Abstract
Myelofibrosis is a rare myeloproliferative neoplasm (MPN) with high risk for progression to acute myeloid leukemia. Our integrated genomic analysis of up to 933 myelofibrosis cases identifies 6 germline susceptibility loci, 4 of which overlap with previously identified MPN loci. Virtual karyotyping identifies high frequencies of mosaic chromosomal alterations (mCAs), with enrichment at myelofibrosis GWAS susceptibility loci and recurrently somatically mutated MPN genes (e.g., JAK2). We replicate prior MPN associations showing germline variation at the 9p24.1 risk haplotype confers elevated risk of acquiring JAK2V617F mutations, demonstrating with long-read sequencing that this relationship occurs in cis. We also describe recurrent 9p24.1 large mCAs that selectively retained JAK2V617F mutations. Germline variation associated with longer telomeres is associated with increased myelofibrosis risk. Myelofibrosis cases with high-frequency JAK2 mCAs have marked reductions in measured telomere length – suggesting a relationship between telomere biology and myelofibrosis clonal expansion. Our results advance understanding of the germline-somatic interaction at JAK2 and implicate mCAs involving JAK2 as strong promoters of clonal expansion of those mutated clones.
Similar content being viewed by others
Introduction
Myelofibrosis (MF) is a rare myeloproliferative neoplasm (MPN), with an incidence of ~1 per 100,000 per year1,2, characterized by the development of abnormal hematopoietic stem cell (HSC) clones and altered bone marrow microenvironment, leading to fibrosis3,4. Patients with MF typically develop cytopenia due to proliferation of aberrant HSC clones and hepatosplenomegaly due to extramedullary hematopoiesis4. Individuals with MF are at a high risk of developing acute myeloid leukemia5. MF can present as primary disease (primary MF) or progress from another MPN (secondary MF) such as polycythemia vera or essential thrombocythemia6.
Recurrent somatic driver mutations have been identified in MF, particularly in JAK2, MPL, and CALR7,8,9; current evidence also indicates a heritable component as well10,11. So far, the JAK2 46/1 haplotype and a single nucleotide polymorphism (SNP) in the TERT gene region have been established as predisposition alleles for MPNs, including both primary and secondary MF12,13,14,15,16, which have led to the use of JAK inhibitors and telomerase inhibitors as potential therapeutic agents for MF17,18,19. A genome-wide association study (GWAS) of MPN that included 136 MF patients identified additional MPN-associated loci (e.g., 3q21.3, 3q25.33, 6p21.31, 13q14.11, 18q11.2, 21q22.12)20. Current knowledge of MF genetic etiology is inferred from the MPN data.
In this work, we undertake an integrated approach to investigate the genetics of MF which includes analysis of germline variation, somatic JAK2 point mutations, somatic mosaic chromosomal alterations (mCAs), and leukocyte telomere length among MF patients who underwent hematopoietic cell transplantation (HCT) and reported to the Center for International Blood and Marrow Transplant Research (CIBMTR), representing a clinically important subset of MF patients. We report here six MF susceptibility loci, four of which replicate prior MPN findings at 9p24.1 (JAK2), 5p15.33 (TERT), 3q25.33 (IFT80), and 4q24 (TET2). We show germline variation at the 9p24.1 risk haplotype confers elevated risk of acquiring cis JAK2V617F mutations which are selectively retained by recurrent 9p24.1 mCAs. MF is dynamically associated with telomere length in which longer inherited telomere length increases MF risk, and clonal expansion of 9p24.1 mCAs markedly reduces measured telomere length.
Results
CIBMTR MF case characteristics
In total, 937 MF cases met the criteria for inclusion in our study, the majority of which were male (58.06%) and had DNA collected at an average age of 56.9 years (median = 58.4, IQR = 52.3–63.9; Supplemental Table 1). Most cases were primary (68.84%) MF, with intermediate 1 or 2 disease (49.30%) based on the Dynamic International Prognostic Scoring System (DIPSS)21 score. The average time from diagnosis to transplant in the full cohort was 63.5 months (median = 25.1, IQR = 9.0–88.3). Compared to primary MF cases, secondary MF cases were more likely to be female (54.45% vs. 36.28%) and had longer average time from diagnosis to transplant (122.8 months vs. 36.8 months).
MF susceptibility loci identified with estimated large effect size
We performed a GWAS using 827 MF cases and 4135 ancestry-matched cancer-free controls drawn from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Screening Trial22 (Methods section). The liability scale heritability of MF was estimated to be 11.4% (s.e.= 5.8%). The genomic inflation (λ) and intercept from linkage disequilibrium score regression (LDSC)23 showed minimal evidence for systematic inflation (λ = 1.02, LDSC intercept= 1.01, Fig. 1).
The association -log10 P-values are plotted for each tested genetic variant on the y-axis (two-sided, logistic regression) and chromosomal position on the x-axis. The nearest gene for each identified locus is labeled. The red line indicates the genome-wide significance threshold (5 × 10−8). The quantile–quantile plot displays observed versus expected −log10 P-values. Source data are provided as a Source Data file.
We analyzed 9,672,066 genotyped and imputed germline variants after filtering on control minor allele frequency (>0.5%) and imputation quality score (>0.7; Methods section) and identified six independent genome-wide significant loci (P < 5 × 10−8) (Supplemental Table 2 and Fig. 1), four of which replicated prior MPN findings at 9p24.1 (JAK2), 5p15.33 (TERT), 3q25.33 (IFT80), and 4q24 (TET2)20. We observed rs7851556 in 9p24.1 as the most significant variant (odds ratio (OR) = 2.39, 95% confidence interval (CI) = 2.13–2.68, P = 5.75 × 10−51), which is in strong linkage disequilibrium (LD) with the lead variant reported for MPN, rs1327494 (R2EUR = 0.95, D′EUR = 0.98)20 and located in an intron of JAK2, a gene which promotes cellular proliferation through the JAK/STAT pathway8,24. As chromosomal alterations are common at 9p24.1 (see mCA section below), we performed a sensitivity analysis in individuals with no detectable 9p24.1 mCAs to ensure no miscalling of germline variants resulting from mCAs in the 9p24.1 region; the rs7851556 signal remained significant (OR = 1.65, 95% CI = 1.41–1.92, P = 1.99 × 10−10), although the effect estimate was attenuated due to removal of MF cases that carried the JAK2 risk haplotype (see below section on JAK2–mCA relationship). rs7705526 had the strongest association (OR = 1.65, 95% CI = 1.48–1.84, P = 7.62 × 10−19) in the 5p15.33 locus. Previously identified in MPN20, this intronic variant is located in TERT, which encodes telomerase, the reverse transcriptase that extends telomeric DNA repeats, and has been associated with CD34+ to CD45+ ratio25. The 3q25.33 variant rs201009932 (OR = 5.78, 95% CI = 3.67–9.11, P = 4.06 × 10−14) (Supplemental Table 2) is in moderate LD with the MPN variant rs77249081 (R2EUR = 0.25, D′EUR = 1.00)20 and resides in an intron variant of IFT80, a part of the IFT complex essential for the assembly and maintenance of cilia as well as differentiation through the Sonic Hedgehog pathway26,27. At 4q24, rs1548483, located near TET2, a putative tumor suppressor gene and common somatic driver mutation in clonal hematopoiesis28,29, was significant (OR = 2.27, 95% CI = 1.71-3.01, P = 1.42 × 10−8); notably, this variant is in high LD with the previously identified MPN variant rs62329718 (R2EUR = 0.94, D′EUR = 1.00)20. Conditional analyses controlling for the lead GWAS variant for the 9p24.1 and 5p15.33 loci identified no evidence for additional independent signals (Supplemental Fig. 1).
