Abstract
Determining cost-effective semiconductors exhibiting desirable properties for commercial photoelectrochemical water splitting remains a challenge. Herein, we report a Sb2Se3 semiconductor that satisfies most requirements for an ideal high-performance photoelectrode, including a small band gap and favourable cost, optoelectronic properties, processability, and photocorrosion stability. Strong anisotropy, a major issue for Sb2Se3, is resolved by suppressing growth kinetics via close space sublimation to obtain high-quality compact thin films with favourable crystallographic orientation. The Sb2Se3 photocathode exhibits a high photocurrent density of almost 30 mA cm−2 at 0 V against the reversible hydrogen electrode, the highest value so far. We demonstrate unassisted solar overall water splitting by combining the optimised Sb2Se3 photocathode with a BiVO4 photoanode, achieving a solar-to-hydrogen efficiency of 1.5% with stability over 10 h under simulated 1 sun conditions employing a broad range of solar fluxes. Low-cost Sb2Se3 can thus be an attractive breakthrough material for commercial solar fuel production.
Similar content being viewed by others
Introduction
Molecular hydrogen production via photoelectrochemical (PEC) splitting of water is a promising solution toward a zero-carbon-based society. In order to produce sustainable H2 for a scale commensurate with the global energy demand, high efficiency and low cost, both of which highly depend on the semiconductor materials used in the PEC system, should be achieved. As the solar-to-hydrogen (STH) efficiency of PEC devices using expensive photovoltaic-grade III–V semiconductors has approached the theoretical maximum1, the research community is now recognising the importance of exploring low-cost materials exhibiting good optoelectronic properties2. With this consideration, nearly all recent critical evaluations3,4,5 pertaining to artificial photosynthesis have urged the development of new light absorbers. According to theoretical calculations6,7, two different light absorbers—having a band gap (Eg) of ~1.8 eV for the top electrodes and 1.0–1.3 eV for the bottom electrodes—are required for realising STH efficiencies of >23% in the D4 (dual light absorber: four photons to one H2) tandem cell. Further requirements for suitable semiconductors for PEC water splitting include a large light absorption coefficient (α), high mobility, easy processability without secondary phases, and stability to photocorrosion. In this regard, most emerging cost-competitive photoelectrode materials for PEC water splitting, such as TiO2 (Eg ~ 3.2 eV)8, Fe2O3 (Eg ~ 2.2 eV)9, BiVO4 (Eg ~ 2.4 eV)10, and Cu2O (Eg ~ 2.0 eV)11, are deemed unsuitable for the bottom photoelectrode due to their large Eg. Although the Eg of Cu2ZnSn(S,Se)4 semiconductor, which is another earth-abundant material applicable for PEC water splitting, varies from 1.0 to 1.5 eV depending on the composition, the pure-phase compound suffers from severe difficulty of synthesis due to the narrow stoichiometric window12. It is worth mentioning that the secondary phase issue, i.e. the formation of undesirable detrimental phases during processing, could pose a major obstacle to large-scale commercialisation. In recent years, various low-cost semiconductors have emerged, such as Cu2S (Eg ~ 1.5 eV)13, CuFeO2 (Eg ~ 1.5 eV)14, CuBi2O4 (Eg ~ 1.7 eV)15, CuSbS2 (Eg ~ 1.5 eV)16, and SnS (Eg ~ 1.3 eV)17. However, none of them have satisfied all the requirements for an ideal semiconductor for PEC water splitting. Therefore, a breakthrough material for realising practical STH conversion is urgently required4.
Sb2Se3 offers many advantageous properties that make it a nearly ideal semiconductor material for PEC water splitting. First, it has a small Eg of 1.1–1.2 eV. In addition, it is a low-cost semiconductor (the cost of Sb is similar to that of Cu)18 and exhibits attractive optoelectronic properties (α > 105 cm−1 and high mobility of ~10 cm2 V−1 s−1)19,20. Moreover, the thermodynamic properties of the Sb-Se system allow only stable orthorhombic Sb2Se3 phases without any secondary phases21, avoiding severe secondary phase issues commonly observed in other multivalent compound materials. It has also been reported that Sb2Se3 is intrinsically stable towards photocorrosion in strong acidic media unlike Cu2O, which is vulnerable to photocorroson22. Owing to these merits, the photocurrent density of the Sb2Se3 photocathode has improved rapidly from the initially reported value of 2.5 mA cm−2 to 17.5 cm−2 at 0 V against the reversible hydrogen electrode (RHE) over the short period of its development23,24,25,26. However, it is still far below the theoretical maximum (about 40 mA cm−2, assuming a 1.2 eV Eg and a 100% incident photon-to-current efficiency (IPCE)). In addition, it has been reported that the onset potential of Sb2Se3 photocathodes can be enhanced up to 0.47 VRHE27, but the photovoltage and fill factor should be further improved to realise unassisted water splitting.
