Abstract
Epidemiological studies have reported a link between essential tremor (ET) and Parkinson’s disease (PD). Recent studies have suggested ET as a possible neurodegenerative disease whose subgroup contained Lewy bodies in the brainstem, as in PD. PD with antedated ET (PDconv) might exhibit traits different from those of the pure form of ET or PD. This study aimed to unveil the interplay between PD and premorbid ET, which might be the core pathobiology that differentiates PDconv from PD. The study included 51 ET, 32 PDconv, and 95 PD patients who underwent positron emission tomography using 18F-N-(3-fluoropropyl)-2beta-carbon ethoxy-3beta-(4-iodophenyl) nortropane and 123I-meta-iodobenzylguanidine myocardial scintigraphy to analyze central dopaminergic and peripheral noradrenergic integrity. The results show that PDconv group followed the typical striatal pathology of PD but with a delay in noradrenergic impairment as it caught up with the denervating status of PD a few years after PD diagnosis. Whereas the two PD subtypes displayed similar patterns of presynaptic dopamine transporter deficits, ET patients maintained high densities in all subregions except thalamus. Presynaptic dopaminergic availability decreased in a linear or quadratic fashion across the three groups (ET vs. PDconv vs. PD). The age at onset and duration of ET did not differ between pure ET and PDconv patients and did not influence the striatal monoamine status. The myocardium in PDconv patients was initially less denervated than in PD patients, but it degenerated more rapidly. These findings suggest that PDconv could be a distinctive subclass in which the pathobiology of PD interacts with that of ET in the early phase of the disease.
Similar content being viewed by others
Introduction
Essential tremor (ET) is a chronic progressive disease that can occur with or without non-motor features such as depression, cognitive deficits, and sleep disorders1,2. Accumulating evidence indicates that ET might be a neurodegenerative disease with an emphasis on the cerebellum, leading some to refer to it as ‘Purkinjopathy,’ despite some challenges to that view3,4,5.
Epidemiologic and neuropathologic reports also suggest a close relationship between ET and PD, although some disagreements exist6,7. Researchers argue that some ET patients develop PD, but not vice versa, indicating unidirectional disease conversion7,8. The pathophysiology of ET might predispose some individuals to PD by spreading of Lewy body pathology found in the brainstem8,9. Given that various sites of origin can lead to diverse PD phenotypes, the evolution of ET into PD might contribute to the development of tremor-dominant PD.
123I-Meta-iodobenzylguanidine (123I-MIBG) myocardial scintigraphy has been used to explain the pathobiology of PD by visualizing preclinical incidental Lewy body disease and rostrocaudal temporal gradients of Lewy bodies10,11. Denervated myocardium, which reflected central dopaminergic deterioration, was investigated to predict motor complications12. Those findings indicate that repeated 123I-MIBG myocardial scintigraphy measurements can be used to evaluate the overall pathologic burden of PD and its dynamic changes. Cardiac sympathetic integrity also has been employed to discriminate among neurodegenerative disorders13.
Based on the assumption that a subtype of ET could be prone to develop into PD8, this study investigated the interplay of ET and PD by assessing dopaminergic and noradrenergic biomarkers. PD patients with antedated ET (PDconv) might exhibit traits different from those in the pure forms of ET and PD; analyses of longitudinal changes in myocardial innervation, in particular, might show differences between PDconv and PD. If the pre-existing ET of PDconv shapes the course of cardiac denervation differently from that found in pure PD, the study of PDconv might show how ET influences PD pathobiology.
Results
Baseline characteristics
Tables 1 and 2 summarize the baseline characteristics of the study populations. PD patients with antedated ET were the oldest group at the time of diagnosis. The distribution of late-onset ET (LOET) did not differ between the ET and PDconv groups, nor did the preceding duration of ET. Within the LOET group (n = 39), the distribution of ET durations (≤5 vs. ≤10 vs. >10) did not differ (Fisher’s exact test; P-value 0.104). The proportions of males and females did not differ significantly among the groups. A family history of ET was observed more frequently in both the ET and PDconv groups than in the PD group. The PDconv and PD groups showed a higher association with PD inheritance than the ET group, but that tendency did not reach statistical significance. The median Unified Parkinson’s Disease Rating Scale (UPDRS) Part III score did not differ between PD groups.