Our GWAS identified two additional MF germline susceptibility loci: 6p21.32 and 17p13.1 (Supplemental Table 2 and Fig. 1). The 6p21.32 variant, rs28442287, (OR = 1.63, 95% CI = 1.38–1.93, P = 9.34 × 10−9) is a downstream variant of HLA-DRB9, within the human leukocyte antigen system30,31, and has been implicated in bone marrow related diseases32,33. The 17p13.1 variant, rs78378222, (OR = 4.47, 95% CI = 2.75–7.27, P = 1.56 × 10−9) is a 3′ UTR variant of the commonly mutated tumor suppressor gene TP53 that increases susceptibility to different types of cancer (e.g., skin basal cell carcinoma, prostate cancer, glioma, and lymphocytic leukemia)34,35,36,37. Future studies are warranted to validate these two MF germline susceptibility loci.
We performed stratified GWAS investigating primary MF (569 cases, 2845 controls) and secondary MF (258 cases, 1290 controls) to investigate possible differences (Supplemental Table 2), and no informative differences in association signals were observed between the two MF types (Supplemental Table 2, Supplemental Fig. 2). We further stratified secondary MF analyses into post-polycythemia vera MF (119 cases, 595 controls) and post-essential thrombocythemia MF (139 cases, 695 controls; Supplemental Table 3). We observed a strong genome-wide significant signal at 9p24.1 (JAK2) only in post-polycythemia vera MF (Supplemental Table 3 and Supplemental Fig. 3), which is consistent with prior reports of higher JAK2 involvement in patients with polycythemia vera38,39,40.
MF-associated germline variants near JAK2 increase expression
We performed expression quantitative trait locus (eQTL) analyses using genome-tissue expression (GTEx) whole blood data41 and identified eQTLs with JAK2 expression (e.g., rs7847141; OR = 1.05, 95% CI = 1.03-1.08, P = 3.69 × 10−5). Germline variants at the lead 9p24.1 locus additionally colocalized with JAK2 expression with rs7851556 having the highest Posterior Probability (PP; PP = 0.59; Supplemental Fig. 4)42. MF risk alleles were associated with increased levels of JAK2 expression, explaining 35% of the shared association signal at 9p24.1 (PPSNP = 0.35)42. Colocalization was also detected at 6p21.32 with HLA-DRB9 (colocalization PP = 0.36)43.
In analysis of whole blood GTEx expression data, a transcriptome-wide association study (TWAS)41,44 identified JAK2 (Z = 9.00, P = 2.18 × 10−19) and RP11-39K24.4 (Z = 10.78, P = 4.26 × 10−27) as significant genes (P < 3.59 × 10−6; Supplemental Figure 5), with positive JAK2 expression associated with increased MF risk. Conditional analyses were performed with predicted RP11-39K24.4 expression, and JAK2 remained an independent expression signal (rs7851556 conditional GWAS P = 9.90 × 10−26; Supplemental Fig. 6).
JAK2 germline risk haplotype confers elevated risk of cis JAK2 V617F mutations
Targeted PacBio long-read sequencing of the JAK2 region identified 562 (60.82%) individuals with the commonly observed activating JAK2V617F mutation, a known MPN driver mutation7,8. Secondary MF following a prior diagnosis of polycythemia vera or essential thrombocythemia were more likely to have the JAK2V617F mutation than primary MF cases (67.93% vs. 57.57%, P = 2.85 × 10−3), with post-polycythemia vera MF having the highest frequency of the JAK2V617F mutation (97.04% vs. 57.57%, P = 5.82 × 10−10). The estimated average mutation allelic fraction on background haplotypes was 62.54% (median = 69.76, IQR = 37.58-92.74), suggesting high clonal expansion of JAK2V617F mutated clones. MF cases carrying the risk allele (T) of rs7851556 (our lead GWAS SNP) were more likely to acquire a somatic JAK2V617F mutation (P = 9.41 × 10−14) (Supplemental Table 4). Additionally, individuals with the JAK2 46/1 germline risk haplotype (GGC, from rs3780367, rs10974944, rs12343867, Methods section) were substantially more likely to acquire JAK2V617F mutations (OR = 2.69, 95% CI = 2.02-3.58, P = 1.16 × 10−11), with 634 (68.61%) individuals carrying the JAK2 46/1 germline risk haplotype, and post-polycythemia vera MF (88.15%) having a higher frequency than both primary MF (65.98%) and post-essential thrombocythemia MF (66.45%). Furthermore, when examining phase information, we observed a strong cis relationship between the germline risk haplotype and JAK2V617F mutations acquired on the same risk haplotype (binomial P = 1.23 × 10−26; Supplemental Table 5), as previously observed in MPN patients12,13,14. These results were consistently observed when stratified by type of MF: primary MF (binomial P = 1.86 × 10−13), post-polycythemia vera MF (binomial P = 4.88 × 10−10), and post-essential thrombocythemia MF (binomial P = 1.90 × 10−4). Of the 562 individuals carrying a JAK2V617F mutation, 370 (65.84%) had the mutation in cis with the risk haplotype (binomial P = 7.68 × 10−33). Interestingly, during our JAK2V617F mutation calling (Methods section), we identified 5 individuals with evidence of the somatic mutation potentially acquired independently on both germline haplotypes (Supplemental Table 6) which were replicated in independent sequencing runs on new libraries. Future studies are needed to further explore the frequency of independent JAK2V617F mutations on both germline haplotypes in MF cases.
