation, electrically charged sites on the surface of the droplet are attracted to species with the opposite charge. Additionally, the adsorption properties of growing metal hydroxides can be utilized for the elimination of oil (Fe and Al hydroxides). It was found that the adsorptive layers of anions and cations of growing hydroxides, in addition to its nucleus, produce positively charged particles that have high adsorption of oil droplets148,149. Also, destabilization by non-absorbing polymers can happen by depletion flocculation148. It has been found that the instability of oil emulsions and the removal of COD can only be attained for values of pH in the range of 5–9.
The aqueous behavior of Al includes the production of polymeric hydroxoaluminum ions, monomeric hydroxoaluminum ions, and Al(OH)3 precipitates. Monomeric cationic hydroxoaluminum are the predominant species at low range of pH. When the pH is close to neutrality, aluminum hydroxides precipitates are the predominant species. The dissolution of the precipitates into monomeric anionic hydroxoaluminum occurs with an increase in pH150. The inability of the monomeric Al ionic species to destabilize oil emulsions can also be attributed to their steric constraints; monomeric Al ionic species have a smaller size in comparison to the size of the droplet; thus, enabling to act over oil droplets. On the other hand, the size of hydroxide precipitates, and polymeric ions can help more than one droplet in the attraction to the coagulant species151. When the electrical charge exceeds a certain threshold, de-emulsification occurs, which slightly decreases the removal efficiency. This is because the coagulant at an excess concentration reduces the efficiency, since it increases the concentration of Al(OH)3 particles, and thus decreases the chance of attracting more than one droplet on the same particle of coagulant.
EC has been used to treat industrial wastewater rich in heavy metals such as Cr6+. Hexavalent Cr removal is achieved by reducing Cr6+ to Cr3+, which then later precipitates in the form of neutral hydroxide. It was found that a higher CD corresponds to faster removal of Cr. Additionally, unlike Fe electrodes, COD removal was not affected by the presence of Cr6+ by using Al electrodes152. 5 min was sufficient for the removal of 99% of Cr6+, but 10 min is required with using Al electrodes153. After 60 min of EC, flecks of aggregates were observed (Fig. 3). Cr6+ ions can react with Fe2+ to produce Cr3+ and Fe3+ 154. By using Fe electrodes, the residual Cr concentration is dependent on the residual Fe concentration, because the removal of Cr ions is carried out by co-precipitation of Cr ions and Fe ions. Hence, complete precipitation of Fe ions is preferred154.
Flecks showed by scanning electron microscope (SEM) after 60 min of EC153.
EC has also been used recently to treat industrial wastewater containing Mn, Cu, and Zn at concentrations of 5 mg L–1, 5 mg L–1, and 10 mg L–1, respectively, and at a pH of 6155. Cu and Zn were removed entirely, and more than 95% removal of Mn was achieved. Decreasing the initial pH resulted in a decrease in removal efficiency. Also, the higher applied current was associated with higher efficiency. The use of different initial concentrations did not affect the removal of heavy metals. Another study reported a 96% arsenic (As) reduction by EC when Fe electrodes were used156. Cu, Cr, Pb, and Zn removal from billet industry wastewater was also studied. Around 99% of these heavy metals were removed at pH 5, CD of 98 A m−2, and 30 min treatment time157.
EC has also been utilized to extract fluoride (F−) from treated industrial wastewater, particularly that generated from steel industry158. F− is additionally present in wastewater generated from semiconductor, electroplating, glass, and ceramic industries159,160. By using EC, F− concentration can be reduced to 0.5 mg L–1 from a concentration of 4.0–6.0 mg L−1 using optimum HRT of 5 min158. Although an increase in HRT is associated with increased removal efficiency, this increase in removal efficiency is negligible after 5 min. Another study reported that increasing the number of Al plates in a reactor from one to three, increased the F− removal from around 90% to 93% at a constant potential of 30 V153. However, increasing the number of plates to about 6 had a negative impact on the F− removal efficiency. This can be explained by Ohm’s law; the current and resistance are inversely proportional at a fixed voltage. It can also be observed that increasing the number of plates resulted in producing more coagulants, resulting in increased resistance, and reduced current. The CD determines the coagulant dosage and size of the bubble production, which affects the growth of flocs.
EC process has also been tested for the removal of organics from the textile industry wastewater. A study reported COD reduction from 1316 mg L–1 to 42.9 mg L–1 by using RuO2/IrO2/TaO2 and titanium electrodes161. In another study, COD and turbidity removal, the effect of electrode material, cell voltage, and other parameters were investigated in textile wastewater treatment using Al and Fe electrodes152. EC removes organic matters by two mechanisms. The first mechanism involves the removal of organic matters through indirect oxidation by utilizing chloride. The second mechanism is adsorptive/entrapment of organic matter, particularly colloids, and SS on metal hydroxide flocs162,163. Apart from the removal of organics, several studies have been conducted to investigate the extraction of color from textile wastewater. The extraction efficiency of color was found to be 95%-99% by EC using Fe and Al electrodes164. The decolorization kinetics followed the first-order law. The highest color extraction efficiency was attained at neutral and slightly acidic pH values164. Another study tested EC’s color removal efficiency for both synthetic and real wastewaters. It was found that the removal of color from synthetic wastewater was higher than that of real wastewater. This was due to the higher organic content in real wastewater, in addition to the complexity165. Table 6 summarizes the recent advances in industrial wastewater treatment by EC.
The specific energy consumption is directly proportional to the current and time166. It was predicted that US $1.86 is required for treating 1 m3 of dye by EC process167. The operating cost increases with the increase in operating time for iron and aluminum electrode, while the operating cost is double for aluminum electrode EC as compared to iron electrode EC for the same operating time168. The operating cost of EC process for the treatment of industrial wastewater is minimal as compared with other process like Fenton and chemical coagulation. Furthermore, the cost of adsorption process is less compared to that of EC, where the generation of huge quantity of sludge is the major problem169.
Electrochemical oxidation (EO) of pollutants in industrial wastewater can be achieved via two main mechanisms. The first is direct anodic oxidation, where oxygen or OH• in the oxide lattice MOx+1 is generated170. The other mechanism is by indirect electrooxidation process in which the oxidation process is carried out via a generated mediator, such as chlorine, hypochlorite, ozone, and H2O2171. Generally, the EO technique can remove a wide variety of pollutants, such as nitrogen species, microorganisms, and refractory organic matter, which are often found in industrial wastewater. Additionally, it is effective in treating non-biodegradable, toxic organic pollutants, nitrite, and nitrate, and some micro contaminants such as pharmaceuticals135. Advanced technologies that are based on chemical oxidation are usually used to treat biologically recalcitrant effluents172. Electrochemical reactions are additionally utilized for disinfection purposes by generating oxidizing species. High disinfection efficiency would be obtained from waters that contain chloride ions because the generation of oxidizing species would be achieved173.
EO is affected by cell design, pH, electrolyte composition, CD, temperature, and electrode materials. Electrodes should be stable and should display low activity toward oxygen evolution reaction and high activity towards organic oxidation174.
The most common electrochemical oxidant is chlorine, which is formed by the oxidation of chloride at the anode. The electrochemical oxidation of ammonia has also been reported, specifically that present in saline industrial wastewater175. An electrochemical cell consisting of circular BDD on a stainless-steel cathode and silicon anode was used. A high level of chloride ions in wastewater increased the ammonia elimination, and the TOC removal was reached up to 90%175. It was also found that the highest efficiency was associated with the lowest CD175. If the chloride content in wastewater is not high enough, salt must be added to increase the treatment efficiency135. The removal of dyes, solvents, and surfactants has also been achieved by EO111. BDD was used as an anode and stainless steel (AISI 304) as a cathode. A complete COD removal was achieved with very high current efficiency, depending on CD and the type of anions in the wastewater. The treatment of dyes is more efficient with using chlorine, while phosphates are better suited for the extraction of aliphatic compounds.
EO has also been utilized for the disinfection and treatment of latex wastewater. The initial concentration of COD and microorganism was 3820 mg L–1 and 180 CFU mL–1, respectively. COD was reduced to a level of 78 mg L–1, while the microorganism was completely removed. This was achieved through the hydrochlorites acid produced from electrolytic reactor that utilizes graphite as an anode and stainless steel as cathode. Equations (3–5) illustrates the electrochemical generation of chlorine/hydrochlorite in a solution that contains chloride ions176:
Nowadays, due to their high stability and efficiency, conductive-diamond anodes have been gaining more attention in the treatment of industrial wastewater containing organic pollutants177. This can be justified by the fact that the anodic activity depends on the value of the overpotential of oxygen evolution. High oxygen evolution overpotential is essential to avoid undesired side reactions that reduce the current efficiency of oxidizing organics135. IrO2, graphite, and Pt exhibit low values of overpotential of oxygen evolution in EO when compared to conductive-diamond anodes. This necessitates the application of a very low CD to remove pollutants effectively or the use of this anode when there is a high concentration of chlorides or metallic mediators. BDD film on titanium substrate also gives a high value of oxygen evolution overpotential. When this anode is used, oxidation can take place with a low amount of oxygen evolved when high values of current densities are present.
EO has been additionally used to remove tetrahydrofuran (THF) from rubber manufacturing wastewater178. THF is a cyclic ether used as a solvent and raw material for synthesizing polymers in the industry. The wastewater has an initial THF concentration of 688 ± 140 mg L–1. THF was treated by EO by using four anodic materials, namely, BDD, RuO2, PbO2, and Pt. The CD applied was 300–1200 A m–2. The experiment resulted in a fast THF removal when BDD anode was used using sodium sulfate as an electrolyte to prevent the formation of organochloride secondary products. Also, COD removal was higher than 98% and TOC elimination higher than 95%. However, the mineralization of organic pollutants was not achieved by using RuO2 as an anode and sodium chloride as an electrolyte. The reason why sodium chloride was chosen as an electrolyte was that DSA, such as RuO2 generates chlorine when NaCl is the electrolyte. The reduced efficiency of Ti/RuO2 in NaCl electrolyte showed that the free chlorine generated is not an active oxidant for THF removal and mineralization179.
EO has also been used to remove COD from vegetable tannery wastewater by using a graphite anode180. The initial COD value was 9600 mg L−1, and the final value obtained was 59 mg L−1 at a CD of 34 mA cm–2 and 120 min of electrolysis. In the case of chrome tanning wastewater, Cr3+ was oxidized to Cr6+ with a conversion of 96 ± 3% at a pH of 2.5–5.5. Cr6+ was then converted to Cr2(SO4)3 for reuse in tanning operation180. EO treatment of tannery wastewater has also been carried out by using Ti/Pt and Ti/Pt/Ir anodes181. It was found that COD removal was not enough to meet the discharge regulations. Hence, the EO process via these electrodes cannot be used alone in the treatment of tannery wastewater181. Moreover, EO has been utilized to extract organic pollutants from textile and finishing wastewater182. The wastewater produced from the textile industry is challenging since it has a high pH, high temperature, intense color, high COD, and low degradability. Usually, dyeing wastewater can be treated by biological oxidation and adsorption. The effect of EO on finishing wastewater and textile dye was investigated using a stainless-steel cathode and titanium or platinum anode. The organic pollutants present in the wastewater were oxidized to water and carbon dioxide when passing through the cell. This is because of the high oxidizing ability of the chemicals generated in the cell, which include hydroxyl, chlorine, oxygen, and other oxidants. In total, 2 mL of 36% hydrochloric acid was added, and electrolysis was allowed to take place for 18 min at a current of 0.89 A cm−2. The COD/BOD ratio decreased from 2.16 to 1.52, indicating that the biodegradability of wastewater was improved. Additionally, COD removal was found to be 86%, BOD removal was 71%, and ADMI color units were reduced completely182.
One comparative study that investigated energy consumption of EC and photocatalytic process for textile dye wastewater treatment has concluded that EC was more economically feasible as the energy consumption of EC process was less than 0.01 kWh m−3 per unit COD removed when compared to >100 kWh m−3 for the photocatalytic process183. Photocatalytic ozonation is considered to be one of the least energy-demanding amongst AOPs technologies. It was reported that the specific energy consumption of the photocatalytic ozonation system in the decomposition of oxalic acid and dichloroacetic acid was 0.007 kWh mM–1 and 0.024 kWh mM–1, respectively. These values were less than those of catalytic ozonation (0.017 kWh/mM and 0.050 kWh mM–1) and photocatalytic oxidation (0.063 kWh mM–1 and 0.350 kWh mM–1)184.