As Sb2Se3 has a one-dimensional (1D) crystal structure, it tends to grow with the morphology of 1D nanostructures, which makes synthesis of compact Sb2Se3 thin films difficult28. In the case wherein a light-absorbing material has a low absorption coefficient and poor electrical properties (e.g. short carrier diffusion length), 1D nanostructuring could be an attractive strategy for enhancing the performance. However, in the previously reported Sb2Se3 photocathodes, probably owing to its good optoelectronic properties, planar-type Sb2Se3 exhibited better performance compared with that of its elongated 1D structured counterpart24,27. One possible reason is that the incomplete coverage of Sb2Se3 films, due to the complicated 1D morphology, can result in direct contact between the substrate and an n-type layer, which acts as a recombination centre. In addition, because the carrier transport in Sb2Se3 along the [001] direction is more efficient than that along the [010] and [100] directions owing to its anisotropic crystallographic nature20, the p–n junction should be formed along the [001] direction to maximise the separation of the photo-generated charges. In the 1D Sb2Se3 system, however, the p–n junction generally is formed along the [010] or [100] directions, resulting in inefficient charge separation. Thus compact film-type Sb2Se3 with favourable crystallographic orientation for efficient charge separation (i.e. vertically aligned (Sb4Se6)n nanoribbons) should be obtained to achieve high-performance Sb2Se3 photocathodes. However, as molecular inks for Sb2Se3 already contain 1D [Sb4Se7]2− chains in the solution24,27, it is a daunting task to obtain compact thin Sb2Se3 films via solution processing.
Here we report high-quality dense Sb2Se3 thin films obtained by the close space sublimation (CSS) method, which is known to be a low-cost, large-area, high-yield deposition technique for preparing light-absorbing semiconductors, such as cadmium telluride29 and CH3NH3PbI330. We implement a fast cooling strategy as well as the two-step CSS method31, enabling pin-hole-free and smooth Sb2Se3 thin films with well-oriented (Sb4Se6)n ribbons. The resulting Sb2Se3 thin-film-based photocathodes reveal a highest photocurrent density close to 30 mA cm−2 at 0 VRHE. The performance of the newly developed Sb2Se3 photocathode is utilised by unassisted overall water splitting PEC cells after judiciously optimising the photovoltage and electrolyte compatibility to the BiVO4 photoanode. The D4 tandem cell of Sb2Se3–BiVO4 in a neutral phosphate buffer successfully demonstrates an impressive STH efficiency of 1.5% with 10 h of stability. Realisation of the Sb2Se3-based tandem device, capable of harvesting a broad range of photons with the readily obtained earth-abundant semiconductor, allows us to envision practical solar hydrogen production via efficient and cost-competitive PEC water splitting.
Results
Fabrication of compact and well-oriented Sb2Se3 thin films
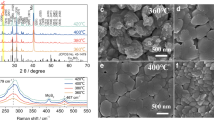
In order to avoid the formation of the thermodynamically favourable 1D structure as well as induce the metastable thin film morphology of Sb2Se3, we tried to kinetically suppress the rearrangement of (Sb4Se6)n nanoribbons by employing a two-step deposition where a low-temperature (340 °C) seed layer was deposited prior to the high-temperature (460 °C) deposition31. In general, the CSS system employs one-step deposition at a high temperature (e.g. 400–550 °C for CdTe) to achieve a suitable growth rate29. Owing to the highly anisotropic nature of Sb2Se3, however, the low-temperature deposition is an imperative step to obtain a compact morphology. At the first deposition step of 340 °C, a compact thin film could be obtained, whereas an elongated 1D structure with incomplete coverage was observed when a temperature of 460 °C was applied directly (Supplementary Fig. 1). Figure 1a–c show the microstructures of Sb2Se3 thin films deposited on an Au/fluorine-doped tin oxide (FTO) substrate by the two-step CSS deposition process (i.e. successive depositions at 340 and 460 °C followed by cooling the chamber naturally after deposition). The role of the Au layer in Sb2Se3 photoelectrodes, which facilitates the transfer of photo-generated holes, has been discussed in previous reports on Sb2Se3 for PEC water splitting26,32 and thin-film solar cells33. Despite the film-type morphology, there were some pin-holes resulting in exposure of the substrate (red circles in Fig. 1b) with the faceted morphology of the Sb2Se3 thin films. Interestingly, it was found that a much smoother pin-hole-free surface was achieved when N2 gas was passed during the cooling process after deposition under the same conditions (i.e. successive depositions at 350 and 450 °C), as shown in Fig. 1d–f. We denoted the sample prepared with N2-assisted cooling as the ‘fast-cooling’ sample, while the naturally cooled sample was denoted as the ‘slow-cooling’ sample. The cooling rates for the fast cooling and the slow cooling until it has reached 200 °C are approximately 15.7 and 11.3 °C min−1, respectively. The detailed differences between the two samples, including grain size distribution and crystallographic orientations, are shown in Supplementary Figs. 2–4.