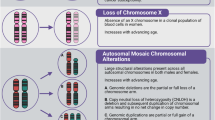
Chromosomal alterations are abundant in MF and preferentially expand JAK2 V617F clones
At least one detectable autosomal mCA was detected in 684 (73.31%) individuals which is in contrast to ~3% in population-based surveys45,46. An elevated frequency of mCAs in secondary MF compared to primary MF was also observed (78.35% vs. 71.03%, OR = 1.48, 95% CI = 1.06-2.05, P = 0.0196), with post-polycythemia vera MF having the highest frequency of mCAs (94.12% vs. 71.03%, OR = 6.53, 95% CI = 3.13-13.60, P = 5.54 × 10−7). Recurrent copy neutral loss of heterozygosity (CNLOH) events were detected on chromosome 9p (N = 298; 31.94%), and recurrent loss events were observed on chromosome 13q (N = 89; 9.54%) and 20q (N = 92; 9.86%; Fig. 2). Each GWAS susceptibility locus showed enrichment for mCAs compared to age and sex-matched cancer-free individuals in the UK Biobank45,46 (binomial P < 1 × 10−8; Supplemental Table 7), suggesting mCAs could clonally expand MF risk conferring alleles at susceptibility loci.
Since the JAK2 9p24.1 locus was the most notable in our GWAS and contains a hotspot of JAK2V617F mutations, we closely examined mCAs in this region. In total, 378 (40.51%) individuals had a detectable autosomal mCA across the 9p24.1 region. MF cases carrying the risk allele (T) of rs7851556 (our lead GWAS SNP) were more likely to have mCAs within the 9p24.1 region (P = 2.28 × 10−19), with CNLOH representing over 75% of the observed mCAs (N = 294; Supplemental Table 8). These results replicate previous reports of an association between 9p CNLOH and the JAK2 46/1 risk haplotype45. We performed allelic shift analyses in heterozygous individuals to test for a cis relationship between the JAK2 risk haplotype and mCAs spanning 9p24.145,46. Using highly correlated genotyped proxy SNPs to our lead GWAS SNP rs785155647, we found that the JAK2 risk haplotype is predominantly amplified by gains (23 of 28, binomial P = 9.12 × 10−4, proxy SNP rs10815167), retained by losses (14 of 16, binomial P = 4.18 × 10−3, proxy SNP rs2230724), and duplicated by CNLOH (51 of 59, binomial P = 9.05 × 10−9, proxy SNP rs10815167), providing strong evidence for preferential clonal expansion of mCAs with the JAK2 MF risk haplotype.
Using long-read PacBio sequencing data, we noted substantial allelic imbalance of heterozygous variants in the vicinity of JAK2 in individuals with mCAs spanning the region, providing independent confirmation of mCA calls in the region (Supplemental Fig. 7). Of the 374 individuals with detectable mCAs spanning JAK2 who also had long-read sequencing data, 97.86% had a JAK2V617F mutation, indicating a strong relationship between mCAs and JAK2V617F mutations (P = 4.37 × 10−80; Supplemental Table 9). To investigate the clonal evolutionary history of JAK2 mutations, we examined mutated cellular fractions of JAK2V617F and JAK2 mCAs. We found substantially higher JAK2V617F allelic fractions on background haplotypes compared to estimated mCA cellular fractions, suggesting the acquisition of a JAK2V617F mutation occurred prior to acquiring a JAK2 chromosomal alteration for the vast majority of MF cases (binomial P = 3.30 × 10−54; Supplemental Fig. 8). The estimated JAK2V617F allelic fractions and mCA cellular fractions may be lower than the true somatic fraction in the actual diseased myeloid cells because we used whole blood DNA from most of the patients. However, this would not affect the ratio of JAK2V617F allelic fraction to mCA cellular fraction. mCAs were also enriched across MPL and CALR gene positions (binomial P < 5 × 10−32; Supplemental Table 10), suggesting mCAs may also clonally expand other MPN driver mutations similar to the JAK2 locus, although this hypothesis needs to be further studied.
Inherited longer telomere length associated with MF risk
In addition to the JAK2 MF susceptibility region, the 5p15.33 locus near TERT implicates telomere length in MF risk. To evaluate the role of telomere length, we used a panel of germline variants associated with measured telomere length to develop a polygenic risk score (PRS) for inherited telomere length48 and observed a strong positive association between increased genetically-inferred leukocyte telomere length and increased MF risk (OR = 1.33, 95% CI = 1.23-1.44, P = 2.56 × 10−13). Of the 19 telomere-length associated variants imputed, seven (rs4691895, rs7705526, rs2853677, rs228595, rs62053580, rs75691080, and rs34978822) were nominally associated (P < 0.05) with MF risk (binomial P = 2.31 × 10−5). The allele related to longer telomere length was associated with increased risk of MF for five of these seven variants (Fig. 3 and Supplemental Table 11). The telomere length PRS was associated with the presence of JAK2V617F mutations (OR = 1.20, 95% CI = 1.04–1.37, P = 0.01) as well as mCAs (OR = 1.17, 95% CI = 1.01–1.36, P = 0.04), suggesting longer telomere length may afford cells the ability to clonally expand to detectable clonal fractions, after acquiring somatic mutations.
Estimates for the SNP-telomere length (Li et al.) and SNP-myelofibrosis associations are presented in Supplemental Table 11. A linear model estimated trend line and calculated 95% confidence interval around the trend (gray fill) are plotted (two-sided P = 5.48 × 10−4).
In a Mendelian randomization (MR) analysis to evaluate a directional relationship between the telomere length-associated variants and MF risk, the intercept from MR-Egger regression was non-significant (P = 0.65, Table 1) after removing five potentially pleiotropic variants (including the lead TERT variant identified in our GWAS) (Supplemental Table 11)49, suggesting no pleiotropy50. Each MR method utilized indicated a strong increasing effect between the telomere length genetic instrument and MF risk (Table 1 and Supplemental Fig. 9).
The genetic correlation between leukocyte telomere length and MF using LD score regression51 was estimated based on summary statistics from a published telomere length GWAS48 along with summary statistics from our MF GWAS. A marginally significant genome-wide genetic correlation was observed between telomere length and MF (LDSC r = 0.23, s.e.m. = 0.11, P = 0.038), similar in magnitude to what was reported for telomere length and MPN (LDSC r = 0.19, s.e.m. = 0.09, P = 0.037)20. Together, our results indicate longer telomere length is associated with increased risk of MF when evaluated by established leukocyte telomere length variants and genome-wide.