EF technique is an electrokinetic phenomenon that has been used for the treatment of various types of industrial wastewater treatment185,186,187,188. It involves the flotation by electrically generated bubbles to separate two liquid phases or solid from liquid phases. Colloidal or finely dispersed particles in wastewater are removed by the small bubbles of O2 and H2 lifting contaminants to clarify the solution. The best recoveries could be obtained in the particle size range of 20–50 µm. The advantages of EF include simplicity, efficiency, environmental compatibility, safety, selectivity, reduction in sludge generation, minimization of added chemicals, and relatively little space requirement due to its shorter residence time189. The efficiency of EF in treating wastewater rich in heavy metals was studied by preparing a solution of NiSO4.6H2O, CuSO4.5H2O, PbSO4, ZnSO4.7H2O, CdSO4.8H2O, and FeSO4.7H2O at an initial concentration of 100 mg L–1 for each salt, initial pH of 8, and conductivity of 2.7 mS cm−1 in the presence of sulfate189. EF showed an average heavy metal removal efficiency of 93%, and the kinetics was found to be fast (around 15 min) except for Ni. Another study investigated the feasibility of EC/EF process for heavy metals removal such as Pb, Ba, and Zn190. Up to 97% removal was achieved with stainless steel mesh electrodes. EC/EF has also been used to remove F− and CaF2 nanoparticles from semiconductor industry wastewater191. Suspended matter and F− were eliminated by the combined methods. Additionally, the high turbidity removal efficiency was achieved by EF technique. Stainless steel was the cathode, and DSA titanium coated with RuO2 (Ti/RuO2) was the anode of the EF cell. The turbidity and F− removals were 97% and 73%, respectively.
EF is the most effective method for the separation of low-density SS and oil185,187,188. Up to 99.71% oil removal from an initial concentration of 1050 mg L–1 emulsified oil has been achieved by using an EF cell with DSA anode composed of Ti/Ru0.34Ti0.66O2 and a stainless-steel cathode187. EF has also been used to treat wastewater from the dairy industry192, palm oil effluent using Pb dioxide-coated titanium anode193, mining wastewater194, and others. Moreover, EF can be a part of a hybrid process. One example of a hybrid EF process is composed of three stages: (i) adsorption by a bonding agent; (ii) wastewater filtration to separate the loaded bonding agent by two variants, i.e. crossflow MF for low-contaminated wastewater or a hybrid process combining flotation and submerged MF for highly contaminated wastewater; and (iii) bonding agent regeneration195.
Photodegradation by nano-scale TiO2
Nano-TiO2 can be utilized to photocatalytically degrade the residual organic contaminants in treated effluents. Nano-TiO2 is useful for the degradation of endocrine disruptors, organic contaminants, micropollutants, and in water filtration membranes. Nano-TiO2 structure and performance would depend on the preparation method. However, certain limitations such as inefficiency under visible light illumination, post-recovery, incomplete removal of toxic byproducts (in some cases), and low mechanical strength still need to be addressed for enhanced performance. TiO2 post-treatment of secondary treated effluents from industrial wastewater treatment plants is a possible solution for the extraction of toxic organics. Nanocrystals of TiO2 possess a high surface-area-to-volume ratio, making them suitable for photocatalysis and adsorption196,197. This means that nano-TiO2 has a higher number of delocalized carriers on its surface, which ensures better-charged transport and efficient generation and separation of photo-generated electrons and holes. Photo-generated holes in TiO2 nanocrystals are powerful oxidants198. Nanocrystals exhibit these properties because of their low dimensionality and quantum size effects199. TiO2 is an n-type semiconductor with a relatively wide bandgap, and has three crystalline phases: rutile (tetragonal), brookite (orthorhombic), and anatase (tetragonal). TiO2 has become the most popular photocatalyst at the nanoscale, and a lot of energy can be saved with photocatalysis without secondary pollution through process control. The types of TiO2 nanostructures include: nanoparticles, nanotubes, nanorods, nanofibers, nanoflowers, and nanowires, in accordance with the preparation method and desired characteristics200. Nano-TiO2 can be prepared through sol-gel201, hydrothermal202, solvothermal203, anodic oxidation204, hard template205, and reverse microemulsion206 methods.
Nano-TiO2 prepared from a sol-gel method has been used for phenol degradation207. Phenols possess endocrine-disrupting properties. Zeng et al. synthesized the nano-TiO2 via the sol-gel process208. Titanium n-butoxide was dissolved in anhydrous ethanol to obtain the solution A. DI water, glacial CH3COOH, and C2H6O were mixed to obtain solution B. Solution A was mixed with solution B to get the sol. The sol was aged for 72 h and then dried at 100 °C and annealed. Regular sizes of anatase nano-TiO2 were obtained. The TiO2 was doped with B, Ni, and Ce for increased phenol degradation under visible light illumination. The best performance was attributed to BNiCeTiO2. In the work of Liu et al.209, porous TiO2 hollow aggregates were synthesized through the hydrothermal method for the photocatalytic degradation of Rhodamine B. NH4F and Ti(SO4)2 were dissolved in DI water, and then the mixture was added to a Teflon-lined autoclave. Hydrothermal synthesis was carried out at 160 °C for 6 h. It was observed that the obtained photocatalyst were more effective than the commercially available photocatalyst P25 for Rhodamine B degradation.
Nano-TiO2 has also been used for chloroform decomposition. In the work of Kang et al. (2001), C12H28O4Ti was dispersed in 1,4-butanediol under 300 °C for 50 min210. The anatase powder was efficient in chloroform degradation. In chloroform decomposition under the UV-light (254 nm, 24 W m–2) with O2 bubbling (500 mL min–1), more than 95% of the chloroform was removed. Nano-TiO2 has been employed for ethylene decomposition. In the work of Praserthdam et al., titanium n-butoxide was added to toluene211. The mixture was autoclaved at 300 °C for 2 h. Spherical shaped particles obtained promoted ethylene decomposition. A high amount of Ti3+ surface defect with Ti3+/OH was found in the TiO2 sample that was quenched in the air at 77K. The sample also exhibited the highest photocatalytic activity for ethylene decomposition. However, despite its versatility for photodegradation of trace pollutants in water, the use of nano-TiO2 still faces some limitations212. Currently, the exploitation of readily available visible light for photodegradation via nano-TiO2 is still inefficient for large-scale treatment, as most studies on large-scale TiO2 photocatalysis have focused on the use of UV light213. Secondly, there is a low adsorption capacity of nano-TiO2 for hydrophobic contaminants because TiO2 is hydrophilic214. Therefore, the efficiency of removing hydrophobic contaminants through nano-TiO2 structures is low. Thirdly, there is inadequate post-recovery of TiO2 particles after treatment in water. The process of regenerating the particles after dispersion in water might be tedious and costly. Fourthly, there might be the production of toxic byproducts after the degradation of the primary contaminants by nano-TiO2215. Although these byproducts might subsequently be removed by further photo-degradation, they remain in trace amounts in the final effluents in many cases. Lastly, many nano-TiO2 structures lack mechanical strength or stabilization for long-time utilization in production plants. These structures would become fractured or destabilized under continuous feed system after some time. In case of polymer membranes, there is a danger of destruction of the membrane structure by UV light or OH• and problems of high cost216.
More recent progress in applications of TiO2 and other photocatalysts is captured in the reviews of Giwa and co-authors217,218,219. This progress is mainly in the aspects of artificial neural network (ANN) modeling, plasma activation, functionalization with quantum dots, and use of nanoreactors. ANN modeling has been used to predict the discoloration of maxilon blue 5G dye by catalysts including TiO2, ZnO, and TiO2–ZnO integrated with Fe220. A Multilayer Perceptron neural model consisting of backpropagation algorithm was employed to assess the influence of operating conditions on the discoloration efficiency. An ANN model has also been employed for the prediction of the efficiency of a photocatalyst consisting of TiO2 and Ag/S for 2-nitrophenol degradation221. The degradation efficiency of 2-nitrophenol was considered as the output variable in this model. The ability of TiO2 nanoparticles to oxidize phenol in a system integrated with photo-electro-Fenton process has been predicted via another ANN model222. This model was coupled with genetic algorithm and optimum phenol removal efficiency was predicted by varying operating conditions such as phenol concentration and pH. Photocatalytic disinfection of water has been predicted using ANN modeling. Two back-propagation neural networks were employed by Lin et al. to assess the efficiency of TiO2 coupled with UV for coliform removal from wastewater223. Input variables including the intensity of UV, coliform counts, color and turbidity, temperature and pH were included in the data that was used to train and evaluate the model. The model was also validated experimentally.
On the aspect of plasma activation, photocatalysts can be combined with plasmonic metals including gold and silver to enhance the transfer of incident photon energy to the photocatalysts and improve their ability to degrade organic pollutants under visible light conditions. Ag-AgI plasmonic photocatalyst has been synthesized by Hu et al. and used to degrade chlorophenolic compounds in water224. The plasmonic photocatalyst exhibited better efficiency than undoped TiO2 P25 nanoparticles under visible (or simulated solar) light conditions. An azo dye, Reactive Brilliant Red, has also been removed from water by Jie et al. using plasmonic Ag/AgCl/polydopamine-TiO2 photocatalysts225. The ability of the synthesized plasmonic photocatalyst to degrade Reactive Brilliant Red in water was four times higher than that of pure TiO2 nanofibers.
On the aspect of functionalization of nanostructured photocatalysts with quantum dots, nanostructured TiO2 has been functionalized with Ag2S quantum dots to improve the photocatalytic efficiency of nanostructured TiO2. Quantum dots exhibit the potential to improve the active sites on a catalyst surface, due to their small sizes, and tuning of the energy bandgap to the region required for visible-light photocatalysis. Visible light is abundant in form of natural solar light in the environment, so visible light-driven photocatalysis may be cheaper than UV or near-infra red-driven photocatalysis. The removal of methyl orange pollutant from water by TiO2/Ag2S quantum dots was reported to be 3.5 times higher than that of undoped TiO2 nanobelts under visible light irradiation226. Other ways to improve the performance of photocatalysts under visible light conditions are well-documented in the work of Giwa et al. [268]. These ways involve the use of visible light-responsive nanocubes including reduced graphene oxide/mesoporous copper ferrite aerogel, porous Fe2O3 nanocube-impregnated graphene aerogel, Au/Au/Ag nanocubes/polyvinylchloride substrate, and 3D/2D In2O3 nanocube/ZnIn2S4 nanosheet. These nanocubes are preferred due to their high adsorption ability, which influences their effective application under the visible range of the solar spectrum.
It is often problematic to use photocatalysts to remove organic pollutant in saline water due to the inference of salts on the photocatalytic process. An emerging technique, which has been tested for degradation of pollutants in seawater, is the use of nanoreactors. In nanoreactors, photocatalysts are synthesized by the adsorbed-layer nanoreactor synthesis (ANS). ANS improves photocatalysis by selective adsorption. ANS and solvothermal synthesis have been employed by Wang et al. to synthesize La3+ or Yb3+- doped TiO2 for the removal of phenol from simulated saline water227. Photocatalyst crystal lattice distortion and oxygen vacancies required for enhanced photocatalysis efficiency were impacted by ANS. About 90% removal of phenol was achieved. This high removal efficiency was as a result of enhanced adsorption, leading to a reduction in the adverse effect of the salt ions in the saline water. Acid fuchsine, a printing dye, has also been removed using zero-valent iron nanoparticles on polyacrylic acid/polyvinyl alcohol fiber mat228. The mat was employed as the nanoreactor. The synthesized nanoreactor showed superior activity when used to decolorize dye wastewater.
Hybrid systems
Hybrid systems consist of two or more treatment methods used together to provide better energy and treatment efficiencies, and/or to overcome the challenges associated with using stand-alone technology. The subsequent sections highlight the recent developments of such hybrid systems for wastewater treatment.
Adsorption on AC/MBR
The hybrid AC/MBR system integrates biological activity, membrane separation, and adsorption on PAC or minerals for the removal of pollutants. An application of this process is the treatment of sugarcane molasses-based distillery wastewater.
The vermiculite/MBR process resulted in a combined attached and suspended growth system since biomass growth occurred both in the mixed liquor and on the surface of vermiculite. Furthermore, it resulted in the removal of 88% Cu, 85% Zn, and 60% Ni for influent metal concentrations in the range of 3-15 mg L−1, which were periodically spiked to municipal wastewater. Also, the addition of vermiculite in the bioreactor mitigated the inhibition of autotrophic and heterotrophic biomass229. Another process that utilized MBR, specifically zeolite/AnMBR process, was tested for the treatment of dyeing wastewater. The zeolite addition gave a reduction in the membrane filtration resistance and improved the decolorizing rate of the system230.