The overall heart-to-mediastinum (H/M) uptake ratios were below the reference level for both the early and delayed ratios. The initial H/M uptake ratio was higher in PDconv patients than PD patients, particularly in the early phase (PDconv vs. PD: Early H/M ratio, 1.65 ± 0.37 vs. 1.51 ± 0.27; Delayed H/M ratio, 1.60 ± 0.41 vs. 1.48 ± 0.33; P-values, 0.024, 0.077, respectively; Supplementary Fig. 1). A significant portion of PD patients exhibited a high prevalence of abnormal denervation, especially when assessed using the early H/M uptake ratio.
Presynaptic dopamine transporter density characteristics across disease groups
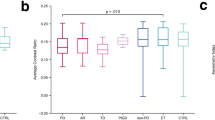
The results of an analysis of covariance of subregional presynaptic monoamine availability partialized by the age at diagnosis are summarized in Table 3. The standardized uptake value ratios (SUVRs) in both PDconv and PD patients were comparable, whereas ET patients had noticeably higher SUVRs. The SUVRs of the thalamus were similar across all three groups. The differences in SUVRs between ET and PDconv patients were not influenced by LOET status (Supplementary Table 1).
Polynomial contrasts were calculated to observe any trends across the disease groups. The caudate, putamen, globus pallidus, and thalamus subregions were analyzed (ET vs. PDconv vs. PD; Fig. 1 and Supplementary Table 2). Linear trends were found, signifying that the degree of monoamine density in PDconv patients was between those of ET and PD patients. The putamen and globus pallidus in PDconv patients manifested a sharp decrease of SUVRs compared with ET patients and decelerated decrements when contrasted with PD patients (quadratic trends).
a Bilateral caudate nucleus, b right caudate nucleus, c left caudate nucleus, d bilateral putamen, e right putamen, f left putamen, g bilateral globus pallidus, h right globus pallidus, i left globus pallidus, j bilateral thalamus, k right thalamus, l left thalamus. Polynomial contrasts, with age at diagnosis as a covariate, were performed in an analysis of covariance to identify between-group trends. The star represents the estimated means of each group with 95% confidence intervals. SUVR standardized uptake value ratio, Bilat. bilateral, ET essential tremor, PDconv Parkinson’s disease converter, PD Parkinson’s disease.
Longitudinal changes in the 123I-MIBG H/M ratio
A linear mixed model was applied to assess the temporal progression of myocardial scintigraphy. The estimated cardiac denervation trajectory was contrasted between PDconv and PD (Fig. 2).
a Estimated early H/M ratio, b estimated delayed H/M ratio. Shaded areas represent the standard error of the regression lines. The zero on the x-axis signifies the average of the cluster-wise mean-centered disease duration, which is equivalent to 32.3 months. The slope of each regression line was calculated while keeping other independent variables in the model constant. Linear mixed model: Cardiac denervation = Intercept + age at diagnosis + disease duration + disease duration x disease group (reference, PD). Age at diagnosis was grand-mean centered, and disease duration was cluster-wise mean-centered for the analyses. PD Parkinson’s disease, PDconv Parkinson’s disease converter.