Telomere length attrition and mCA-induced clonal expansion
In an analysis of leukocyte relative telomere length (rTL) measured before HCT, on average, telomere length was significantly shorter among older subjects (P = 0.0037), peripheral blood mononuclear cell (PBMC)-derived DNA (P = 0.0031), secondary MF (P = 0.0016), and those with a longer time from diagnosis to HCT transplant (P = 0.0171) (Supplemental Table 12). To further investigate the reported association between telomere length and mCAs in the general population52, individuals with autosomal mCAs had significantly shorter rTL compared to individuals without autosomal mCAs (P = 7.93 × 10−5; Supplemental Table 13). Multivariable analyses demonstrated negative associations between rTL and the presence of any autosomal mCA (OR = 0.14, 95% CI = 0.04–0.44, P = 7.39 × 10−4; Table 2). These results were consistent when restricted to individuals with whole blood-derived DNA (OR = 0.19, 95% CI = 0.06–0.62, P = 6.05 × 10−3). A close inspection of mCAs in the 9p24.1 region demonstrated stronger attenuation of rTL (OR = 0.04, 95% CI = 0.01–0.19, P = 2.60 × 10−5), indicating individuals with autosomal mCAs, especially those spanning JAK2, exhibit signatures of clonal expansion. Multivariable Poisson regression also demonstrated rTL was negatively associated with a greater number of autosomal mCAs (incidence rate ratio= 0.33, 95% CI = 0.22–0.48, P = 1.98 × 10−8). Associations between measured telomere length and mCAs were consistent when stratified by chromosomal location (e.g., telomeric or interstitial) (Phet = 0.91) and copy number state (e.g., gain, loss, CNLOH, undetermined; Phet = 0.90, Table 2). In sensitivity analyses, adjusting for MF type, DIPSS score, and time to transplant, negative associations with rTL were observed both overall and stratified by chromosomal region and copy number state (overall OR = 0.19, 95% CI = 0.06–0.64, P = 7.44 × 10−3; Table 2).
Analyses of the rTL and mCA clonal fractions indicated a strong relationship in which increasing mCA cellular fraction was associated with a substantial decrease in measured rTL (β = −0.57, 95% CI = -0.74 to −0.39, P = 4.76 × 10−10), especially for mCAs spanning JAK2 (β = −1.17, 95% CI = -1.44 to −0.91, P = 1.39 × 10−16). Interestingly, we did not observe an association between measured rTL and JAK2V617F mutation presence (OR = 0.84, 95% CI = 0.29-2.42, P = 0.74), but we did observe an association with JAK2V617F clonal fraction (β = −1.11, 95% CI = −1.31 to −0.91, P = 2.26 × 10−25). After removing individuals with mCAs, rTL was no longer associated with JAK2V617F clonal fraction (β = −0.27, 95% CI = -0.71–0.16, P = 0.22), suggesting JAK2V617F alone may not be a strong driver of rapid clonal expansion in MF (Supplemental Table 14). These findings indicate mCAs, many of which selectively retain or duplicate JAK2V617F mutations, promote rapid clonal expansion of mutated clones resulting in significant reductions in telomere length.
Discussion
We conducted an integrated genomic characterization of MF by investigating both germline susceptibility alleles and somatic events, particularly mosaic chromosomal events. Our study supports a key role of JAK2 events as a critical driver of MF and underscores important interactions of germline susceptibility with JAK2V617F mutations and mCAs12,13,14,45. We observed that 68% of MF cases carry at least one germline JAK2 risk allele, whereas somatic events are also critical to MF development - in our study 61% of MF cases carried a somatic JAK2V617F mutation, and 41% with an mCA spanning JAK2. Overall, we observed that ~85% of cases involve JAK2. Using independent sequencing runs, we identified five individuals with evidence of acquiring independent JAK2V617F mutations on both germline haplotypes. The frequency and consequences of independent JAK2V617F mutations on both germline haplotypes should be further studied. In a GWAS, we identified six MF susceptibility loci (one including JAK2 on 9p24.1) with two independent signals unique to MF20. The estimated MF heritability is 11.4% (s.e. = 5.8%), and all MF susceptibility loci had high effect sizes (OR > 1.6) relative to those typically found by GWAS, suggesting a strong germline component at multiple genomic loci for MF risk. Global assessment of mCAs demonstrated a high frequency in MF cases and demonstrated enrichment at each GWAS locus and across other MPN driver mutations, providing potential evidence for genome-wide germline-somatic interactions beyond that observed for JAK2.
Our integrated study demonstrates a complex germline-somatic interaction in MF patients at the 9p24.1 susceptibility locus, which confirmed prior findings and revealed insights into MF etiology. The observed effect of telomere length on disease risk in concert with 9p24.1 indicates that the presence of a germline susceptibility locus involving JAK2 directly influences the probability of developing a somatic event in the same region, presumably on the same haplotype (Fig. 4). We observed that individuals with the germline JAK2 risk haplotype tagged by rs7851556 were predisposed to acquiring a somatic JAK2V617F mutation in cis, as previously reported12,13,14. The cis relationship could not be checked directly due to the distance between our lead GWAS variant (rs7851556) and the JAK2V617F mutation, but this relationship is supported by the high LD between rs7851556 and variants (rs3780367, rs10974944, rs12343867) in the 46/1 risk haplotype (R2 > 0.93). We also observed mCAs lead to preferential over-representation of this risk haplotype containing the JAK2V617F mutation45. Prior studies demonstrate JAK2 is a strong activator of cellular growth and proliferation8,24, promotes cell surface localization53, is activated in response to a variety of cytokines54,55,56, and induces bone marrow fibrosis57. Altered JAK2 activity conferred by the germline JAK2 risk haplotype, JAK2V617F activating mutation, and 9p24.1 mCAs could lead to a cellular phenotype characterized by increased clonal expansion. While we were able to connect germline susceptibility and somatic mutations in cross-sectional data from MF patients, future longitudinal assessment will be key in follow-up of our study. Likewise future studies characterizing germline functional variation are needed to better understand how the germline JAK2 MF susceptibility locus leads to altered JAK2 expression and acquisition of JAK2V617F mutations.
Both longer inherited telomere length and the germline JAK2 risk haplotype tagged by rs7851556 are associated with increased risk of myelofibrosis. The germline JAK2 risk haplotype further predisposes to somatically acquire the JAK2V617F mutation in cis, and mosaic chromosomal alterations in the 9p24.1 region lead to over-representation of the germline risk haplotype and JAK2V617F somatic mutation. We hypothesize that increasing JAK2 activity underlies each of these processes which leads to increased clonal expansion and proliferation, and accelerated telomere length shortening in myelofibrosis patients.