Combined filtration – adsorption
The integrated adsorption–filtration process has been successfully applied to remove heavy metals from industrial wastewater. The combined mineral-UF system for industrial pre-wastewater treatment at pH = 8 resulted in effective removal of Pb, Cu, Ni and Zn using vermiculite, bentonite, and zeolite as minerals21. Another combined adsorption-UF process was also tested for the treatment of textile wastewater. The examined process produced an effluent with a low metal concentration that can safely and efficiently be cleared into municipal sewers. The COD removal was between 76% to 92%, and color removal ranging between 45% and 70%21. This process was also applied to treat several different industrial wastewater streams from the chemical, metal plating, and textile industries. The performance of this combined process in terms of heavy metal removal varied according to the wastewater characteristics, and the type of sorbent used [24]. This adsorption-UF process was also tested for the removal of heavy metals from electroplating wastewater, which resulted in more 97% heavy metal removal at a pH greater than 8 due to combined adsorption and UF.
Combined EC-EF treatment
EC is an efficient method for the removal of suspended and colloidal particles, but ineffective to remove persistent organic compounds. On the other hand, EO is very effective in breaking down organic compounds by oxidation, but it necessitates more time than EC (30 min vs 21 h). As a result, the applicability of EO is limited. Hence, a synergistic combination of the two technologies can be used to achieve the removal of persistent organic compounds at a reasonable period. The electrooxidative mineralization of electrocoagulated wastewater reduces the treatment time134. In the hybrid technology, EC removes colloids, TSS, and charged species while EO oxidizes the remaining persistent organics. In the case of EC treatment alone, there was a drastic drop in the COD for the initial 30 min of treatment, in which a restrictive value was reached and no additional increase in efficiency was observed134. When EO was applied alone, the sample was totally mineralized after 21 h of treatment. About 3 hours were needed to reduce the COD to about half with EO. In the combined system, the initial feed of wastewater for EO was electrocoagulated wastewater (COD = 425 mg L−1 and pH 8). At a pH of 8, almost a complete removal of COD was achieved after 70 min of treatment. Also, the fastest rate for total mineralization by EO was found to occur at pH 8134.
Photocatalytic membrane reactors
Photocatalytic membranes (PMRs) are hybrid reactors in which photocatalysis is coupled with membrane process; the catalyst can be immobilized on a membrane (i.e., photocatalytic membrane) or suspended in the mixture216. Recently, nano-TiO2 has been shown to be effective for the photodegradation of contaminants in the PMRs using entrapped or suspended catalyst216. The schematic of a PMR is shown in Fig. 4.
An illustration of lab-scale PMR system operating via recirculating batch mode (inspired from309).
PMRs have been employed to remove various pollutants present in industrial wastewater, such as pharmaceuticals121, dyes231, textile and wood processing232, organics such as phenols231,233, and others234,235. The main advantages of PMRs with TiO2 immobilized on the membrane are the reduction in fouling due to the presence of TiO2, and the ability to use the membrane without recycling and regenerating the catalyst. On the other hand, some disadvantages include a lower degradation efficiency when compared to suspended TiO2 processes, a necessity to change the membrane when the catalyst’s activity is lowered, a risk of membrane damage by UV light or OH•, and inability to change the catalyst’s loading due to the fixed amount of immobilized catalyst on the membrane216. A pilot-scale PMR employing UV/TiO2 photocatalysis has been applied recently to remove some of the pollutants that can be present in industrial effluents121. The removal of 32 different pharmaceuticals, endocrine disrupting compounds, and estrogenic activity was evaluated. The degradation of all compounds followed pseudo-first-order kinetics. More than 70% of 29 compounds were removed while 50% of 3 compounds were removed121.
Most of the applications that combine photocatalysis with pressure-driven membrane processes employ MF or UF216. The application of a hybrid RO/photocatalysis system is limited since RO should not be used when the feed contains SS. Considering the above, more studies should be conducted on the possible applications of the RO/photocatalysis system. Some studies should have been carried out to find better catalysts that can enhance the catalytic effect236,237,238. Future research should be directed towards the modifications of the substrate to lower the bandgap required to activate the whole generation sites in PMRs.
Recently, a “fungal membrane bioreactor” (FMBR) using Phanerochaete chrysosporium was integrated with PMR for the treatment of textile industry wastewater239. It was observed that COD abatement and color removals were 53% and 58% for photocatalytic degradation, respectively, while the values were 56% and 60% for fungal biodegradation. The hybrid process achieved total removal efficiencies of 93% and 99% for color and COD, respectively. These results suggest that using photocatalysis as a post-treatment technique to the fungal biodegradation process is more effective than applying photocatalysis as a pre-treatment technique for the advanced treatment in the textile industry wastewater.
Sonication or hydrodynamic cavitation-assisted AOP
Hot spots, highly reactive free radicals, and liquid-circulation associated turbulence, can be used for the intensification of various physical/chemical operations240. Hydrodynamic cavitation ensures bubble dynamics and creates optimum operating parameters in reactor configurations. The use of this method may reduce chemical consumption, but it may be costlier. It has been shown that it is an energy-efficient method when compared with ultrasonication and high-speed homogenization241. Recently, the oxidants obtained from sonication have provided positive feedback242. Although the chemical process gave higher removal efficiency, the chemical-free sonication process is more environmentally friendly. For example, the use of Fe2+ increased the degradation of 1,4-dioxane by 98.1%, but 79% removal efficiency was obtained when sonication was employed for the removal of 1,4-dioxane from water242.
The integration of photocatalysis and sonication has been used to achieve high removal efficiency of dye. The behavior of the hybrid method can be explained by two main reasons: (1) production of OH• from H2O2 by photocatalyst, and (2) improvement of mass transfer between the liquid and the surface of catalyst as a result of sonolysis243.
Hydrodynamic cavitation has been used together with OH• for the degradation of Escherischia coli (E. Coli)244. A logarithmic equation based on the survived cells was used to calculate the dying-off rate of the E. Coli. It was observed that the OH• breaks the carbon bonds in the cell, therefore breaking its DNA chain and resulting in its eventual death. The disinfection property of H2O2 also decreased protein synthesis in the cell. It was observed that the intensity and efficiency of the process are highly dependent on the cavitation field, energy consumed, gas content, and the initial concentration of the microbes. This positive effect of hydrodynamic cavitation on E. Coli was also confirmed by245, where it was shown that the concerned bacteria stopped dividing after only 3 min of treatment. However, further analysis showed that these bacteria did not actually die, but they were in an inactive state.
Hydrodynamic cavitation has been used to degrade volatile organic compounds such as ethylbenzene, toluene, benzene, and xylenes246. The highest degradation was achieved by toluene, i.e. 21% in 240 min. The conversion rate of the pollutants was influenced by an increase in the diameter of the hydrodynamic pipe. Sonication has also been used to remove polyaromatic hydrocarbon from industrial wastewater247. Low and high-molecular-weight pollutants were degraded. Meanwhile, it was observed that a rise in temperature played a significant role in improving the removal efficiency. In addition, hydrodynamic cavitation has been used recently to degrade carbamazepine248. 96% degradation was achieved in only 15 min by using hydrodynamic acoustic cavitation, as compared to 27% degradation when only hydrodynamic cavitation was employed249. A batch mode sonication was implemented on olive mill factory wastewater by250. The main observations in this study were as follows: (1) the increase in the initial temperature affects the degradation rates, and (2) COD was not entirely removed due to an insufficient amount of formed OH•. The study also emphasized the economic impact of using sonication for the treatment of olive mill factory wastewater. The total cost of sonication was estimated to be 665 Euros m−3 yr−1. The nature and extent of pollutants removed by sonication and hydrodynamic cavitation-assisted AOPs are shown in Table 7.
Electrically-enhanced membrane bioreactor (eMBR)
Reduction of membrane fouling and enhancing effluent’s quality is possible with using an electrically-enhanced membrane bioreactor (eMBR), a wastewater treatment technology that combines electrochemical processes, biodegradation, and membrane filtration in one system251. A schematic is shown in Fig. 5. The electrochemical treatment works on increasing pollutants removal efficiency and controlling the mobility of foulants and their deposition on the membrane surface via electrochemical mechanisms, such as electrophoresis, electrocoagulation, and electroosmosis. Various metal species in the system are released due to electric dissociation, which can contribute to the aggregation and destabilization of collides and suspended solids through the coagulation process136. The metal species characteristics depend on the pH of the media. Moreover, extracellular polymeric substances and extracellular polymeric substances can be transported to the oppositely charged electrodes, away from the membrane, via electrophoretic motion198. This helps in reducing membrane fouling and the formation of biofilms.
eMBR has been employed to remove organics (COD), nutrients, color, and turbidity252,253. Most of the conducted studies were performed at a lab-scale. Also, most of the experimental studies were limited to low-to-medium strength wastewater252.
The concept of circular economy in metal recovery
Circular economy is defined as a transition in which the generation of waste is minimized while resources, material, and products are maintained in the economy for extended period and utilized as much as possible254. The concept of circular economy has come into place in response to the drawbacks of the conventional ‘take-make-consume-and dispose’ model. The idea of a circular economy can be applied to wastewater treatment by metal recovery via electroplating, galvanizing, and anodizing and its utilization in various applications. For instance, aluminum ores contain ~30% alumina whereas wastewater sludge may contain up to 10–15%255. Conventional metal recovery methods include physical (e.g., ion exchange by ED, membrane filtration, etc.), chemical (e.g., precipitation, electrochemical methods, etc.), and biological (e.g., biosorption, bioremediation, etc.) methods. However, these methods are energy and chemical-intensive. Biological processes, for instance, have been used intensively for heavy metals extraction. Most heavy metals are easily adsorbed onto membrane lips and proteins such as phospholipids, peptidoglycan, lipopolysaccharides, teichoic acids, and teichuronic, in addition to anionic functional groups that are present in EPS. Most of the biovolume fraction is unoccupied since it is a surface process, which limits the potential of heavy metal immobilization. Nevertheless, by some kind of chemical transformation inside the cells, fungi, and bacteria can actively bioaccumulate heavy metals. Furthermore, the biological recovery of radionuclides is primarily driven by biosorption. Uranium has been previously immobilized by using organisms such as Rhodotorula glutinis256. Previous comprehensive review papers have addressed metal extraction using biological methods256. One of the challenges of using biological methods for metals extraction is the relatively low concentration of metals in wastewater effluents, which necessitates a step of pre-concentration of metals by nanofiltration, electrodialysis or reverse osmosis for instance. These technologies are costly especially at large scale treatment, which is an issue that future studies should address. Membrane filtration, for example, includes MF, UF, NF, and RO, which physically retain metals while allowing water to pass through. Chemical sorbents can also be used to modify membranes to increase the removal selectively of metal ions. Polycysteine functionalized MF membranes were efficient in removing Hg and Cd. However, the main disadvantages associated with using membrane technology for heavy metal recovery are membrane fouling, high energy consumption, and the high operational cost. On the other hand, ion exchange by ED requires solid resin to exchange the metals in wastewater with other cations such as H+. The solid resins are not selective; however, they can preferentially bind ions. Additionally, although ion exchange has fast kinetics and high removal efficiency, it is not suitable for high concentrations of metal ions due to resins saturation. Another technique that is widely used for metals recovery is chemical precipitation, due to its simple operation and low capital cost. However, the challenge associated with chemical precipitation is toxic sludge generation, which requires additional processing for disposal257. The recent development of bioelectrochemical technologies offers a platform for both oxidation and reduction related reactions, which provides an alternative approach for efficient metal recovery. These technologies employ microorganisms to produce chemical and electric current by utilizing the chemical energy stored in biodegradable materials. Many recent studies have discussed using bioelectrochemical technology in metal recovery from wastewater257.
Concluding remarks and future perspectives
A comprehensive review of various technologies for industrial wastewater treatment has been carried out, with a particular focus on effluents with high heavy metal content. The development of technologies for industrial wastewater treatment that can remove toxic pollutants is pivotal for meeting the growing water demands and for providing water security. As can be seen from this review, the focus of most research is to develop membranes and nanomaterials for water treatment, to optimize operating conditions of existing technologies, to investigate the potential use of low-cost adsorbents, and to develop hybrid technologies for water recycle and reuse. Recent breakthroughs in membrane technologies have emerged as significant innovations for the treatment and reclamation of industrial wastewater. Membrane bioreactors and low-pressure membranes have been used for wastewater treatment; however, the biggest challenge with these technologies is their high potential of fouling, which leads to reduced efficiency and shortened membrane life. Other technical problems include the complexity and cost of residuals disposal, especially when using membrane technologies. Adsorption is another method that is recently preferred for the removal of low concentrations and non-degradable organic compounds. It has advantages over the other methods due to its simple design and low initial costs. Recently, researchers have been investigating low-cost adsorbents such as natural materials, agricultural and industrial wastes, and others. The physio-chemical treatment processes are effective and quick; however, the high chemical and operational costs associated with them as well as sludge generation limit their applications. It can also be concluded that the treatment process is strongly dependent on the characteristics of wastewater. Also, although many techniques can be employed for industrial effluent treatment, each technology has its drawbacks. Thus, the selection of the most suitable treatment method depends on some parameters such as pH, initial pollutants concentration, the types of targeted pollutants, potential environmental impacts, as well as the process economic feasibility.