A repeated measures mixed model of the 123I-MIBG H/M ratio revealed a significant inverse linear growth curve against disease duration (Early H/M ratio vs. Delayed H/M ratio; coefficients, −0.003 vs. −0.003; P-value, <0.001 vs. 0.002; Supplementary Table 3). The negative association between the H/M ratio and disease duration differed across disease subtypes, particularly in the early H/M ratio group (Early H/M ratio vs. Delayed H/M ratio; coefficients, −0.004 vs. −0.002; P-values, 0.005 vs. 0.163; Supplementary Table 3). The difference in early H/M ratios between PDconv and PD neared zero after approximately 77.3 months (x = 0 equals 32.3; the difference is estimated to be zero at x = 45, which is equivalent to a disease duration of 77.3 months; Fig. 2). The between-group contrast estimations in the delayed H/M ratio did not converge within the study period, but they did demonstrate a narrowing growth curve. The decay rate of PD was the same across H/M ratios, whereas PDconv had a steeper decline (Fig. 2). H/M ratios showed significant random variance among patients (Early H/M ratio vs. Delayed H/M ratio; Intercept; Intraclass correlation 0.758 vs. 0.776; χ2 (1) = 72.5 vs. 81.3; P-value, <0.001; Supplementary Table 3).
Discussion
In this study, central and peripheral monoamine integrity was compared among ET, PD with preceding ET (ET → PD; PDconv), and PD patients. Presynaptic dopaminergic transporter densities did not differ between the two PD subtypes, but ET patients maintained the highest monoamine densities in all subregions except the thalamus. Incremental presynaptic dopaminergic denervation gradients were observed in the striatum, globus pallidus, and thalamus across ET, PDconv, and PD patients. The myocardium in patients with pure PD showed more severe initial denervation than in those with PDconv, but the rate of denervation was estimated to be more rapid in the PDconv group. These attributes suggest that PDconv could be a distinctive subtype of PD in which the latent influence of ET affects its pathobiology during its progression.
PD patients with preceding ET were significantly older than PD patients, but the age at PD diagnosis was comparable to the age of ET diagnosis. This might reflect the biased nature of the disease entity: individuals with prolonged ET and older onset whose disease converted to PD would be included in the PDconv group. The older onset of PDconv accorded with previous studies7,14.
The tremor preceding PDconv might be assessed as merely aging-related tremor15, but ET should not be excluded based on age. Pathologic evidence failed to differentiate between ET with younger and older onset, and no suitable age cutoff has been established to separate ET based on age-related symptoms16,17. Our data about the age at PDconv onset refer to the age at which PD was confirmed, not ET. About 75% of this group endured more than five years of ET before PD developed, demonstrating comparable onset of ET in PDconv patients to pure ET patients. Therefore, age was not considered to confound the interpretation of data in this study.
Aging and the prolongation of ET were observed to affect clinical progression1,17,18,19. These features were important indicators of neurodegeneration because advanced age and disease duration correlated with increased prevalence, rapid progression, and pathologic changes4,17,19. The definition of LOET was carefully designated to imply the underpinning significance of age for both ET and PD. The cutoff age of 65 years was chosen to encompass the increased prevalence of PD, the incidence of PD from ET, and accelerated clinical progression6,17,19,20. In a sub-analysis, ET and PDconv did not differ in terms of late-onset age or duration of ET, which were presumed to have no influence on the interpretation of central and peripheral monoamine status. Moreover, disease duration at PD diagnosis did not differ significantly between PDconv and PD patients. This null difference was also not considered to affect the interpretations.
ET patients retained the highest dopamine transporter densities among the three groups in all subregions except the thalamus. This result was expected because a biochemical study measuring striatal tyrosine hydroxylase, a dopaminergic neuron marker, showed no difference in ET patients compared with controls21. When comparing PDconv and PD, similar uptake patterns were observed. This finding suggests that some ET patients with Lewy pathology could be triggered into PD pathobiology, resulting in analogous patterns8,9,22.