The MF susceptibility signal near TERT together with our PRS, MR, and genetic correlation analyses, suggest that polygenetic effects of genetic variants associated with telomere length are important contributors to MF predisposition, especially in light that telomere length is required for hematopoietic stem cell self-renewal58. A prior study on a smaller number of variants suggested longer genetically determined telomere length, as derived by telomere length associated variants, increases MPN risk59. Our observations further suggest inherited genome-wide variation promoting longer telomeres could fuel MF clonal expansion, especially JAK2-mCA mediated expansion. A downstream consequence of this increased cellular proliferation is shorter telomeres as observed in the reduced rTL in patients with mCAs. As previously demonstrated in MPN patients, and replicated in our MF study, measured rTL was not associated with JAK2V617F mutation presence, but was inversely associated with JAK2V617F clonal fraction60. Our results indicate that mCAs are driving this observed association between measured rTL and JAK2V617F clonal fraction, suggesting mCAs, particularly mCAs spanning JAK2, promote clonal expansion in MF. A recent trial identified that telomerase inhibitor therapy may be an effective treatment for MF; although the mechanism is poorly understood19. It is possible that patients with MF and JAK2 mCAs who have expanded clones may benefit from telomerase inhibition through senescence or apoptosis of these MF clones, potentially leading to better clinical outcomes61. Future functional and clinical investigations, using the flow-FISH assay to measure leukocyte cell-type specific telomere length, are required to test this hypothesis. Furthermore, our study included MF patients who received HCT between 2000 and 2016, and may represent a specialized clinical subset, which may not generalize to all MF patients.
The large number of cases for this rare disease allowed for investigation of potential differences between primary and secondary MF, and secondary subtype. We noted no major differences in germline susceptibility by type of MF, and observed a significant signal at 9p24.1 (JAK2) in post-polycythemia vera MF. We did observe differences in their somatic profiles, with both JAK2V617F mutations and mCA acquisition more commonly observed in secondary MF compared to primary MF. This observation underscores a higher somatic load of mutations in secondary MF, which could be due to prior MPN diagnosis and longer disease duration, and is in agreement with prior reports38,39,40. This higher frequency of somatic mutations could have implications for MF treatment approaches, with JAK2 inhibitors potentially inducing a greater response in individuals with higher mutation allele burden62.
In conclusion, our integrated genomic investigation of MF highlights a strong inherited genetic component of MF, which, in turn, influences the somatic profile of abundant mCAs in MF. These findings underscore the critical role of JAK2 and telomere biology in MF susceptibility, which could inform avenues for improved clinical management of MF.
Methods
Study population
This study utilized blood samples and clinical information from the Center for International Blood and Marrow Transplant Research (CIBMTR) database and repository (https://www.cibmtr.org). The study was approved by the National Marrow Donor Program institutional review board. All patients provided written informed consent for the research use of their samples and clinical data. Patients eligible for inclusion were those who underwent hematopoietic cell transplantation (HCT) for primary idiopathic or secondary MF between 2000 and 2016 and have a pre-HCT blood sample available for genomic analysis. Blood samples, from either whole blood or peripheral blood mononuclear cells (PBMCs), were collected within 30 days before administering HCT conditioning regimen. In total, 937 MF patients met the criteria for inclusion in our study, of which 863 (92.1%) contributed whole blood and 74 (7.9%) PBMCs (Supplemental Table 1).
SNP genotyping and quality control assessment
We used Qiagen QIAsymphony for DNA extraction. All genomic laboratory work included in this study was conducted by the NCI Cancer Genomics Research Laboratory. Genotyping on MF cases was completed using the Illumina Infinium Global Screening Array-24v1-0. Genotypes were called using standard Illumina microarray data analysis workflows. Controls were selected from 62,880 previously genotyped cancer-free individuals within the Prostate, Lung, Colorectal, and Ovarian (PLCO) Screening Trial22, who were genotyped on the same array as the cases. To minimize technical artifacts, genotyping quality control steps were performed on the joint set of MF cases and PLCO controls. Standard quality control checks were performed to ensure high completion rates (≥95%), no sample contamination, sex concordance, no unexpected duplicates or replicates, normal rates of heterozygosity, and no instances of high relatedness (IBD < 0.2). Genetic ancestry was inferred using SNPWEIGHTS63, which estimates the percentage of European, West African, and East Asian ancestry for each subject. After filtering based on quality control steps and European ancestry (>80%), 833 MF cases and 56,929 cancer-free controls were eligible for the GWAS analysis.
Genome-wide association study
We genetically matched the MF cases and PLCO controls using PCAmatchR to minimize the effects of confounding due to potential population stratification bias64. For the combined set of cases and controls, we extracted linkage disequilibrium filtered variants (R2 < 0.1) from the array manifest file (GSA-24v2-0_A1) and performed principal component (PC) analyses using PLINK65. The first 20 PCs and eigenvalues were used to 1:5 match MF cases and PLCO controls based on eigenvalue weights (Supplemental Figure 10). After matching, six MF cases did not have well matching controls and were removed from the analysis (Supplemental Figure 10), resulting in a total of 827 cases and 4135 genetically matched controls. Imputation was carried out using the Michigan Imputation Server with the TOPMed reference panel (https://imputationserver.sph.umich.edu). Following imputation, association analyses were conducted under an additive model using SNPTEST (https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html). Any PCs that remained significant after matching were additionally adjusted for in the GWAS association tests. For the main GWAS, germline variants were filtered based on control minor allele frequency (>0.5%) and imputation quality score (>0.7). In GWAS stratified by MF type (Supplemental Table 1), variants were filtered based on a more stringent control minor allele frequency (>5%) to remove potential spurious associations arising due to small sample size. Manhattan plots for results visualization were generated using the “qqman” and “hudson” R packages66,67.
Linkage disequilibrium score regression
Linkage disequilibrium score regression (LDSC) was used to estimate the narrow-sense heritability estimate of MF risk as well as compute genetic correlations51. We computed LD scores from European individuals within 1000 Genomes Project data68. Variants were filtered by minor allele frequency (>1%); 5,956,602 variants were retained for all calculations51. Heritability calculations were based on a MF sample prevalence of 0.1667 in our GWAS and a population prevalence of 5.69 × 10−51.
Colocalization of MF GWAS and QTLs
Colocalization analyses of the MF GWAS signals were performed using expression quantitative trait locus (eQTL) whole blood genome-tissue expression (GTEx) (version 8) data41 with eCAVIAR and Hyprcoloc42,43. We extracted GWAS and QTL summary statistics 100 kb upstream and downstream of the lead GWAS SNP as input for colocalization analyses, with the exception of chromosome 6, which was limited to 10 kb, due to the number of variants in the region. For each MF GWAS locus, eCAVIAR colocalization posterior probability (colocalization PP > 0.01)43 or Hyprcoloc posterior probability (PP > 0.50)42 was used to identify colocalization.