Membrane-based processes offer potential performance benefits, especially when coupled with electrochemical advanced oxidation procedures such as photoelectron-catalysis, electro-Fenton and electro-catalysis, and other processes. However, membrane fouling remains a big challenge. Also, utilizing membrane biofilms for the transformation of emerging chemicals should be explored. In doing so, the microbial strains have to be carefully selected, and operating conditions have to be precisely optimized such that the growth of biofilm on the membranes does not cause an increase in trans-membrane pressure. Future studies should aim to develop genetically engineered cultures to improve microbial strains to exhibit desirable characteristics that help reduce membrane fouling. Moreover, more research should focus on developing anti-fouling membranes specifically for MBRs as there is only limited work on this aspect. Efficient membrane cleaning techniques should be developed, incorporating recent advances in biochemical- and sonication-enabled cleaning strategies. Integrating self-cleaning membranes should also be investigated. Adsorption systems should be fully integrated with the existing treatment technologies such that synergies could be tapped on in ensuring the treated water quality standards. Another area to focus on is the green regeneration techniques to increase the recyclability of adsorbents. Similarly, the selectivity of the adsorbents may be further fine-tuned to capture emerging contaminants, which are otherwise leaked through the system untreated. Similarly, in the case of advanced oxidation processes, hydrogen peroxide dosage should be optimized such that excess of them do not scavenge the free radicals in the system and reduce the overall efficiency of the pollutant degradation. The toxicity of the by-products must also be considered. The potential of coupling should be investigated to tackle the growing concerns of emerging pollutants and toxic by-products. This requires additional studies for finding the best couplings and optimizing operating conditions to maximize the capabilities of these advanced treatment technologies. Research should also focus on integrating these processes within the existing treatments plants infra-structure with minimum disruption to the plant operations.
Data availability
The authors declare that the data supporting the findings of this study are available within the manuscript. Further data can be requested (if need be) by contacting the corresponding author.
References
Water, U. N. Water and Jobs. The United Nations World Water Development Report 2016 https://www.unwater.org/publications/world-water-development-report-2016/ (2016).
Mutamim, N. S. et al. Membrane bioreactor: Applications and limitations in treating high strength industrial wastewater. Chem. Eng. J. 225, 109–119 (2013).
Lin, H. et al. Membrane bioreactors for industrial wastewater treatment: a critical review. Crit. Rev. Environ. Sci. Technol. 42, 677–740 (2012).
Hai, F. I., Yamamoto, K. & Lee, C.-H. Membrane Biological Reactors Theory, Modeling, Design, Management and Applications to Wastewater Reuse. (IWA Publishing, 2013).
Smythe, G., Matelli, G., Bradford, M. & Rocha, C. Biological treatment of salty wastewater. Environ. Prog. 16, 179–183 (1997).
UN. Wastewater management a UN-water analytical brief Analytical Brief. UN Water (2014).
UNESCO. Water in a changing world. The United Nations World Water Development Report 3. United Nations Educational, scientific and Cultural Organization (2009).
Ferella, F., De Michelis, I., Zerbini, C. & Vegliò, F. Advanced treatment of industrial wastewater by membrane filtration and ozonization. Desalination 313, 1–11 (2013).
Eccles, H. Treatment of metal-contaminated wastes: Why select a biological process? Trends Biotechnol. 17, 462–465 (1999). vol.
Barakat, M. A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 4, 361–377 (2011).
Zhao, M., Xu, Y., Zhang, C., Rong, H. & Zeng, G. New trends in removing heavy metals from wastewater. Appl. Microbiol. Biotechnol. 100, 6509–6518 (2016).
Adib, H., Hassanajili, S., Sheikhi-Kouhsar, M. R., Salahi, A. & Mohammadi, T. Experimental and computational investigation of polyacrylonitrile ultrafiltration membrane for industrial oily wastewater treatment. Korean J. Chem. Eng. 32, 159–167 (2014).
Deowan, S. A. et al. Novel low-fouling membrane bioreactor (MBR) for industrial wastewater treatment. J. Memb. Sci. 510, 524–532 (2016).
Qdais, H. A. & Moussa, H. Removal of heavy metals from wastewater by membrane processes: a comparative study. Desalination 164, 105–110 (2004).
Mohsen-Nia, M., Montazeri, P. & Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 217, 276–281 (2007).
Galambos, I., Mora Molina, J., Járay, P., Vatai, G. & Bekássy-Molnár, E. High organic content industrial wastewater treatment by membrane filtration. Desalination 162, 117–120 (2004).
Barakat, M. A. & Schmidt, E. Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination 256, 90–93 (2010).
Juang, R. S. & Shiau, R. C. Metal removal from aqueous solutions using chitosan-enhanced membrane filtration. J. Memb. Sci. 165, 159–167 (2000).
Trivunac, K. & Stevanovic, S. Removal of heavy metal ions from water by complexation-assisted ultrafiltration. Chemosphere 64, 486–491 (2006).
Aroua, M. K., Zuki, F. M. & Sulaiman, N. M. Removal of chromium ions from aqueous solutions by polymer-enhanced ultrafiltration. J. Hazard. Mater. 147, 752–758 (2007).
Katsou, E., Malamis, S. & Haralambous, K. J. Industrial wastewater pre-treatment for heavy metal reduction by employing a sorbent-assisted ultrafiltration system. Chemosphere 82, 557–564 (2011).
Malamis, S., Katsou, E., Kosanovic, T. & Haralambous, K. J. Combined adsorption and ultrafiltration processes employed for the removal of pollutants from metal plating wastewater. Sep. Sci. Technol. 47, 983–996 (2012).
Malamis, S., Katsou, E., Stylianou, M., Haralambous, K. J. & Loizidou, M. Copper removal from sludge permeate with ultrafiltration membranes using zeolite, bentonite and vermiculite as adsorbents. Water Sci. Technol. 61, 581–589 (2010). vol.
Katsou, E., Malamis, S., Haralambous, K. J. & Loizidou, M. Use of ultrafiltration membranes and aluminosilicate minerals for nickel removal from industrial wastewater. J. Memb. Sci. 360, 234–249 (2010).
Afonso, M. D. & Bórquez, R. Review of the treatment of seafood processing wastewaters and recovery of proteins therein by membrane separation processes — prospects of the ultrafiltration of wastewaters from the fish meal industry. Desalination 142, 29–45 (2002).
Turano, E., Curcio, S., De Paola, M. G., Calabrò, V. & Iorio, G. An integrated centrifugation–ultrafiltration system in the treatment of olive mill wastewater. J. Memb. Sci. 209, 519–531 (2002).
Lester, N. Heavy Metals in Wastewater and Sludge Treatment Processes. vol. 2 (CRC Press, 1987).
Santos, A. et al. Fate and behaviour of copper and zinc in secondary biological wastewater treatment processes: II Removal at varying sludge age. Environ. Technol. 31, 725–743 (2010).
Clara, M., Kreuzinger, N., Strenn, B., Gans, O. & Kroiss, H. The solids retention time—a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res 39, 97–106 (2005).
Noble, J. GE ZeeWeed MBR technology for pharmaceutical wastewater treatment. Membr. Technol. 2006, 7–9 (2006).
Scholz, W. & Fuchs, W. Treatment of oil contaminated wastewater in a membrane bioreactor. Water Res 34, 3621–3629 (2000).
Kim, B. R. et al. Design and startup of a membrane-biological-reactor system at a Ford-engine plant for treating oily wastewater. Water Env. Res 78, 362–371 (2006).
Di Fabio, S. et al. Are centralized MBRs coping with the current transition of large petrochemical areas? A pilot study in Porto-Marghera (Venice). Chem. Eng. J. 214, 68–77 (2013).
Battistoni, P., Cola, E., Fatone, F., Bolzonella, D. & Eusebi, A. L. Micropollutants removal and operating strategies in ultrafiltration membrane systems for municipal wastewater treatment: Preliminary results. Ind. Eng. Chem. Res. 46, 6716–6723 (2007).
Nelson, P. O., Chung, A. K. & Hudson, M. C. Factors affecting the fate of heavy metals in the activated sludge process. J. Water Pollut. Control Fed. 53, 1323–1333 (1981).
Fatone, F., Eusebi, A. L., Pavan, P. & Battistoni, P. Exploring the potential of membrane bioreactors to enhance metals removal from wastewater: Pilot experiences. Water Sci. Technol. 57, 505–511 (2008). vol.
Robles, A., Ruano, M. V., Ribes, J. & Ferrer, J. Sub-critical long-term operation of industrial scale hollow-fibre membranes in a submerged anaerobic MBR (HF-SAnMBR) system. Sep. Purif. Technol. 100, 88–96 (2012).
Tazi-Pain, A., Schrotter, J. C., Bord, G., Payreaudeau, M. & Buisson, H. Recent improvement of the BIOSEP® process for industrial and municipal wastewater treatment. Desalination 146, 439–443 (2002).
Tao, Y., Gao, D. W., Fu, Y., Wu, W. M. & Ren, N. Q. Impact of reactor configuration on anammox process start-up: MBR versus SBR. Bioresour. Technol. 104, 73–80 (2012).
Kanai, M., Ferre, V., Wakahara, S., Yamamoto, T. & Moro, M. A novel combination of methane fermentation and MBR — Kubota Submerged Anaerobic Membrane Bioreactor process. Desalination 250, 964–967 (2010).
Saravanane, R. & Sundararaman, S. Effect of loading rate and HRT on the removal of cephalosporin and their intermediates during the operation of a membrane bioreactor treating pharmaceutical wastewater. Water Sci. Technol. 61, 1907–1914 (2010).
Qu, Y. Y. et al. Population dynamics in bioaugmented membrane bioreactor for treatment of bromoamine acid wastewater. Bioresour. Technol. 100, 244–248 (2009).
Shrout, J. D. & Nerenberg, R. Monitoring bacterial twitter: does quorum sensing determine the behavior of water and wastewater treatment biofilms? Env. Sci. Technol. 46, 1995–2005 (2012).
Ergön-Can, T., Köse-Mutlu, B., Koyuncu, İ. & Lee, C.-H. Biofouling control based on bacterial quorum quenching with a new application: Rotary microbial carrier frame. J. Memb. Sci. 525, 116–124 (2017).
Davies, D. G. et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Sci. (80-.) 280, 295–298 (1998).
Stuckey, D. C. Recent developments in anaerobic membrane reactors. Bioresour. Technol. 122, 137–148 (2012).
Ren, T., Li, X. & Yu, H. Effect of N-acy-l-homoserine lactones-like molecules from aerobic granules on biofilm formation by Escherichia coli K12. Bioresour. Technol. 129, 655–658 (2013).
Lade, H., Paul, D. & Kweon, J. H. Quorum quenching mediated approaches for control of membrane biofouling. Int J. Biol. Sci. 10, 550–565 (2014).
Zhang, W. & Li, C. Exploiting quorum sensing interfering strategies in gram-negative bacteria for the enhancement of environmental applications. Front. Microbiol. 6, 1535 (2015).
Kim, S.-R. et al. Biofouling control with bead-entrapped quorum quenching bacteria in membrane bioreactors: physical and biological effects. Environ. Sci. Technol. 47, 836–842 (2013).
Kim, H. W. et al. Microbial population dynamics and proteomics in membrane bioreactors with enzymatic quorum quenching. Appl Microbiol Biotechnol. 97, 4665–4675 (2013).
Guo, W., Ngo, H.-H. & Li, J. A mini-review on membrane fouling. Bioresour. Technol. 122, 27–34 (2012).
Malaeb, L., Le-Clech, P., Vrouwenvelder, J. S., Ayoub, G. M. & Saikaly, P. E. Do biological-based strategies hold promise to biofouling control in MBRs? Water Res 47, 5447–5463 (2013).
Gnirss, R. & Dittrich, J. Microfiltration of Municipal Wastewater for Disinfection and Advanced Phosphorus Removal: Results from Trials with Different Small-Scale Pilot Plants. Water Environ. Res. 72, 602–609 (2000).