Imaging evidence has suggested that ET could be a neurodegenerative disorder. ET patients were found to have a subtle dopaminergic deficit throughout the whole striatum, and their estimates of dopamine uptake were located below the control level and above the level seen in PD7,22. Within the context of the unidirectional evolution of ET into PDconv and post-mortem reports that tremor-dominant PD had milder neurodegeneration8,9,23, trend analyses were conducted with PDconv placed between the pure forms of ET and PD. Those analyses revealed a descending gradient of central monoamine density, with accelerated denervation observed in the putamen and globus pallidum. The accelerating presynaptic degeneration of the putamen and globus pallidus as ET progressed to PD was in line with previous studies that showed the greatest deficits in those areas in PD22,23. These findings imply that some ET could be a pre-stage of PD that might surpass the threshold for syndromic advancement. By the time it does, nigrostriatal degeneration could reach the sub-level of pure PD, with some characteristic areas such as the putamen and globus pallidus degenerating at levels comparable to those in PD. These results might support the observation that ET could have a neurodegenerative cause as reflected in PDconv. Similar findings were observed when the age at diagnosis was squared and included as a covariate in the models to account for the additional effect of age (Supplementary Tables 4 and 5). The results might further strengthen that the observations were independent of age, despite the fact that the effect of age on the brain would be biologically inseparable.
The markers of cardiac denervation not only sub-classify endophenotypes of PD, but also reflect disease burden and deterioration13,24,25. In this research, longitudinal measurements of 123I-MIBG were used to assess the progression of PDconv and PD. The baseline peripheral noradrenergic integrity was less affected in PDconv, but its denervation progressed more rapidly, catching up with the denervated myocardium seen in pure PD after approximately six years. The milder impairment observed in PDconv patients indicated a lesser burden shaped by its development from ET, while its faster deterioration hinted at the transfer of influential pathobiology from ET to PD. A previous study asserted that PD patients suffered abrupt vulnerability five years after diagnosis due to nonlinear deterioration26. The progression in PDconv patients of our study appears to lag by one year compared to that of the previous report. The intersection between the two PD subtypes was interpreted to be delayed by the interplay of ET before its influence was gradually weakened as PD biology progressed.
Central dopaminergic (cross-sectional) and peripheral noradrenergic (longitudinal) biomarkers indicate that ET might act as a neurodegenerative disease that evolves into a milder form of PD; however, after the mid-phase of the disease span, PD pathology appeared to dominate the disease biology. This possible transition of dominance underpinned the accelerated degeneration of the myocardium seen in PDconv. The disputable transition could also support the potential neurodegenerative nature of some ET3,4,8,9. The transformation to PD would not be conceivable if converted ET did not already possess the same degenerative properties as PD.
This study has several strengths. The diagnosis of ET was strengthened by strictly including only individuals with at least three years of tremor. Previous diagnostic criteria had limitations that resulted in heterogeneity in ET classification, but this study applied the most recent criteria, which minimized this issue2,27. Additionally, the total disease duration of PD, which is 86 months on average, consolidates its diagnosis by clinically filtering out neurodegenerative PD-mimics. The mean duration of PDconv was shorter than that of PD but was sufficient to exclude atypical PD because the timely development of PD from ET substantiated the diagnosis of PD8. These durations adequately safeguard each diagnosis, so disease-specific homogeneity was maintained for these analyses. Unlike epidemiological studies, ET and PD patients with pre-existing ET were also substantiated by presynaptic dopamine transporter imaging, rather than relying solely on clinical diagnosis. The influential roles of older age and disease duration in neurodegeneration were sub-analyzed and interpreted in this study. The null difference between ET and preceding ET of PDconv is critical to the interpretation of monoamine comparisons, which could influence the pathophysiology of both diseases. Finally, this study used both cross-sectional and longitudinal objective biomarkers in its interpretation. The dopamine (intracranial) and noradrenergic (extracranial) markers are crucial surrogates of PD. These neuroimaging techniques could be used in clinical practice to not only differentiate diagnoses but also predict the risk of conversion and progression. However, the cost-effectiveness and clinical utility of those techniques need further substantiation in future studies.