Transcriptome-wide association study
We performed a transcriptome-wide association study (TWAS) on the MF GWAS summary statistics using FUSION (http://gusevlab.org/projects/fusion/)44. TWAS was performed using GTEx whole blood gene expression data (version 7)41 by imputing the gene expression phenotypes for GWAS data overlapping the LD reference panel (1000 Genomes European)51. FUSION was used to test associations using pre-computed expression reference weights. A Bonferroni corrected level of significance of 3.59 × 10−6 (0.05/13,909 GTEx available features) was used to assess statistical significance.
Targeted PacBio sequencing and mutation calling
Targeted PacBio Single Molecule Real-Time (SMRT) sequencing was performed on MF cases to detect JAK2V617F mutations. We followed the SMRT sequencing process protocols as described in the PacBio sequencing manual (https://www.pacb.com/wp-content/uploads/Procedure-checklist-Preparing-SMRTbell-libraries-using-PacBio-barcoded-M13-primers-for-multiplex-SMRT-sequencing.pdf). Briefly, a 2-step PCR, using 10 ng of DNA as input, was conducted. In step 1, template-specific primers amplified the region of chr9:5067166-5074380 in GRCh38. Next, in step 2, unique barcode sequences were incorporated onto each sample, using universal tags, for multiplexing. Products were purified and quantified with an additional normalization step to ensure equal concentration of each sample prior to pooling, and hairpin adapters were ligated to the ends of each amplicon pool (up to 384 samples) during the SMRT bell library preparation. Each pooled library had primer annealing and polymerase binding performed according to the protocol and sequenced on 1 Sequel II SMRT Cell 8 M. After sequencing, Circular Consensus Sequencing (CCS) read generation was performed with set criteria, including a minimum of 3 passes and accuracy of 99%. CCS reads were used as input to lima (https://github.com/PacificBiosciences/barcoding) to demultiplex the pooled samples, and then aligned to GRCh38 using pbmm (https://github.com/PacificBiosciences/pbmm2).
Haplotypes were then generated based on aligned CCS reads, including 3 SNPs within the JAK2 46/1 haplotype (rs3780367, rs10974944, rs12343867) and the JAK2V617F mutation (chr9:5073770). For each MF case, any haplotype with frequency < 1% was removed. Additionally, cases were removed if they did not have sufficient DNA quantity (N = 1), total number of CCS reads < 1000 (N = 1), more than two germline haplotypes (based on rs3780367, rs10974944, rs12343867; N = 4), or JAK2V617F mutations called on more than one germline haplotype (N = 49). We repeated sequencing of 53 individuals that failed QC; 1 individual did not have sufficient DNA for resequencing. We also resequenced an additional 43 MF cases who passed the above QC steps to further validate our PacBio sequencing and QC procedure. We followed the same protocol as detailed above for the resequenicng effort, with new aliquots of the genomic DNA taken and reamplified to generate a new library. Again, haplotypes were generated based on aligned CCS reads, including the 3 SNPS within the JAK2 46/1 haplotype and the JAK2V617F mutation, and any haplotype with frequency < 1% was removed. We observed a high degree of concordance (>93%) between the first and second sequencing efforts for the subjects who originally passed our QC procedure (N = 43). Of those individuals that originally failed our sequencing QC (N = 53), we removed cases that were again identified with more than two germline haplotypes (N = 2) or JAK2V617F mutations called on more than one germline haplotype (N = 5). Overall, 924 MF cases were retained for downstream analyses with average read depth of 8,623.2 (median = 6872, min = 3155, max = 27,402).
Mosaic chromosomal alterations QC and calling
Using genotype data, MF cases were called for mCAs. Before mCA calling, the same GWAS quality control measures were performed, with completion rates relaxed to ≥90%. MoChA software (https://github.com/freeseek/mocha) was used to detect somatic copy number aberrations, a similar approach has been previously implemented45,46. Briefly, MoChA utilizes hidden Markov models (HMM) to integrate B allele frequency (BAF) and log2 R ratio (LRR), and leverage haplotype information to detect subtle imbalances between maternal and paternal allelic fractions in a cell population. The BAF was calculated as the ratio of signal intensity between two alleles at each genotyped variant in relation to estimated genotype clusters and was used to detect allelic imbalances as well as calculate the proportion of cells with a deletion, duplication, and copy neutral loss of heterozygosity (CNLOH)69. Contiguous genomic stretches of BAF values for heterozygous SNPs that deviate from 0.5 are indicative of mosaic chromosomal alterations. LRR calculates the log base 2 of the ratio of observed total signal intensities to expected signal intensities for a genotyped variant69. Contiguous genomic stretches with LRR > 0 indicate copy gain, <0 indicate loss and around 0 indicate CNLOH. Furthermore, phase data was used to detect subtle over or under representation of haplotypes indicating the presence of a mCA. Eagle2, a software utilizing a population-based approach to infer phase (1000 Genomes reference panel), was used to infer haplotypes70,71.
Chromosome 9 had a high frequency of CNLOH events with high cell fractions. These events are poorly detected by phase-based methods, due to lack of heterozygous sites in the event region. Although MoChA applies a non-phased-based model to detect high-level mosaic events, this approach only detects mosaic gains or losses, not CNLOH. Therefore, we additionally applied a custom software pipeline that utilized the BAFSegmentation software (http://baseplugins.thep.lu.se/wiki/se.lu.onk.BAFsegmentation) to recover additional events not detected in MoChA72. Chromosome 9 was segmented for mosaic events using circular binary segmentation on BAF values. All potential events from both detection methods were plotted and visualized, and false positive calls were excluded from the analysis based on manual review of each plot.
Samples with called mCAs on the merged set from MoChA and BAFSegmentation were classified by copy number state (gain, loss, CNLOH, or undetermined events), cellular fraction (the percentage of sampled leukocytes carrying the detected mCA), and chromosomal region (e.g., telomeric, interstitial, or whole chromosome event). Events that only occurred around telomeric ends (±1 Mb from chromosome ends) were defined as telomeric, events that spanned an entire chromosome were defined as whole chromosome events, and all other events were defined as interstitial.
Leukocyte telomere length polygenetic risk score and Mendelian randomization
We generated a telomere length polygenic risk score (PRS) by aggregating variants previously found to be associated with measured telomere length in GWAS into a weighted genetic instrument (Supplemental Table 11)48. The inherited telomere length PRS was standardized to have mean 0 and standard deviation 1. PRS analyses were adjusted for ancestry principal components within MF risk analyses, and sex, age, age-squared, genetic ancestry, and DNA source within JAK2V617F mutation and mCA analyses. Mendelian randomization (MR) analyses were performed using the telomere length associated variants48 within the “Mendelian Randomization” R package50. We utilized the “GLIDE” R package to investigate any potential evidence of pleiotropy among the included variants49. Any variant found to have heterogeneous effects between measured telomere length and MF (false discovery rate <0.2) was removed from the analysis49.