Pellegrin, M. L. & Kinnear, D. J. MBR Energy Consumption: Comparing Operating Full-Scale Plants. in In Proceedings of 84th Annual Water Environment Federation Technical Exposition & Conference (ed. In Proceedings of 84th Annual Water Environment Federation Technical Exposition & Conference) 5544–5560 (Water Environment Federation, 2011).
K, M. & M, S. Benchmarking energy consumption in municipal wastewater treatment plants in Japan. Water Sci. Technol. 62, 2256–2262 (2010).
Hua, M. et al. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 211–212, 317–331 (2012).
Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling. (De Gruyter, 2012).
Adeleye, A. S. et al. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 286, 640–662 (2016).
Doke, K. M. & Khan, E. M. Adsorption thermodynamics to clean up wastewater; critical review. Rev. Environ. Sci. Bio/Technol. 12, 25–44 (2013).
Cecen, F. & Aktas, Ö. Activated Carbon for Water and Wastewater Treatment: Integration of Adsorption and Biological Treatment (1). (Wiley-VCH, 2011).
Singh Vats, S., Tyagi, P. & Industrial, R. Wastewater treatment by bilogical activated carbon - a review. Res J. Pharm. Biol. Chem. Sci. 2, 1053–1058 (2011).
Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 30, 38–70 (2005).
Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon N. Y. 42, 83–94 (2004).
Al-Jiboury, K. Adsorption of phenol from industrial wastewater using commercial powdered activated carbon. ICOEST (2013).
Zhang, K., Gao, N., Deng, Y., Shui, M. & Tang, Y. Granular activated carbon (GAC) adsorption of two algal odorants, dimethyl trisulfide and β-cyclocitral. Desalination 266, 231–237 (2011).
Sarabadan, M., Bashiri, H. & Mousavi, S. M. Removal of crystal violet dye by an efficient and low cost adsorbent: Modeling, kinetic, equilibrium and thermodynamic studies. Korean J. Chem. Eng. 2019 3610 36, 1575–1586 (2019).
Zhou, Y.-F. & Haynes, R. J. Sorption of heavy metals by inorganic and organic components of solid wastes: significance to use of wastes as low-cost adsorbents and immobilizing agents. Crit. Rev. Environ. Sci. Technol. 40, 909–977 (2010).
Malamis, S. & Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 252–253, 428–461 (2013).
Şölener, M., Tunali, S., Özcan, A. S., Özcan, A. & Gedikbey, T. European Desalination Society and Center for Research and Technology Hellas (CERTH), Sani Resort 22–25 April 2007, Halkidiki, GreeceAdsorption characteristics of lead(II) ions onto the clay/poly(methoxyethyl)acrylamide (PMEA) composite from aqueous soluti. Desalination 223, 308–322 (2008).
Vhahangwele, M. & Mugera, G. W. The potential of ball-milled South African bentonite clay for attenuation of heavy metals from acidic wastewaters: Simultaneous sorption of Co2+, Cu2+, Ni2+, Pb2+, and Zn2+ ions. J. Environ. Chem. Eng. 3, 2416–2425 (2015).
Inglezakis, V. J., Stylianou, M. A., Gkantzou, D. & Loizidou, M. D. Removal of Pb(II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination 210, 248–256 (2007).
Quan, D. X. et al. 2013 International Symposium on Environmental Science and Technology (2013 ISEST)Immobilization of Pb and Cd in Contaminated Soil Using Nano-Crystallite Hydroxyapatite. Procedia Environ. Sci. 18, 657–665 (2013).
Amalhay, M. et al. SYMPHOS 2013 - 2nd International Symposium on Innovation and Technology in the Phosphate IndustryRemoval of Cadmium from Water Using Natural Phosphate as Adsorbent. Procedia Eng. 83, 386–393 (2014).
Keleş, E., Özer, A. K. & Yörük, S. Removal of Pb2+ from aqueous solutions by rock phosphate (low-grade). Desalination 253, 124–128 (2010).
Ghahremani, D. et al. Potential of nano crystalline calcium hydroxyapatite for Tin(II) removal from aqueous solutions: Equilibria and kinetic processes. Arab. J. Chem. https://doi.org/10.1016/j.arabjc.2012.10.006.
Barka, N., Qourzal, S., Assabbane, A., Nounah, A. & Ait-Ichou, Y. Removal of Reactive Yellow 84 from aqueous solutions by adsorption onto hydroxyapatite. J. Saudi Chem. Soc. 15, 263–267 (2011).
Wei, W., Sun, R., Cui, J. & Wei, Z. Removal of nitrobenzene from aqueous solution by adsorption on nanocrystalline hydroxyapatite. Desalination 263, 89–96 (2010).
Maretto, M. et al. Adsorption of hydrocarbons from industrial wastewater onto a silica mesoporous material: Structural and thermal study. Microporous Mesoporous Mater. 203, 139–150 (2015).
Pollard, S. J. T., Fowler, G. D., Sollars, C. J. & Perry, R. Low-cost adsorbents for waste and wastewater treatment: a review. Sci. Total Environ. 116, 31–52 (1992).
Zhang, F. S. & Itoh, H. Iron oxide-loaded slag for arsenic removal from aqueous system. Chemosphere 60, 319–325 (2005).
Kanel, S. R., Choi, H., Kim, J. Y., Vigneswaran, S. & Shim, W. G. Removal of arsenic(III) from groundwater using low-cost industrial by-products - Blast furnace slag. Water Qual. Res. J. Can. 41, 130–139 (2006).
Oh, C., Rhee, S., Oh, M. & Park, J. Removal characteristics of As(III) and As(V) from acidic aqueous solution by steel making slag. J. Hazard. Mater. 213–214, 147–155 (2012).
Duan, J. & Su, B. Removal characteristics of Cd(II) from acidic aqueous solution by modified steel-making slag. Chem. Eng. J. 246, 160–167 (2014).
Balsamo, M. et al. Arsenate removal from synthetic wastewater by adsorption onto fly ash. Desalination 263, 58–63 (2010).
Sun, D., Zhang, X., Wu, Y. & Liu, X. Adsorption of anionic dyes from aqueous solution on fly ash. J. Hazard. Mater. 181, 335–342 (2010).
Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 166, 36–59 (2011).
Mofarrah, A., Husain, T. & Chen, B. Optimizing Cr(VI) adsorption on activated carbon produced from heavy oil fly ash. J. Mater. Cycles Waste Manag. 16, 482–490 (2014).
Visa, M., Andronic, L. & Duta, A. Fly ash-TiO2 nanocomposite material for multi-pollutants wastewater treatment. J. Environ. Manag. 150, 336–343 (2015).
Arief, V. O., Trilestari, K., Sunarso, J., Indraswati, N. & Ismadji, S. Recent Progress on Biosorption of Heavy Metals from Liquids Using Low Cost Biosorbents: Characterization, Biosorption Parameters and Mechanism. Stud. CLEAN – Soil, Air, Water 36, 937–962 (2008).
Raj, K. R., Kardam, A., Arora, J. K., Srivastava, S. & Srivastava, M. M. Prediction of the As(III) and As(V) Abatement Capacity of Zea mays Cob Powder: ANN Modelling. Natl Acad. Sci. Lett. 36, 41–47 (2013).
Rajeswari, M. et al. Continuous biosorption of cadmium by Moringa olefera in a packed column. Biotechnol. Bioprocess Eng. 18, 321–325 (2013).
Tan, T. & Cheng, P. Biosorption of metal ions with Penicillium chrysogenum. Appl. Biochem. Biotechnol. 104, 119–128 (2003).
Akar, T., Tunali, S. & Çabuk, A. Study on the characterization of lead (II) biosorption by fungus Aspergillus parasiticus. Appl. Biochem. Biotechnol. 136, 389–405 (2007).
Ahmady-Asbchin, S., Andrès, Y., Gérente, C. & Cloirec, P. L. Biosorption of Cu(II) from aqueous solution by Fucus serratus: Surface characterization and sorption mechanisms. Bioresour. Technol. 99, 6150–6155 (2008).
Panda, G. C., Das, S. K., Bandopadhyay, T. S. & Guha, A. K. Adsorption of nickel on husk of Lathyrus sativus: Behavior and binding mechanism. Colloids Surf. B Biointerfaces 57, 135–142 (2007).
Witek-Krowiak, A., Szafran, R. G. & Modelski, S. Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 265, 126–134 (2011).
Piotrowska-Niczyporuk, A., Bajguz, A., Zambrzycka, E. & Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 52, 52–65 (2012).
Feng, N., Guo, X., Liang, S., Zhu, Y. & Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 185, 49–54 (2011).
Gomez, P. F. et al. Heavy metal biosorption by Rhizopus sp. biomass immobilized on textiles. Water Air Soil Pollut. 225, 1834 (2014).
Al-Anber, Z. A. & Matouq, M. A. D. Batch adsorption of cadmium ions from aqueous solution by means of olive cake. J. Hazard. Mater. 151, 194–201 (2008).
Rahman, M. S. & Sathasivam, K. V. Heavy metal adsorption onto Kappaphycus sp. from aqueous solutions: the use of error functions for validation of isotherm and kinetics models. Biomed. Res. Int. 2015, 126298 (2015).
Malamis, S., Katsou, E. & Haralambous, K. J. Study of Ni(II), Cu(II), Pb(II), and Zn(II) removal using sludge and minerals followed by MF/UF. Water, Air, Soil Pollut. 218, 81–92 (2011).
Putri, K. N. A., Keereerak, A. & Chinpa, W. Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int. J. Biol. Macromol. 156, 762–772 (2020).
Pan, J., Gao, B., Song, W., Xu, X. & Yue, Q. Modified biogas residues as an eco-friendly and easily-recoverable biosorbent for nitrate and phosphate removals from surface water. J. Hazard. Mater. 382, 121073 (2020).
Wang, J. & Chen, C. Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 160, 129–141 (2014).
Phetphaisit, C. W., Yuanyang, S. & Chaiyasith, W. C. Polyacrylamido-2-methyl-1-propane sulfonic acid-grafted-natural rubber as bio-adsorbent for heavy metal removal from aqueous standard solution and industrial wastewater. J. Hazard. Mater. 301, 163–171 (2016).
Lee, S.-T., Mi, F.-L., Shen, Y.-J. & Shyu, S.-S. Equilibrium and kinetic studies of copper(II) ion uptake by chitosan-tripolyphosphate chelating resin. Polym. (Guildf.) 42, 1879–1892 (2001).
Liu, X. D., Tokura, S., Nishi, N. & Sakairi, N. A novel method for immobilization of chitosan onto nonporous glass beads through a 1,3-thiazolidine linker. Polym. (Guildf.) 44, 1021–1026 (2003).
Dotto, G. L. & McKay, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 8, 103988 (2020).
Poyatos, J. M. et al. Advanced oxidation processes for wastewater treatment: state of the art. Water Air. Soil Pollut. 205, 187–204 (2009).
Bautista, P., Mohedano, A. F., Casas, J. A., Zazo, J. A. & Rodriguez, J. J. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol. 83, 1323–1338 (2008).
Andreozzi, R., Caprio, V., Insola, A. & Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 53, 51–59 (1999).
Wang, Y. et al. Dimethyl phthalate degradation at novel and efficient electro-Fenton cathode. Appl. Catal. B Environ. 156–157, 1–7 (2014).
EPA., U. S. Wastewater Technology Fact Sheet Ozone Disinfection. Off. Water Washington, D.C. (1999) doi:EPA 832-F-99-063.
Kommineni, S. et al. Advanced oxidation processes: literature review. Cent. Groundw. Restor. Prot. Natl. water Res. Inst. 110–208 (2000).
Umar, M., Aziz, H. A. & Yusoff, M. S. Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manag 30, 2113–2121 (2010).
Brillas, E., Sirés, I. & Oturan, M. A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 109, 6570 (2009).
Panizza, M. & Cerisola, G. Electro-Fenton degradation of synthetic dyes. Water Res. 43, 339–344 (2009).
Wang, C.-T., Chou, W.-L., Chung, M.-H. & Kuo, Y.-M. COD removal from real dyeing wastewater by electro-Fenton technology using an activated carbon fiber cathode. Desalination 253, 129–134 (2010).
Benotti, M. J., Stanford, B. D., Wert, E. C. & Snyder, S. A. Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res 43, 1513–1522 (2009).
Xia, Q., Jiang, Z., Li, D., Wang, J. & Yao, Z. Green synthesis of a dendritic Fe3O4 @Feo composite modified with polar C-groups for Fenton-like oxidation of phenol. J. Alloys Compd. (2018) https://doi.org/10.1016/j.jallcom.2018.02.311.
Liu, S. et al. The degradation of tetracycline in a photo-electro-Fenton system. Chem. Eng. J. 231, 441 (2013).