This study also has a few limitations that restrict its generalizability. First, the registration of ET was performed retrospectively. The application of the recent consensus criteria and preserved monoamine density, however, substantiates its diagnosis. A prospective study that includes an examination of the non-motor prodromal features of PD in the ET cohort would further strengthen the argument. Longitudinal 123I-MIBG myocardial scintigraphy of pure ET would also provide in-depth insights into the neurodegenerative model if the data could be analyzed with other groups. Moreover, comparisons of associations between central and peripheral denervation in all three groups, not only cross-sectional but also longitudinal, would be of great value in substantiating the argument. Second, the current study did not include disease-specific clinimetrics. To our knowledge, no suitable tools for measuring disease severity are compatible with both ET and PD, to enable direct comparisons. Third, certain subtypes of PD, such as the tremor-dominant phenotype, might differ in their presentations. Contrasting ET with tremor-dominant PD would strengthen this study’s argument, but the sample size was not sufficient for further stratification. Subdividing tremor-dominant PD during enrollment is required in future studies. However, it is noteworthy that postural and action tremors also frequently occur in the non-tremor dominant types of PD. Caution is needed because no validated method is yet available that address this issue in subclassifying motor phenotypes. Fourth, the frequency of family history in ET was lower than in a previous estimation2. The retrospective design used in this study could explain that discordance. However, sporadic onset is more prevalent in older ET17,18, which might account for the lower prevalence of familial forms because the study population experienced older onset. Moreover, there is a scant epidemiologic inference that explicitly applied the most recent consensus diagnostic criteria, as was done in this study2,27. Fifth, this study did not include a control population. If the study had demonstrated differences with the control, it would have bolstered the argument. A future study that enrolls a control population is required. Lastly, the comparisons of presynaptic dopamine transporter density would have been more valuable if the disease duration of each group could be controlled in the model as the disease duration would be an important, indivisible biological factor that affects the integrity and receptor availability.
In conclusion, the Lewy variant subtype of ET might interact with early PD during its development, though its clinical picture might eventually be dominated by PD pathobiology. This PD subtype with preceding ET represents a distinct subclass of PD, and its existence might lend support to the disputable neurodegenerative model of ET.
Methods
This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital. All subjects provided written informed consent to participate. Research was conducted in accordance with relevant guidelines and regulations.
Patients
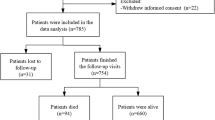
This study enrolled 127 de novo drug-naïve PD (including PD who converted from ET) and 51 ET patients diagnosed between July 2015 and November 2022. PD was diagnosed based on the UK Parkinson’s Disease Society Brain Bank criteria, and its diagnosis was supported with positron emission tomography (PET) imaging studies using 18F-N-(3-fluoropropyl)-2beta-carbon ethoxy-3beta-(4-iodophenyl) nortropane (18F-FP-CIT)28,29. PD patients exhibited decreased dopamine transporter uptake in the striatum, primarily in the posterior putamen (Supplementary Fig. 2). Among the PD patients enrolled, 32 had pre-existing ET.
Fifty-one ET patients were retrospectively registered, and their diagnoses were confirmed based on both previous criteria and the 2018 Consensus Statement27,30. None of the ET patients demonstrated any dopamine transporter uptake deficits in their PET results, which were visually analyzed by a nuclear medicine specialist (S.H.).
Patients were strictly defined as PD-converters (PDconv) if pre-existing ET evolved into PD (ET → PD). Co-existence without a clear temporal association between ET and PD was not allowed. All patients endured at least three years of ET without isolated head/voice tremors or mild parkinsonism before they were diagnosed with ET or PDconv27. Because the exact duration of the tremor could not often be recalled, the preceding period of ET was subclassified into three groups: 3–5 years vs. 6–10 years vs. >10 years. Patients were classified as LOET if ET first emerged after the age of 65 years. That designation was intended to encompass the previously estimated prevalence of both PD and older onset ET6,18,20,31. Patients were monitored every 2–6 months, and their diagnosis was confirmed by two neurologists after the last visit (S.-W.Y. and J.-S.K.).