Telomere length measurement
We utilized a modified qPCR assay to measure leukocyte relative telomere length (rTL) in pre-HCT extracted DNA73. The qPCR assay calculates the ratio between telomeric repeat copy number (T) and that of a single reference gene (beta-globin gene; 36B4) (S). Relative T/S was calculated in relation to a reference curve and final measurements were exponentiated to assure normality. All telomeric and 36B4 reactions were measured in triplicate, and the mean was used for final calculations. A total of 927 patients had DNA available for telomere length measurement. The overall completion rate was high (98.9%), resulting in 916 patients with available measured telomere length. An internal control, which is used to standardize results within the project, had an overall coefficient of variation (CV) of 2.96% and the intraclass correlation coefficient (ICC) and its 95% confidence interval for study technical replicates was 0.982 (0.975, 0.986). All telomere length analyses adjusted for sex, age, age-squared, genetic ancestry, and DNA source, unless otherwise noted.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Raw SNP genotyping data and raw targeted PacBio sequencing data that were generated on this study from the myelofibrosis individuals is available on dbGaP under accession number phs002635.v1.p1. CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality. The mCA calls and phenotypic UK Biobank data used in this study, which were used under license, are available from: http://www.ukbiobank.ac.uk/. Genotype data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Screening Trial is available on dbGaP under accession number phs001286.v2.p2. Source data are provided with this paper.
References
Mehta, J., Wang, H., Iqbal, S. U. & Mesa, R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk. lymphoma 55, 595–600 (2014).
Moulard, O. et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur. J. Haematol. 92, 289–297 (2014).
Vardiman, J. W. et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114, 937–951 (2009).
Tefferi, A. Primary myelofibrosis: 2014 update on diagnosis, risk‐stratification, and management. Am. J. Hematol. 89, 915–925 (2014).
Mesa, R. A. et al. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood 105, 973–977 (2005).
Barosi, G. et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 22, 437–438 (2008).
Tefferi, A. & Pardanani, A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncol. 1, 97–105 (2015).
Tefferi, A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 24, 1128–1138 (2010).
Nangalia, J. et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 369, 2391–2405 (2013).
Sud, A. et al. Familial risks of acute myeloid leukemia, myelodysplastic syndromes, and myeloproliferative neoplasms. Blood 132, 973–976 (2018).
Landgren, O. et al. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24 577 first-degree relatives of 11 039 patients with myeloproliferative neoplasms in Sweden. Blood 112, 2199–2204 (2008).
Jones, A. V. et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat. Genet. 41, 446–449 (2009).
Kilpivaara, O. et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2 V617F-positive myeloproliferative neoplasms. Nat. Genet. 41, 455–459 (2009).
Olcaydu, D. et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat. Genet. 41, 450–454 (2009).
Oddsson, A. et al. The germline sequence variant rs2736100_C in TERT associates with myeloproliferative neoplasms. Leukemia 28, 1371–1374 (2014).
Trifa, A. P. et al. TERT rs2736100 A> C SNP and JAK 2 46/1 haplotype significantly contribute to the occurrence of JAK 2 V617F and CALR mutated myeloproliferative neoplasms–a multicentric study on 529 patients. Br. J. Haematol. 174, 218–226 (2016).
Palmer, J. & Mesa, R. The role of fedratinib for the treatment of patients with primary or secondary myelofibrosis. Ther. Adv. Hematol. 11, 2040620720925201 (2020).
Schieber, M., Crispino, J. D. & Stein, B. Myelofibrosis in 2019: moving beyond JAK2 inhibition. Blood Cancer J. 9, 1–11 (2019).
Tefferi, A. et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N. Engl. J. Med. 373, 908–919 (2015).
Bao, E. L. et al. Inherited myeloproliferative neoplasm risk affects haematopoietic stem cells. Nature 586, 769–775 (2020).
Passamonti, F. et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood 116, 2857–2858 (2010).
Prorok, P. C. et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control. Clin. Trials 21, 273S–309S (2000).
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Ghoreschi, K., Laurence, A. & O’Shea, J. J. Janus kinases in immune cell signaling. Immunol. Rev. 228, 273–287 (2009).
Lopez de Lapuente Portilla, A. et al. Genome-wide association study on 13 167 individuals identifies regulators of blood CD34+cell levels. Blood 139, 1659–1669 (2022).
Irigoín, F. & Badano, L. J. Keeping the balance between proliferation and differentiation: the primary cilium. Curr. Genomics 12, 285–297 (2011).
Yang, S. & Wang, C. The intraflagellar transport protein IFT80 is required for cilia formation and osteogenesis. Bone 51, 407–417 (2012).
Tefferi, A. et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia 23, 905–911 (2009).
Buscarlet, M. et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. J. Am. Soc. Hematol. 130, 753–762 (2017).
Klein, J. & Sato, A. The HLA system. N. Engl. J. Med. 343, 702–709 (2000).
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19, 133–150 (2019).
Gragert, L. et al. Fine-mapping of HLA associations with chronic lymphocytic leukemia in US populations. Blood 124, 2657–2665 (2014).
Savage, S. A. et al. Genome-wide Association Study identifies HLA-DPB1 as a significant risk factor for severe aplastic anemia. Am. J. Hum. Genet. 106, 264–271 (2020).
Stacey, S. N. et al. A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat. Genet. 43, 1098–1103 (2011).
Wang, Y. et al. A novel TP53 variant (rs78378222 A> C) in the polyadenylation signal is associated with increased cancer susceptibility: evidence from a meta-analysis. Oncotarget 7, 32854 (2016).
Enciso-Mora, V. et al. Low penetrance susceptibility to glioma is caused by the TP53 variant rs78378222. Br. J. Cancer 108, 2178–2185 (2013).
Rashkin, S. R. et al. Pan-cancer study detects genetic risk variants and shared genetic basis in two large cohorts. Nat. Commun. 11, 4423 (2020).
Rotunno, G. et al. Epidemiology and clinical relevance of mutations in postpolycythemia vera and postessential thrombocythemia myelofibrosis: A study on 359 patients of the AGIMM group. Am. J. Hematol. 91, 681–686 (2016).
Masarova, L. et al. Patients with post-essential thrombocythemia and post-polycythemia vera differ from patients with primary myelofibrosis. Leuk. Res. 59, 110–116 (2017).
Palandri, F. et al. Differences in presenting features, outcome and prognostic models in patients with primary myelofibrosis and post-polycythemia vera and/or post-essential thrombocythemia myelofibrosis treated with ruxolitinib. New perspective of the MYSEC-PM in a large multicenter study*. Semin. Hematol. 55, 248–255 (2018).