Ferrag-Siagh, F. et al. Electro-Fenton pretreatment for the improvement of tylosin biodegradability. Environ. Sci. Pollut. Res. 21, 8534–8542 (2014).
Komtchou, S., Dirany, A., Drogui, P. & Bermond, A. Removal of carbamazepine from spiked municipal wastewater using electro-Fenton process. Environ. Sci. Pollut. Res. 22, 11513–11525 (2015).
Carra, I. et al. Performance of different advanced oxidation processes for tertiary wastewater treatment to remove the pesticide acetamiprid. J. Chem. Technol. Biotechnol. 91, 72–81 (2015).
Babuponnusami, A. & Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2, 557–572 (2014).
Thirugnanasambandham, K. & Sivakumar, V. Optimization of treatment of grey wastewater using Electro-Fenton technique–Modeling and validation. Process Saf. Environ. Prot. 95, 60–68 (2015).
Grčić, I., Vujević, D., Šepčić, J. & Koprivanac, N. Minimization of organic content in simulated industrial wastewater by Fenton type processes: A case study. J. Hazard. Mater. 170, 954–961 (2009).
Navarro, R. R., Ichikawa, H. & Tatsumi, K. Ferrite formation from photo-Fenton treated wastewater. Chemosphere 80, 404–409 (2010).
Li, R. et al. Removal of triazophos pesticide from wastewater with Fenton reagent. J. Hazard. Mater. 167, 1028–1032 (2009).
Oturan, N., Brillas, E. & Oturan, M. A. Unprecedented total mineralization of atrazine and cyanuric acid by anodic oxidation and electro-Fenton with a boron-doped diamond anode. Environ. Chem. Lett. 10, 165–170 (2012).
Iglesias, O., Meijide, J., Bocos, E., Sanromán, M. Á. & Pazos, M. New approaches on heterogeneous electro-Fenton treatment of winery wastewater. Electrochim. Acta 169, 134–141 (2015).
Linares-Hernández, I., Barrera-Díaz, C., Bilyeu, B., Juárez-GarcíaRojas, P. & Campos-Medina, E. A combined electrocoagulation–electrooxidation treatment for industrial wastewater. J. Hazard. Mater. 175, 688–694 (2010).
Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 38, 11–41 (2004).
Mollah, M. Y. A., Schennach, R., Parga, J. R. & Cocke, D. L. Electrocoagulation (EC) — science and applications. J. Hazard. Mater. 84, 29–41 (2001).
Calvo, L. S. et al. An electrocoagulation unit for the purification of soluble oil wastes of high COD. Environ. Prog. 22, 57–65 (2003).
El-Naas, M. H., Al-Zuhair, S., Al-Lobaney, A. & Makhlouf, S. Assessment of electrocoagulation for the treatment of petroleum refinery wastewater. J. Environ. Manag. 91, 180–185 (2009).
Şengil, İ. A., Kulaç, S. & Özacar, M. Treatment of tannery liming drum wastewater by electrocoagulation. J. Hazard. Mater. 167, 940–946 (2009).
GilPavas, E., Dobrosz-Gómez, I. & Gómez-García, M. Á. The removal of the trivalent chromium from the leather tannery wastewater: the optimisation of the electro-coagulation process parameters. Water Sci. Technol. 63, 385–394 (2011).
Espinoza-Quiñones, F. R. et al. Electrocoagulation efficiency of the tannery effluent treatment using aluminium electrodes. Water Sci. Technol. 60, 2173–2185 (2009).
Moreno-Casillas, H. A. et al. Electrocoagulation mechanism for COD removal. Sep. Purif. Technol. 56, 204–211 (2007).
Bensadok, K., Benammar, S., Lapicque, F. & Nezzal, G. Electrocoagulation of cutting oil emulsions using aluminium plate electrodes. J. Hazard. Mater. 152, 423–430 (2008).
Karhu, M., Kuokkanen, V., Kuokkanen, T. & Rämö, J. Bench scale electrocoagulation studies of bio oil-in-water and synthetic oil-in-water emulsions. Sep. Purif. Technol. 96, 296 (2012).
Ahmadi, S., Sardari, E., Javadian, H. R., Katal, R. & Sefti, M. V. Removal of oil from biodiesel wastewater by electrocoagulation method. Korean J. Chem. Eng. 30, 634–641 (2013).
Hassan, I., Nirdosh, I. & Sedahmed, G. H. Separation of Oil from Oil–Water Emulsions by Electrocoagulation in an Electrochemical Reactor with a Fixed-Bed Anode. Water, Air, Soil Pollut. 226, 1–12 (2015).
Safari, S., Azadi Aghdam, M. & Kariminia, H. R. Electrocoagulation for COD and diesel removal from oily wastewater. Int. J. Environ. Sci. Technol. 13, 231–242 (2016).
Cañizares, P., Martínez, F., Jiménez, C., Sáez, C. & Rodrigo, M. A. Coagulation and electrocoagulation of oil-in-water emulsions. J. Hazard. Mater. 151, 44–51 (2008).
Sahu, O., Mazumdar, B. & Chaudhari, P. K. Treatment of wastewater by electrocoagulation: a review. Environ. Sci. Pollut. Res. 21, 2397–2413 (2014).
Cañizares, P., Martínez, F., Jiménez, C., Lobato, J. & Rodrigo, M. A. Comparison of the aluminum speciation in chemical and electrochemical dosing processes. Ind. Eng. Chem. Res. 45, 8749–8756 (2006).
Cañizares, P., Martínez, F., Lobato, J. & Rodrigo, M. A. Break-up of oil-in-water emulsions by electrochemical techniques. J. Hazard. Mater. 145, 233–240 (2007).
Zongo, I. et al. Electrocoagulation for the treatment of textile wastewaters with Al or Fe electrodes: Compared variations of COD levels, turbidity and absorbance. J. Hazard. Mater. 169, 70–76 (2009).
Cheballah, K., Sahmoune, A., Messaoudi, K., Drouiche, N. & Lounici, H. Simultaneous removal of hexavalent chromium and COD from industrial wastewater by bipolar electrocoagulation. Chem. Eng. Process. Process. Intensif. 96, 94–99 (2015).
Gao, P., Chen, X., Shen, F. & Chen, G. Removal of chromium(VI) from wastewater by combined electrocoagulation–electroflotation without a filter. Sep. Purif. Technol. 43, 117–123 (2005).
Gatsios, E., Hahladakis, J. N. & Gidarakos, E. Optimization of electrocoagulation (EC) process for the purification of a real industrial wastewater from toxic metals. J. Environ. Manag. 154, 117–127 (2015).
Hansen, H. K., Nuñez, P. & Jil, C. Removal of Arsenic from Wastewaters by Airlift Electrocoagulation. Part 1: Batch Reactor Experiments. Sep. Sci. Technol. 43, 212–224 (2008).
Petsriprasit, C., Namboonmee, J. & Hunsom, M. Application of the electrocoagulation technique for treating heavy metals containing wastewater from the pickling process of a billet plant. Korean J. Chem. Eng. 27, 854–861 (2010).
Khatibikamal, V., Torabian, A., Janpoor, F. & Hoshyaripour, G. Fluoride removal from industrial wastewater using electrocoagulation and its adsorption kinetics. J. Hazard. Mater. 179, 276–280 (2010).
Sujana, M. G., Thakur, R. S. & Rao, S. B. Removal of fluoride from aqueous solution by using alum sludge. J. Colloid Interface Sci. 206, 94–101 (1998).
Toyoda, A. & Taira, T. A new method for treating fluorine wastewater to reduce sludge and running costs. IEEE Trans. Semicond. Manuf. 13, 305–309 (2000).
Raju, G. B., Karuppiah, M. T., Latha, S. S., Parvathy, S. & Prabhakar, S. Treatment of wastewater from synthetic textile industry by electrocoagulation–electrooxidation. Chem. Eng. J. 144, 51–58 (2008).
Feng, J. et al. Treatment of tannery wastewater by electrocoagulation. J. Environ. Sci. 19, 1409–1415 (2007).
Benhadji, A., Taleb Ahmed, M. & Maachi, R. Electrocoagulation and effect of cathode materials on the removal of pollutants from tannery wastewater of Rouïba. Desalination 277, 128–134 (2011).
Kabdaşlı, I., Arslan-Alaton, I., Ölmez-Hancı, T. & Tünay, O. Electrocoagulation applications for industrial wastewaters: a critical review. Environ. Technol. Rev. 1, 2–45 (2012).
Merzouk, B. et al. Effect of modification of textile wastewater composition on electrocoagulation efficiency. Desalination 275, 181–186 (2011).
Secula, M. S., Crežescu, I. & Petrescu, S. An experimental study of indigo carmine removal from aqueous solution by electrocoagulation. Desalination 277, 227–235 (2011).
Pirkarami, A. & Olya, M. E. Removal of dye from industrial wastewater with an emphasis on improving economic efficiency and degradation mechanism. J. Saudi Chem. Soc. 21, S179–S186 (2017).
Bayramoglu, M., Eyvaz, M. & Kobya, M. Treatment of the textile wastewater by electrocoagulation: Economical evaluation. Chem. Eng. J. 128, 155–161 (2007).
Syam Babu, D., Anantha Singh, T. S., Nidheesh, P. V. & Suresh Kumar, M. Industrial wastewater treatment by electrocoagulation process. Sep. Sci. Technol. (Phila.) 55, 3195–3227 (2020). vol.
Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 39, 1857–1862 (1994).
Anglada, Á., Urtiaga, A. & Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: fundamentals and review of applications. J. Chem. Technol. Biotechnol. 84, 1747–1755 (2009).
Mantzavinos, D. & Psillakis, E. Enhancement of biodegradability of industrial wastewaters by chemical oxidation pre-treatment. J. Chem. Technol. Biotechnol. 79, 431–454 (2004).
Polcaro, A. M. et al. Characterization of a stirred tank electrochemical cell for water disinfection processes. Electrochim. Acta 52, 2595–2602 (2007).
Sáez, C., Cañizares, P., Llanos, J. & Rodrigo, M. A. The treatment of actual industrial wastewaters using electrochemical techniques. Electrocatalysis. 4, 252–258 (2013).
Anglada, A., Ibañez, R., Urtiaga, A. & Ortiz, I. Electrochemical oxidation of saline industrial wastewaters using boron-doped diamond anodes. Catal. Today 151, 178–184 (2010).
Rajkumar, D. & Palanivelu, K. Electrochemical treatment of industrial wastewater. J. Hazard. Mater. 113, 123–129 (2004).
Cañizares, P., Paz, R., Sáez, C. & Rodrigo, M. A. Costs of the electrochemical oxidation of wastewaters: A comparison with ozonation and Fenton oxidation processes. J. Environ. Manag. 90, 410–420 (2009).
Chadwick, S. S. Ullmann’s encyclopedia of industrial chemistry. Ref. Serv. Rev. 16, 31–34 (1988).
Urtiaga, A., Gómez, P., Arruti, A. & Ortiz, I. Electrochemical removal of tetrahydrofuran from industrial wastewaters: anode selection and process scale-up. J. Chem. Technol. Biotechnol. 89, 1243–1250 (2014).
Vijayaraghavan, K., Ramanujam, T. K. & Balasubramanian, N. In situ hypochlorous acid generation for treatment of tannery wastewaters. J. Environ. Eng. 124, 887–891 (1998).
Szpyrkowicz, L., Naumczyk, J. & Zilio-Grandi, F. Electrochemical treatment of tannery wastewater using Ti Pt and Ti/Pt/Ir electrodes. Water Res. 29, 517–524 (1995).
Vlyssides, A. G. & Israilides, C. J. Electrochemical oxidation of a textile dye and finishing wastewater using a Pt/Ti electrode. J. Environ. Sci. Heal. Part A 33, 847–862 (1998).
Khorram, A. G. & Fallah, N. Comparison of electrocoagulation and photocatalytic process for treatment of industrial dyeing wastewater: Energy consumption analysis. Environ. Prog. Sustain. Energy 39, 13288 (2020).
Mehrjouei, M., Müller, S. & Möller, D. Energy consumption of three different advanced oxidation methods for water treatment: a cost-effectiveness study. J. Clean. Prod. 65, 178–183 (2014).
Hosny, A. Y. Separating oil from oil-water emulsions by electroflotation technique. Sep. Technol. 6, 9–17 (1996).
Chen, W. & Horan, N. J. The treatment of a high strength pulp and paper mill effluent for wastewater re-use. Env. Technol. 19, 173–182 (1998).
Nahui, F. N. B., Nascimento, M. R., Cavalcanti, E. B. & Vilar, E. O. Electroflotation of emulsified oil in industrial wastes evaluated with a full factorial design. Braz. J. Chem. Eng. 25, 435–442 (2008).