On comparing the morphological and crystallographic differences between the fast- and slow-cooling samples, it was obvious that the N2 flow during the cooling process significantly affects the morphology and crystallographic orientation. As mentioned above, because Sb2Se3 has a strong 1D anisotropic nature, thermodynamically favourable sharp and facet morphologies are inevitably obtained when kinetically sufficient time is provided (i.e. slow cooling). In contrast, in case of fast cooling, which offers insufficient time to reach the thermodynamically stable morphology, a metastable smooth morphology can be achieved. This explanation is supported by the solution-processed Sb2Se3, in which 1D nanostructures were observed when sufficient Se precursor was provided, while the metastable planar-type Sb2Se3 was obtained in Se-deficient conditions27. In addition, a slight change in X-ray diffraction (XRD) data implied that the ribbons likely move during the cooling process, presumably due to the low melting temperature of Sb2Se3 (~608 °C). Thus it can be reasonably concluded that, in order to fabricate compact thin-film-type Sb2Se3 with favourably oriented (Sb4Se6)n ribbons, rearrangement of the ribbons should be kinetically suppressed as much as possible to prevent the formation of thermodynamically stable 1D structures. Obtained with kinetically controlled growth, it was possible to obtain Sb2Se3 thin films with a compact structure as well as well-oriented ribbons, both of which are expected to be favourable for better performance.
PEC performance of CSS-Sb2Se3 photocathodes
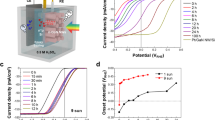
Figure 2a, b show the PEC performance of RuOx/TiO2/Sb2Se3/Au/FTO photocathodes using fast- and slow-cooling Sb2Se3 films measured in pH 1 electrolytes. As we mentioned above, the Au layer acts as a hole-selective contact, which facilitates the transfer of photo-generated holes while blocking the electrons backflow32,33. Without the Au layer, Sb2Se3 photocathodes revealed relatively poor performance while nearly similar morphology of Sb2Se3 was observed (Supplementary Fig. 5), which verifies the role of the Au layer not affecting the growth of Sb2Se3 but assisting the transfer of photo-generated charges. The RuOx catalytic layer was deposited by the PEC method, while atomic layer deposition (ALD) was used for the TiO2 layer, similar to a previous study32. In both samples, the onset potentials shifted towards a positive direction after the first scan due to activation of the RuOx catalyst34. The photocurrent density of the fast-cooling sample approached 30 mA cm−2 at 0 VRHE, which is not only the highest value obtained for a Sb2Se3 photocathode but also among the best observed for all photoelectrodes used in PEC water splitting so far. Note that the data shown in Fig. 2 were obtained from the best-performing device, while normally 25–30 cm−2 at 0 VRHE photocurrent density was observed in the fast-cooling Sb2Se3-based photocathodes. The previously reported photocurrent densities of photoelectrodes for PEC water splitting are shown in Supplementary Fig. 6. Given the low-cost and relatively short history of Sb2Se3 as well as the simple preparation and low material usage due to the high α, the high photocurrent density of ~30 mA cm−2 at 0 VRHE clearly demonstrates the strong potential of Sb2Se3 as a promising photocathode material.
a, b J–V curves of a fast- and b slow-cooling samples under simulated 1 sun air mass 1.5 G chopped illumination at a scan speed of 5 mV s−1 from positive to negative potential and c corresponding HC-STH efficiencies. d Wavelength-dependent IPCE and integrated photocurrent density of fast- and slow-cooling samples measured at 0 VRHE. Source data used to generate this figure can be found in the Source Data file.