Lonsdale, J. et al. The genotype-tissue expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Foley, C. N. et al. A fast and efficient colocalization algorithm for identifying shared genetic risk factors across multiple traits. Nat. Commun. 12, 1–18 (2021).
Hormozdiari, F. et al. Colocalization of GWAS and eQTL signals detects target genes. Am. J. Hum. Genet. 99, 1245–1260 (2016).
Gusev, A. et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 48, 245–252 (2016).
Loh, P.-R. et al. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature 559, 350–355 (2018).
Loh, P.-R., Genovese, G. & McCarroll, S. A. Monogenic and polygenic inheritance become instruments for clonal selection. Nature 584, 136–141 (2020).
Machiela, M. J. & Chanock, S. J. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31, 3555–3557 (2015).
Li, C. et al. Genome-wide association analysis in humans links nucleotide metabolism to leukocyte telomere length. Am. J. Hum. Genet. 106, 389–404 (2020).
Dai, J. Y. et al. Diagnostics for pleiotropy in Mendelian randomization studies: global and individual tests for direct effects. Am. J. Epidemiol. 187, 2672–2680 (2018).
Yavorska, O. O. & Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46, 1734–1739 (2017).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236 (2015).
Brown, D. W. et al. Genetically predicted telomere length is associated with clonal somatic copy number alterations in peripheral leukocytes. PLoS Genet. 16, e1009078 (2020).
Royer, Y., Staerk, J., Costuleanu, M., Courtoy, P. J. & Constantinescu, S. N. Janus kinases affect thrombopoietin receptor cell surface localization and stability*. J. Biol. Chem. 280, 27251–27261 (2005).
Witthuhn, B. A. et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 74, 227–236 (1993).
Silvennoinen, O. et al. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc. Natl Acad. Sci. USA 90, 8429–8433 (1993).
Parganas, E. et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93, 385–395 (1998).
Kennedy, J. A. et al. Expression of TEL-JAK2 in primary human hematopoietic cells drives erythropoietin-independent erythropoiesis and induces myelofibrosis in vivo. Proc. Natl Acad. Sci. USA 103, 16930–16935 (2006).
Morrison, S. J., Prowse, K. R., Ho, P. & Weissman, I. L. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity 5, 207–216 (1996).
Giaccherini, M. et al. Genetic polymorphisms associated with telomere length and risk of developing myeloproliferative neoplasms. Blood Cancer J. 10, 1–7 (2020).
Ruella, M. et al. Telomere shortening in Ph-negative chronic myeloproliferative neoplasms: a biological marker of polycythemia vera and myelofibrosis, regardless of hydroxycarbamide therapy. Exp. Hematol. 41, 627–634 (2013).
Mascarenhas, J. et al. Telomerase activity, telomere length and HTERT expression correlate with clinical outcomes in higher-risk myelofibrosis (MF) relapsed/refractory (R/R) to janus kinase inhibitor treated with imetelstat. Hemasphere 4, 1098 (2020).
Barosi, G. et al. JAK2V617F allele burden ⩾50% is associated with response to ruxolitinib in persons with MPN-associated myelofibrosis and splenomegaly requiring therapy. Leukemia 30, 1772–1775 (2016).
Chen, C.-Y. et al. Improved ancestry inference using weights from external reference panels. Bioinformatics 29, 1399–1406 (2013).
Brown, D. W., Myers, T. A. & Machiela, M. J. PCAmatchR: a flexible R package for optimal case–control matching using weighted principal components. Bioinformatics 37, 1178–1181 (2020).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Turner, S. D. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. J. Open Source Softw. 3, 731 (2018).
Lucas, A., Verma, A. & Ritchie, M. D. Hudson: a user-friendly R package to extend manhattan plots. bioRxiv https://doi.org/10.1101/2022.01.25.474274 (2022).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Machiela, M. J. et al. Characterization of large structural genetic mosaicism in human autosomes. Am. J. Hum. Genet. 96, 487–497 (2015).
Loh, P.-R. et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet 48, 1443–1448 (2016).
Loh, P.-R., Palamara, P. F. & Price, A. L. Fast and accurate long-range phasing in a UK Biobank cohort. Nat. Genet. 48, 811 (2016).
Staaf, J. et al. Segmentation-based detection of allelic imbalance and loss-of-heterozygosity in cancer cells using whole genome SNP arrays. Genome Biol. 9, R136 (2008).
Dagnall, C. L. et al. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLoS ONE 12, e0184098 (2017).
Acknowledgements
This work was supported by the intramural research program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, and National Institutes of Health. The Cancer Genomics Research Laboratory is funded with Federal funds from the National Cancer Institute, National Institutes of Health, under NCI Contract No. 75N910D00024. The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1−2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program. This work used the computational resources of the NIH’s High-Performance Computing Biowulf cluster and was conducted using the UK Biobank resource (application 21552). The UK Biobank was established by the Wellcome Trust, the Medical Research Council, the United Kingdom Department of Health, and the Scottish Government. The UK Biobank has also received funding from the Welsh Assembly Government, the British Heart Foundation, and Diabetes UK. The conceptual framework of myelofibrosis pathogenesis was created with BioRender.com. We thank Dr. Ian D. Buller for his statistical support and guidance. The opinions expressed by the authors are their own and this material should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health or the National Cancer Institute.
Funding
Open Access funding provided by the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
D.W.B., Y.W., S.M.G., and M.J.M. conceived the study. A.S.M., S.R.S., T.W., S.J.L., and W.S. contributed samples. K.J., W.L., C.D., and K.T. performed the experiments. W.Z. carried out the mCA calls. D.W.B., W.Z., A.K., T.Z., and O.W.L. performed computational and statistical analyses. J.L., J.W., B.Z., and N.D.F. contributed to genetic analysis of PLCO. H.J.D., and V.G. provided subject matter expertize. S.M.G. and M.J.M. jointly supervised the study. D.W.B., S.J.C., S.A.S., S.M.G., and M.J.M. drafted the manuscript with input from all authors. D.W.B., W.Z., Y.W., K.J., W.L., C.D., K.T., A.K., T.Z., S.-H.L., O.W.L., S.K., J.B.V., A.H., J.L., J.W., B.Z., B.H., A.S.M., S.R.S., T.W., H.J.D., V.G., S.J.L., N.D.F., M.Y., S.J.C., S.A.S., W.S., S.M.G., and M.J.M. critically read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Frank Stegelmann and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brown, D.W., Zhou, W., Wang, Y. et al. Germline-somatic JAK2 interactions are associated with clonal expansion in myelofibrosis. Nat Commun 13, 5284 (2022). https://doi.org/10.1038/s41467-022-32986-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32986-7
This article is cited by
-
Telomere length and cancer risk: finding Goldilocks
Biogerontology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.