The following baseline characteristics were investigated: age at diagnosis; sex; ET duration before the diagnosis of ET/PDconv; follow-up duration; total disease duration; history of hypertension, diabetes mellitus, or dyslipidemia; smoking status; and family history of first-degree relatives with ET or PD. Motor severity was assessed with UPDRS Part III examination.
Patients were excluded if they had any of the following indications: (1) any symptom or sign of atypical and/or secondary parkinsonism during follow-up; (2) magnetic resonance imaging results suggestive of atypical or secondary parkinsonism; (3) history of diabetic or autonomic neuropathy at the initial evaluation; (4) history of symptomatic stroke that could affect general cognition or performance; (5) history of heart failure or documentation of atrial fibrillation on electrocardiography; and (6) current use of medications known to influence the central dopaminergic, noradrenergic, and/or serotonergic systems at the time of diagnosis.
123I-MIBG myocardial scintigraphy
123I-MIBG scintigraphy was performed using a dual-head camera equipped with a low-energy, high-resolution collimator (Siemens), and data were collected 30 min (early) and 120 min (delayed) after injection of 111 MBq of 123I-MIBG. A static image was obtained with a 128 × 128 matrix. Regions of interest were manually drawn around the heart and mediastinum. Tracer uptake was measured within each region of interest to calculate the H/M ratio at the early and delayed time points. The reference values for abnormal early and delayed H/M ratios were defined as <1.70 and <1.78, respectively32. The washout rate was calculated as follows: [(early H/M ratio – late H/M ratio)/early H/M ratio] x 10032.
All patients were initially evaluated at the time of diagnosis, and they were re-assessed 2–3 times. The interval between clinical and cardiac assessments was 0.9 months (interquartile range, IQR 1.3).
Imaging acquisition and processing of 18F-FP-CIT PET
18F-FP-CIT PET images were assessed once at the initial diagnostic evaluation. Brain computed tomography (CT) and 18F-FP-CIT PET images were acquired using a Discovery STE PET/CT scanner (Discovery PET/CT 710, General Electric Healthcare, Waukesha, WI, USA). Three hours after an intravenous injection of 3.7MBq/kg of 18F-FP-CIT, brain CT scans were obtained for attenuation correction, followed by a 10-minute 18F-FP-CIT PET scan. PET images were reconstructed using a fully 3D-ordered subset-expectation maximization algorithm (VUE Point HD) with 4 iterations and 16 subsets. This reconstruction was combined with a Gaussian filter featuring a 3.3-mm full width at half maximum. The images were processed using a 256 × 256 matrix size, a pixel spacing of 0.976 cm * 0.976 cm, and a slice thickness of 3.27 mm.
Statistical Parametric Mapping 8 software (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK) and an in-house automated pipeline program implemented in MATLAB 2015a (MathWorks, Natick, MA, USA) were used for the processing of 18F-FP-CIT PET images. For the quantification of striatal uptake, a skull-stripped CT (ssCT) guided image processing method validated in the previous paper was utilized33,34. In brief, individual CT images were first skull-stripped by using the SPM segmentation tool with the probabilistic templates for the skull, brain, and cerebrospinal fluid (CSF). 18F-FP-CIT PET images were co-registered to brain CT images and then spatially normalized to MNI space with 1 mm isovoxel, with the parameter normalizing ssCT images to the ssCT template33,34. By overlaying the in-house striatal volume of interest (VOI) templates derived from the FreeSurfer 5.1 (Massachusetts General Hospital, Harvard Medical School; http://surfer.nmr.mgh.harvard.edu) on each spatially normalized PET image, the striatal subregional and cerebellar uptake binding values were measured. Finally, the regional standardized uptake values ratios (SUVRs) for each side of the caudate, putamen, globus pallidus, and ventral striatum were calculated with the cerebellar cortex as a reference. All the image processing was executed by the previously validated method in the previous works, which provided the relevant templates33,34.
The interval between clinical assessment and PET was 0.2 months (IQR 0.8). The interval between 123I-MIBG scintigraphy and 18F-FP-CIT PET was 0.0 months (IQR 0.9).