Vaughan, R., Brian, E., Gar, W. & David, M. Pilot-scale investigation of chemical addition-dissolved air flotation for the treatment of an oily wastewater. Environ. Eng. Sci. 17, 267–277 (2009).
Belkacem, M., Khodir, M. & Abdelkrim, S. Treatment characteristics of textile wastewater and removal of heavy metals using the electroflotation technique. Desalination 228, 245–254 (2008).
de Oliveira da Mota, I., de Castro, J. A., de Góes Casqueira, R. & de Oliveira Junior, A. G. Study of electroflotation method for treatment of wastewater from washing soil contaminated by heavy metals. J. Mater. Res. Technol. 4, 109–113 (2015).
Aoudj, S., Khelifa, A., Drouiche, N. & Hecini, M. Removal of fluoride and turbidity from semiconductor industry wastewater by combined coagulation and electroflotation. Desalin. Water Treat. 57, 18398–18405 (2016).
Melchiors, M. S. et al. Treatment of wastewater from the dairy industry using electroflocculation and solid whey recovery. J. Environ. Manag. 182, 574–580 (2016).
Ho, C. C. & Chan, C. Y. The application of lead dioxide-coated titanium anode in the electroflotation of palm oil mill effluent. Water Res 20, 1523–1527 (1986).
Alexandrova, L., Nedialkova, T. & Nishkov, I. Electroflotation of metal ions in waste water. Int. J. Miner. Process. 41, 285–294 (1994).
Mavrov, V., Erwe, T., Blöcher, C. & Chmiel, H. Desalination and the Environment: Fresh Water for allStudy of new integrated processes combining adsorption, membrane separation and flotation for heavy metal removal from wastewater. Desalination 157, 97–104 (2003).
Anpo, M. & Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 216, 505–516 (2003).
Zheng, X., Chen, D., Wang, Z. W., Lei, Y. & Cheng, R. Nano-TiO2 membrane adsorption reactor (MAR) for virus removal in drinking water. Chem. Eng. J. 230, 180–187 (2013).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
Lin, H. et al. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B-Environ. 68, 1–11 (2006).
Wang, Y., He, Y., Lai, Q. & Fan, M. Review of the progress in preparing nano TiO2: an important environmental engineering material. J. Environ. Sci. (China) 26, 2139–2177 (2014).
Gupta, S. M. & Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Cent. Eur. J. Chem. 10, 279–294 (2012).
Byrappa, K. & Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 53, 117–166 (2007).
Nam, C. T., Yang, W.-D. & Duc, L. M. Solvothermal Synthesis of TiO2 Photocatalysts in Ketone Solvents with Low Boiling Points. J. Nanomater. 2013, 11 (2013).
Paulose, M. et al. Anodic growth of highly ordered TiO2 nanotube arrays to 134 microm in length. J. Phys. Chem. B 110, 16179–16184 (2006).
Zhao, L. & Yu, J. Controlled synthesis of highly dispersed TiO2 nanoparticles using SBA-15 as hard template. J. Colloid Interface Sci. 304, 84–91 (2006).
Chen, Y. et al. Synthesis of BiOI-TiO2 composite nanoparticles by microemulsion method and study on their photocatalytic activities. Scientific World Journal. 2014, 647040 (2014).
de Luna, L. A., da Silva, T. H., Nogueira, R. F., Kummrow, F. & Umbuzeiro, G. A. Aquatic toxicity of dyes before and after photo-Fenton treatment. J. Hazard Mater. 276, 332–338 (2014).
Zeng, P. et al. Efficiency comparison for treatment of amantadine pharmaceutical wastewater by Fenton, ultrasonic, and Fenton/ultrasonic processes. Environ. Earth Sci. 73, 4979–4987 (2015).
Liu, Z., Sun, D. D., Guo, P. & Leckie, J. O. One-step fabrication and high photocatalytic activity of porous TiO2 hollow aggregates by using a low-temperature hydrothermal method without templates. Chem. (Easton) 13, 1851–1855 (2007).
Kang, M. et al. Characterization of a TiO2 photocatalyst synthesized by the solvothermal method and its catalytic performance for CHCl3 decomposition. J. Photochem. Photobiol. A Chem. 144, 185–191 (2001).
Supphasrirongjaroen, P., Praserthdam, P., Panpranot, J., Na-Ranong, D. & Mekasuwandumrong, O. Effect of quenching medium on photocatalytic activity of nano-TiO2 prepared by solvothermal method. Chem. Eng. J. 138, 622–627 (2008).
Tan, Y. N., Wong, C. L. & Mohamed, A. R. An overview on the photocatalytic activity of nano-doped- in the degradation of organic pollutants. ISRN Mater. Sci. 2011, 18 (2011).
Gupta, S. M. & Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 56, 1639–1657 (2011).
Kuwahara, Y. & Yamashita, H. Efficient photocatalytic degradation of organics diluted in water and air using TiO2 designed with zeolites and mesoporous silica materials. J. Mater. Chem. 21, 2407–2416 (2011).
Chen, C.-C. & Lu, C.-S. Mechanistic studies of the photocatalytic degradation of methyl green: an investigation of products of the decomposition processes. Env. Sci. Technol. 41, 4389–4396 (2007).
Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 73, 71–91 (2010).
Giwa, A. et al. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process Saf. Environ. Prot. 146, 220–256 (2021).
Yusuf, A. et al. A review of emerging trends in membrane science and technology for sustainable water treatment. J. Clean. Prod. 266, 121867 (2020).
Giwa, A., Dindi, A. & Kujawa, J. Membrane bioreactors and electrochemical processes for treatment of wastewaters containing heavy metal ions, organics, micropollutants and dyes: Recent developments. J. Hazard. Mater. 370, 172–195 (2019).
Lenzi, G. G. et al. Photocatalytic degradation of textile reactive dye using artificial neural network modeling approach. N. Pub Balaban 57, 14132–14144 (2015).
Bararpour, S. T. et al. Investigation of 2-nitrophenol solar degradation in the simultaneous presence of K2S2O8 and H2O2: Using experimental design and artificial neural network. J. Clean. Prod. 176, 1154–1162 (2018).
Khataee, A. R., Fathinia, M., Zarei, M., Izadkhah, B. & Joo, S. W. Modeling and optimization of photocatalytic/photoassisted-electro-Fenton like degradation of phenol using a neural network coupled with genetic algorithm. J. Ind. Eng. Chem. 20, 1852–1860 (2014).
Lin, C. H., Yu, R. F., Cheng, W. P. & Liu, C. R. Monitoring and control of UV and UV-TiO2 disinfections for municipal wastewater reclamation using artificial neural networks. J. Hazard. Mater. 209–210, 348–354 (2012).
Hu, C. et al. Plasmon-induced photodegradation of toxic pollutants with Ag−AgI/Al2O3 under visible-light irradiation. J. Am. Chem. Soc. 132, 857–862 (2009).
Jie, X., Bao, N., Gong, B. & Zhou, S. Facile synthesis of plasmonic Ag/AgCl/polydopamine-TiO2 fibers for efficient visible photocatalysis. Nano-Struct. Nano-Objects 12, 98–105 (2017).
Hu, X., Li, Y., Tian, J., Yang, H. & Cui, H. Highly efficient full solar spectrum (UV-vis-NIR) photocatalytic performance of Ag2S quantum dot/TiO2 nanobelt heterostructures. J. Ind. Eng. Chem. 45, 189–196 (2017).
Wang, T. et al. Enhanced performance of TiO2/reduced graphene oxide doped by rare-earth ions for degrading phenol in seawater excited by weak visible light. Adv. Powder Technol. 30, 1920–1931 (2019).
Xiao, S., Shen, M., Guo, R., Wang, S. & Shi, X. Immobilization of zerovalent iron nanoparticles into electrospun polymer nanofibers: synthesis, characterization, and potential environmental applications. J. Phys. Chem. C. 113, 18062–18068 (2009).
Katsou, E., Malamis, S. & Loizidou, M. Performance of a membrane bioreactor used for the treatment of wastewater contaminated with heavy metals. Bioresour. Technol. 102, 4325–4332 (2011).
Huang, X., Shen, Y. & X, K. Recent advances in membrane bioreactor technology for wastewater treatment in China. Front. Environ. Sci. Eng. 4, 245–271 (2010).
Ryu, J., Choi, W. & Choo, K. H. A pilot-scale photocatalyst-membrane hybrid reactor: Performance and characterization. Water Sci. Technol. 51, 491–497 (2005).
Doruk, N., Yatmaz, H. C. & Dizge, N. Degradation efficiency of textile and wood processing industry wastewater by photocatalytic process using in situ ultrafiltration membrane. CLEAN – Soil Air Water 44, 224–231 (2016).
Thiruvenkatachari, R., Kwon, T. O. & Moon, I. S. Application of slurry type photocatalytic oxidation-submerged hollow fiber microfiltration hybrid system for the degradation of bisphenol A (BPA). Sep. Sci. Technol. 40, 2871–2888 (2005).
Fu, J., Ji, M., Zhao, Y. & Wang, L. Kinetics of aqueous photocatalytic oxidation of fulvic acids in a photocatalysis-ultrafiltration reactor (PUR). Sep. Purif. Technol. 50, 107–113 (2006).
Le-Clech, P., Lee, E. K. & Chen, V. Hybrid photocatalysis/membrane treatment for surface waters containing low concentrations of natural organic matters. Water Res 40, 323–330 (2006).
Sato, S. Photocatalysts sensitive to visible light. Sci. (80-.) 295, 626–627 (2002).
Ihara, T., Miyoshi, M., Iriyama, Y., Matsumoto, O. & Sugihara, S. Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl. Catal. B Environ. 42, 403–409 (2003).
Nolan, M., Iwaszuk, A., Lucid, A. K., Carey, J. J. & Fronzi, M. Design of novel visible light active photocatalyst materials: surface modified TiO2. Adv. Mater. 28, 5425–5446 (2016).
Deveci, E. Ü., Dizge, N., Yatmaz, H. C. & Aytepe, Y. Integrated process of fungal membrane bioreactor and photocatalytic membrane reactor for the treatment of industrial textile wastewater. Biochem. Eng. J. 105, 420–427 (2016). Part.
Gogate, P. R. Hydrodynamic cavitation for food and water processing. Food Bioprocess Technol. 4, 996–1011 (2011).
Jyoti, K. K. & Pandit, A. B. Water disinfection by acoustic and hydrodynamic cavitation. Biochem. Eng. J. 7, 201–212 (2001).
Son, H.-S., Choi, S.-B., Khan, E. & Zoh, K.-D. Removal of 1,4-dioxane from water using sonication: Effect of adding oxidants on the degradation kinetics. Water Res 40, 692–698 (2006).
Maezawa, A. et al. Treatment of dye wastewater by using photo-catalytic oxidation with sonication. Ultrason. - Sonochem. 14, 615–620 (2007).
Gashchin, O. R. & Viten’ko, T. N. Features of disinfection kinetics of water containing Escherichia Coli in conditions of hydrodynamic cavitation. J. Water Chem. Technol. 30, 322–327 (2008).
Mezule, L., Tsyfansky, S., Yakushevich, V. & Juhna, T. A simple technique for water disinfection with hydrodynamic cavitation: Effect on survival of Escherichia coli. Desalination 248, 152–159 (2009).
Braeutigam, P., Wu, Z. ‐L., Stark, A. & Ondruschka, B. Degradation of BTEX in Aqueous Solution by Hydrodynamic Cavitation. Chem. Eng. Technol. 32, 745–753 (2009).
Sponza, D. T. & Oztekin, R. Effect of sonication assisted by titanium dioxide and ferrous ions on polyaromatic hydrocarbons (PAHs) and toxicity removals from a petrochemical industry wastewater in Turkey. J. Chem. Technol. Biotechnol. 85, 913–925 (2010).
Musmarra, D. et al. Degradation of ibuprofen by hydrodynamic cavitation: Reaction pathways and effect of operational parameters. Ultrason. Sonochem. 29, 76–83 (2016).
Braeutigam, P. et al. Degradation of carbamazepine in environmentally relevant concentrations in water by Hydrodynamic-Acoustic-Cavitation (HAC). Water Res 46, 2469–2477 (2012).
Oztekin, R. & Sponza, D. T. Treatment of wastewaters from the olive mill industry by sonication. J. Chem. Technol. Biotechnol. 88, 212–225 (2013).
Bani-Melhem, K. & Elektorowicz, M. Performance of the submerged membrane electro-bioreactor (SMEBR) with iron electrodes for wastewater treatment and fouling reduction. J. Memb. Sci. 379, 434–439 (2011).
Ensano, B. M. B. et al. Combination of electrochemical processes with membrane bioreactors for wastewater treatment and fouling control: a review. Front. Environ. Sci. 4, (2016). https://doi.org/10.3389/fenvs.2016.00057.
Ahmed, M. A. L. & Hasan, S. W. Fe and Zn removal from steel making industrial wastewater by electrically enhanced membrane bioreactor. Desalin. Water Treat. 6–8 (2017). https://doi.org/10.5004/dwt.2017.21305.
Hagelüken, C., Lee-Shin, U. J., Carpentier, A. & Heron, C. The EU circular economy and its relevance to metal recycling. Recycling 1, 242–253 (2016).
(IWA), T. international water association. Water Utility Pathways in a Circular Economy. (2016).
Puyol, D. et al. Resource recovery from wastewater by biological technologies: opportunities, challenges, and prospects. Front. Microbiol. 7, 2106 (2017).
Wang, H. & Ren, Z. J. Bioelectrochemical metal recovery from wastewater: A review. Water Res 66, 219–232 (2014).
Bae, T.-H., Han, S.-S. & Tak, T.-M. Membrane sequencing batch reactor system for the treatment of dairy industry wastewater. Process Biochem 39, 221–231 (2003).
Lu, S. G. et al. The performance of fermentation wastewater treatment in ultrafiltration membrane bioreactor by contiuous and intermittent aeration processes. Water Sci. Technol. 42, 323–329 (2000).
Tsilogeorgis, J., Zouboulis, A., Samaras, P. & Zamboulis, D. European Desalination Society and Center for Research and Technology Hellas (CERTH), Sani Resort 22 –25 April 2007, Halkidiki, GreeceApplication of a membrane sequencing batch reactor for landfill leachate treatment. Desalination 221, 483–493 (2008).
Hall, E. R., Onysko, K. A. & Parker, W. J. Enhancement of bleached kraft organochlorine removal by coupling membrane filtration and anaerobic treatment. Env. Technol. 16, 115–126 (1995).
Hai, F. I., Yamamoto, K. & Fukushi, K. International Congress on Membranes and Membrane ProcessesDevelopment of a submerged membrane fungi reactor for textile wastewater treatment. Desalination 192, 315–322 (2006).
Yigit, N. O. et al. The Third Membrane Science and Technology Conference of Visegrad Countries (PERMEA); part 1Treatment of a denim producing textile industry wastewater using pilot-scale membrane bioreactor. Desalination 240, 143–150 (2009).
Delgado, L. F. et al. Removal of a cytostatic drug by a membrane bioreactor. Desalin. Water Treat. 9, 112–118 (2009).
Torres, A. P. R., Santiago, V. M. J. & Borges, C. P. Performance evaluation of submerged membrane bioreactor pilot units for refinery wastewater treatment. Environ. Prog. 27, 189–194 (2008).
Qin, J.-J., Oo, M. H., Tao, G. & Kekre, K. A. Feasibility study on petrochemical wastewater treatment and reuse using submerged MBR. J. Memb. Sci. 293, 161–166 (2007).
Goltara, A., Martinez, J. & Mendez, R. Carbon and nitrogen removal from tannery wastewater with a membrane bioreactor. Water Sci. Technol. 48, 207–214 (2003).
Munz, G. et al. The role of tannins in conventional and membrane treatment of tannery wastewater. J. Hazard. Mater. 164, 733–739 (2009).
Chegrouche, S., Mellah, A. & Barkat, M. Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235, 306–318 (2009).
Barkat, M., Nibou, D., Chegrouche, S. & Mellah, A. Kinetics and thermodynamics studies of chromium(VI) ions adsorption onto activated carbon from aqueous solutions. Chem. Eng. Process. Process. Intensif. 48, 38–47 (2009).
Kannan, N. & Rengasamy, G. Comparison of Cadmium Ion Adsorption on Various ACTIVATED CARBONS. Water Air. Soil Pollut. 163, 185–201 (2005).
Çetinkaya Dönmez, G., Aksu, Z., Öztürk, A. & Kutsal, T. A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem 34, 885–892 (1999).
Dakiky, M., Khamis, M., Manassra, A. & Mer’eb, M. Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 6, 533–540 (2002).
Anjana, K., Kaushik, A., Kiran, B. & Nisha, R. Biosorption of Cr(VI) by immobilized biomass of two indigenous strains of cyanobacteria isolated from metal contaminated soil. J. Hazard. Mater. 148, 383–386 (2007).
Srinivasa Rao, P., Kalyani, S., Suresh Reddy, K. V. N. & Krishnaiah, A. Comparison of biosorption of Nickel (II) and Copper (II) ions from aqueous solution by sphaeroplea algae and acid treated sphaeroplea algae. Sep. Sci. Technol. 40, 3149–3165 (2005).
Green-Ruiz, C., Rodriguez-Tirado, V. & Gomez-Gil, B. Cadmium and zinc removal from aqueous solutions by Bacillus jeotgali: pH, salinity and temperature effects. Bioresour. Technol. 99, 3864–3870 (2008).
Ucun, H., Bayhana, Y. K., Kaya, Y., Cakici, A. & Algur, O. F. Biosorption of lead (II) from aqueous solution by cone biomass of Pinus sylvestris. Desalination 154, 233–238 (2003).
Ahluwalia, S. S. & Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 98, 2243–2257 (2007).
Pérez, M., Torrades, F., Domènech, X. & Peral, J. Fenton and photo-Fenton oxidation of textile effluents. Water Res 36, 2703–2710 (2002).
Sahinkaya, S., Ozdemir, C. & Karatas, M. USE OF FENTON’S REAGENT FOR REMOVAL OF PESTICIDES FROM INDUSTRIAL WASTEWATER. in (SGEM Scientific GeoConference, 2007). citeulike-article-id:13484962.
Peternel, I. T., Koprivanac, N., Božić, A. M. L. & Kušić, H. M. Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J. Hazard. Mater. 148, 477–484 (2007).
Rivas, F. J., Beltrán, F. J., Gimeno, O. & Alvarez, P. Optimisation of Fenton’s reagent usage as a pre-treatment for fermentation brines. J. Hazard. Mater. 96, 277–290 (2003).
Sun, J.-H. et al. Oxidative decomposition of p-nitroaniline in water by solar photo-Fenton advanced oxidation process. J. Hazard. Mater. 153, 187–193 (2008).
Kušić, H., Koprivanac, N., Božić, A. L. & Selanec, I. Photo-assisted Fenton type processes for the degradation of phenol: A kinetic study. J. Hazard. Mater. 136, 632–644 (2006).
Tekin, H. et al. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J. Hazard. Mater. 136, 258–265 (2006).
Cui, X. et al. Pilot-scale treatment of pharmaceutical berberine wastewater by Fenton oxidation. Environ. Earth Sci. 73, 4967–4977 (2015).
Pérez, M., Torrades, F., Garcı́a-Hortal, J. A., Domènech, X. & Peral, J. Removal of organic contaminants in paper pulp treatment effluents under Fenton and photo-Fenton conditions. Appl. Catal. B Environ. 36, 63–74 (2002).
Catalkaya, E. C. & Color, F. K. TOC, and AOX removals from pulp and mill effluent by advanced oxidation processes: a comparative study. J. Hazard. Mater. 139, 244–253 (2007).
Neyens, E. & Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 98, 33–50 (2003).
Kochany, J. & Lipczynska-Kochany, E. Utilization of landfill leachate parameters for pretreatment by Fenton reaction and struvite precipitation-A comparative study. J. Hazard. Mater. 166, 248–254 (2009).
De la Cruz, N. et al. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Res 46, 1947–1957 (2012).
Li, J., Luan, Z., Yu, L. & Ji, Z. Pretreatment of acrylic fiber manufacturing wastewater by the Fenton process. Desalination 284, 62–65 (2012).
Ma, X.-J. & Xia, H.-L. Treatment of water-based printing ink wastewater by Fenton process combined with coagulation. J. Hazard. Mater. 162, 386–390 (2009).
Minella, M. et al. Photo-Fenton oxidation of phenol with magnetite as iron source. Appl. Catal. B Environ. 154–155, 102–109 (2014).
Bagher Miranzadeh, M. et al. Comparison of Fenton and Photo-Fenton Processes for Removal of Linear Alkyle Benzene Sulfonate (Las) from Aqueous Solutions. vol. 25 (2016).
Iglesias, O., Gómez, J., Pazos, M. & Sanromán, M. Á. Electro-Fenton oxidation of imidacloprid by Fe alginate gel beads. Appl. Catal. B Environ. 144, 416–424 (2014).
Kongjao, S., Damronglerd, S. & Hunsom, M. Simultaneous removal of organic and inorganic pollutants in tannery wastewater using electrocoagulation technique. Korean J. Chem. Eng. 25, 703–709 (2008).
Wang, C.-T. & Chou, W.-L. Performance of COD removal from oxide chemical mechanical polishing wastewater using iron electrocoagulation. J. Environ. Sci. Heal. Part A 44, 1289–1297 (2009).
Kumar, M., Ponselvan, F. I. A., Malviya, J. R., Srivastava, V. C. & Mall, I. D. Treatment of bio-digester effluent by electrocoagulation using iron electrodes. J. Hazard. Mater. 165, 345–352 (2009).
Kushwaha, J. P., Srivastava, V. C. & Mall, I. D. Organics removal from dairy wastewater by electrochemical treatment and residue disposal. Sep. Purif. Technol. 76, 198–205 (2010).
Valero, D. et al. Electrocoagulation of wastewater from almond industry. Chemosphere 84, 1290–1295 (2011).
Akyol, A. Treatment of paint manufacturing wastewater by electrocoagulation. Desalination 285, 91–99 (2012).
Chou, W. L., Wang, C. T. & Huang, K. Y. Investigation of process parameters for the removal of polyvinyl alcohol from aqueous solution by iron electrocoagulation. Desalination 251, 12–19 (2010).
Heidmann, I. & Calmano, W. Removal of Ni, Cu and Cr from a galvanic wastewater in an electrocoagulation system with Fe- and Al-electrodes. Sep. Purif. Technol. 71, 308–314 (2010).
Vasudevan, S., Lakshmi, J. & Sozhan, G. Effects of alternating and direct current in electrocoagulation process on the removal of cadmium from water. J. Hazard. Mater. 192, 26–34 (2011).
Mansouri, K., Elsaid, K., Bedoui, A., Bensalah, N. & Abdel-Wahab, A. Application of electrochemically dissolved iron in the removal of tannic acid from water. Chem. Eng. J. 172, 970–976 (2011).
Li, P., Song, Y. & Yu, S. Removal of Microcystis aeruginosa using hydrodynamic cavitation: performance and mechanisms. Water Res 62, 241 (2014).
Nidheesh, P. V. & Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 299, 1 (2012).
Horovitz, I. et al. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 310, 98–107 (2016).
Acknowledgements
We would like to acknowledge the support, guidance, and assistance provided by the library services team at Khalifa University of Science and Technology, Abu Dhabi, United Arab Emirates. This review would not have been possible without the access to the different library database and e-sources. We would also like to acknowledge the funding from Khalifa University through the Center for Membranes and Advanced Water Technology (CMAT), under grant number RC2-2018-009.
Author information
Authors and Affiliations
Contributions
M.A.: Conceptualization, Methodology, Writing—original draft. M.O.M.: Writing—review & editing. A.G.: Conceptualization, Methodology, Writing—original draft. M.E.: Review & editing. E.K.: Writing—review & editing. O.K.: Writing—review & editing. V.N.: Review & editing. S.W.H.: Conceptualization, Validation, Resources, Supervision, Review & editing, Administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, M., Mavukkandy, M.O., Giwa, A. et al. Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. npj Clean Water 5, 12 (2022). https://doi.org/10.1038/s41545-022-00154-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-022-00154-5
This article is cited by
-
Assessment of toxicity and antimicrobial performance of polymeric inorganic coagulant and evaluation for eutrophication reduction
Scientific Reports (2024)
-
Construction of flake ball-shaped Bi2WO6 embedded on phenyl functionalized g-C3N4 nanosheet for efficient degradation insight of colorless pollutants and its biological application
Environmental Science and Pollution Research (2024)
-
WO3–NiO/rGO Based Photocatalyst for Effectively Degradation of Colored and Colorless Pollutants Using Solar Light Irradiation
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Experimental and Theoretical Investigation of the Effect of Cellulose Nanowhiskers on the Pb(II) Adsorption by Superabsorbent Hydrogel Nanocomposites
Water, Air, & Soil Pollution (2024)
-
Visible light-induced H2 production and pollutant degradation by copper oxide nanosphere embedded zinc-cadmium-sulfide composite
Emergent Materials (2024)