Statistical analyses
Statistical analyses were performed with jamovi software (version 2.3.22; retrieved from https://www.jamovi.org) for Mac. The software is a graphical user interface for R and includes a GAMLj module (version 2.6.6; https://gamlj.github.io) that allows linear mixed modeling.
Descriptive statistics and independent t-test, Welch’s t-test, analysis of variance (ANOVA), or Kruskal-Wallis test were performed for continuous variables. Categorical variables were examined using Fisher’s exact test. The Mantel-Haenszel test was used to detect any trends between nominal and ordinal scales.
Analysis of covariance (ANCOVA) was applied to assess the between-group differences in subregional SUVRs with age at diagnosis as a covariate. Polynomial contrasts were used to investigate trends across the disease groups: ET vs. PDconv vs. PD. A linear mixed model by restricted maximum likelihood estimation was performed to investigate the pattern of cardiac denervation progression. The intercept was set as a random effect to reflect individual variance. Multiple comparisons were adjusted using the Tukey and Dwass-Steel-Critchlow-Fligner methods for ANOVA and Kruskal-Wallis testing, respectively. In region-based analyses, the false discovery rate method was applied to the multiple ANCOVA tests. The Scheffe method was used when adjusting for post hoc pairwise comparisons among the disease groups. Statistical significance was defined as a two-tailed p-value < 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Anonymized data generated during the current study are available from the corresponding author on request from individuals affiliated with research or healthcare institutions.
References
Benito-Leon, J. Essential tremor: a neurodegenerative disease? Tremor Other Hyperkinet. Mov. 4, 252 (2014).
Welton, T. et al. Essential tremor. Nat. Rev. Dis. Prim. 7, 83 (2021).
Grimaldi, G. & Manto, M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov. Disord. 28, 1759–1761 (2013).
Louis, E. D. & Faust, P. L. Essential tremor pathology: neurodegeneration and reorganization of neuronal connections. Nat. Rev. Neurol. 16, 69–83 (2020).
Rajput, A. H., Adler, C. H., Shill, H. A. & Rajput, A. Essential tremor is not a neurodegenerative disease. Neurodegener. Dis. Manag. 2, 259–268 (2012).
Benito-León, J., Louis, E. D. & Bermejo-Pareja, F. Risk of incident Parkinson’s disease and parkinsonism in essential tremor: a population based study. J. Neurol. Neurosurg. Psychiatry 80, 423–425 (2009).
Thenganatt, M. A. & Jankovic, J. The relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat. Disord. 22, S162–S165 (2016).
Louis, E. D. & Ottman, R. Is there a one-way street from essential tremor to Parkinson’s disease? Possible biological ramifications. Eur. J. Neurol. 20, 1440–1444 (2013).
Louis, E. D. et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 130, 3297–3307 (2007).
Orimo, S. et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131, 642–650 (2008).
Borghammer, P. & Van Den Berge, N. Brain-first versus gut-first Parkinson’s disease: a hypothesis. J. Parkinsons Dis. 9, S281–S295 (2019).
Lee, J. E. et al. Cardiac sympathetic denervation can predict the wearing-off phenomenon in patients with Parkinson disease. J. Nucl. Med. 59, 1728–1733 (2018).
Orimo, S., Yogo, M., Nakamura, T., Suzuki, M. & Watanabe, H. (123)I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in α-synucleinopathies. Ageing Res. Rev. 30, 122–133 (2016).
Tan, E. K., Lee, S. S., S, F. C. & Lum, S. Y. Evidence of increased odds of essential tremor in Parkinson’s disease. Mov. Disord. 23, 993–997 (2008).
Deuschl, G., Petersen, I., Lorenz, D. & Christensen, K. Tremor in the elderly: Essential and aging-related tremor. Mov. Disord. 30, 1327–1334 (2015).
Louis, E. D. The evolving definition of essential tremor: What are we dealing with? Parkinsonism Relat. Disord. 46, S87–S91 (2018).
Louis, E. D. The roles of age and aging in essential tremor: an epidemiological perspective. Neuroepidemiology 52, 111–118 (2019).
Hopfner, F. et al. Early- and late-onset essential tremor patients represent clinically distinct subgroups. Mov. Disord. 31, 1560–1566 (2016).
Louis, E. D. et al. Older onset essential tremor: more rapid progression and more degenerative pathology. Mov. Disord. 24, 1606–1612 (2009).
Alty, J. E. & Kempster, P. A. A practical guide to the differential diagnosis of tremor. Postgrad. Med. J. 87, 623–629 (2011).
Shill, H. A. et al. Brain biochemistry in autopsied patients with essential tremor. Mov. Disord. 27, 113–117 (2012).
Isaias, I. U. et al. Striatal dopamine transporter abnormalities in patients with essential tremor. Nucl. Med. Commun. 29, 349–353 (2008).
Helmich, R. C., Hallett, M., Deuschl, G., Toni, I. & Bloem, B. R. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 135, 3206–3226 (2012).
Kim, J. S. et al. Normal ‘heart’ in Parkinson’s disease: is this a distinct clinical phenotype? Eur. J. Neurol. 24, 349–356 (2017).
Yoo, S. W. et al. Cardiac sympathetic burden reflects Parkinson disease burden, regardless of high or low orthostatic blood pressure changes. Npj. Parkinsons Dis. 7, 71 (2021).
Prange, S. et al. Age and time course of long-term motor and nonmotor complications in Parkinson disease. Neurology 92, e148–e160 (2019).
Bhatia, K. P. et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 33, 75–87 (2018).
Gibb, W. R. & Lees, A. J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 51, 745–752 (1988).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Brin, M. F. & Koller, W. Epidemiology and genetics of essential tremor. Mov., Disord. 13, S55–S63 (1998).
Bermejo-Pareja, F., Louis, E. D. & Benito-León, J. Risk of incident dementia in essential tremor: a population-based study. Mov. Disord. 22, 1573–1580 (2007).
Ryu, D. W. et al. Initial versus follow-up sequential myocardial 123I-MIBG scintigraphy to discriminate Parkinson disease from atypical parkinsonian syndromes. Clin. Nucl. Med. 44, 282–288 (2019).
Cho, H., Kim, J. S., Choi, J. Y., Ryu, Y. H. & Lyoo, C. H. A computed tomography-based spatial normalization for the analysis of [18F] fluorodeoxyglucose positron emission tomography of the brain. Korean J. Radiol. 15, 862–870 (2014).
Kim, J. S. et al. Feasibility of computed tomography-guided methods for spatial normalization of dopamine transporter positron emission tomography image. PLoS. One 10, e0132585 (2015).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01050492 awarded to S.-W.Y.), and also by the Ministry of Science, ICT and Future Planning (NRF-2017R1D1A1B06028086 awarded to J.-S.K.). This research was also supported by the “Korea National Institute of Health” research project (2021-ER1008-02 awarded to S.-W.Y. and J.-S.K.).
Author information
Authors and Affiliations
Contributions
S.-W.Y. and J.-S.K. contributed to the conception and design of the study; S.-W.Y., C.H.L., S.H., Y.K., J.-Y.Y. and J.-S.K. contributed to the acquisition and analysis of data; S.-W.Y., C.H.L., S.H., and J.-S.K. contributed to the interpretation of results, drafting the text and preparing figure; S.-W.Y. drafted the manuscript; C.H.L., S.H., Y.K., J.-Y.Y., and J.-S.K. revised the manuscript. S.-W.Y., and J.-S.K. obtained funding. All authors read and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoo, SW., Ha, S., Lyoo, C.H. et al. Exploring the link between essential tremor and Parkinson’s disease. npj Parkinsons Dis. 9, 134 (2023). https://doi.org/10.1038/s41531-023-00577-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-023-00577-y