While the fast-cooling sample revealed a record photocurrent density, the photocurrent density of the slow-cooling sample with a RuOx co-catalyst, which had a facet morphology, was relatively low (~17 mA cm−2 at 0 VRHE, Fig. 2b). Figure 2c exhibits the half-cell STH conversion efficiencies (HC-STH) calculated from the third scans in Fig. 2a, b according to the equation HC-STH = Iph × (ERHE − EH+/H2)/PSUN × 100%, where Iph is the photocurrent density obtained under an applied bias of ERHE, EH+/H2 is 0 VRHE, and PSUN is 100 mW cm−2. It is worth noting that the maximum value of HC-STH in the fast cooling was observed at a more positive potential of 0.16 VRHE compared with that of the slow-cooling counterpart (0.09 VRHE), indicating that the fast-cooling strategy is advantageous for not only the photocurrent but also the photovoltage and fill factor. As shown in Fig. 2d, both fast- and slow-cooling Sb2Se3 photocathodes were able to harvest photons over 1000 nm, but the IPEC was much higher for the fast-cooling sample over the entire wavelength. Because the optical properties (e.g. surface reflection of Sb2Se3/Au/FTO samples) for the fast- and slow-cooling samples were nearly identical (Supplementary Fig. 7), optical properties can be excluded from the potential origin of the difference noted in the performance. In addition, a similar performance was observed when a Pt co-catalyst was used (Pt/TiO2/Sb2Se3/Au/FTO, Supplementary Fig. 8): thus we can conclude that the performance difference arose not from the co-catalyst but presumably from the TiO2/Sb2Se3 junction.
In order to understand the performance difference observed for the fast- and slow-cooling samples, we performed Kelvin probe force microscopy (KPFM) analyses, which allowed us to investigate the topography and surface potential distribution. Figure 3a, c and b, d show the surface topography and surface potential of TiO2/Sb2Se3 devices, respectively. The fast-cooling TiO2/Sb2Se3 revealed relatively uniform distribution of surface topography and potential, as shown in Fig. 3a, b. On the other hand, some noticeable high-potential regions, as represented by the bright yellow colour in Fig. 3d, were observed for the slow-cooling TiO2/Sb2Se3 sample. Topography and potential line profiling for the fast-cooling sample suggested that the potential resembles the topography of TiO2/Sb2Se3 (Fig. 3e), indicating a lower surface potential at the grain boundaries of Sb2Se335. In the fast-cooling sample, the separation of the photo-generated electrons and holes efficiently occurred along the vertically aligned [Sb4Se6]n ribbons, as shown in Fig. 3g, owing to the p–n junction and relatively small lateral potential difference. In contrast, in the slow-cooling sample, the surface potential increased significantly with a rapid drop in the topography (Fig. 3f), indicating direct contact between the n-type TiO2 layer and substrate due to pin-holes. It might be worth to note that the height recorded by AFM and KPFM ranged from a few nm to hundreds nm and even sometimes μm scale36,37. In such a case, the photo-excited electrons can be extracted laterally to the ribbons and they can recombine with the holes at the back contact as shown in Fig. 3h due to the large electric field across the p–n junction. It is widely known that direct contact between the top and bottom contact (i.e., in this case, the TiO2 and Au layers) can cause significant degradation of the performance, even in the case of the chemical composition and optoelectronic properties have negligible differences. For example, Luo et al. reported the effect of a thin blocking layer to prevent shunt pathways, thereby enabling much higher performance in Cu2O nanowire photocathodes without any noticeable differences in chemical composition and morphology38. The KPFM results clearly demonstrated the importance of pin-hole-free compact thin films in preventing the recombination and the performance degradation mechanism in the presence of pin-holes, which had not yet been experimentally demonstrated.
a, c Topography of a fast- and c slow-cooling samples. b, d Surface potential of b fast- and d slow-cooling samples. e, f Topography and potential line profile (obtained from scanning the black lines in Fig. 3a–d) for e fast- and f slow-cooling samples. g, h Schematics showing possible charge separation and recombination in g fast- and h slow-cooling samples. Source data used to generate this figure can be found in the Source Data file.
Sb2Se3 photocathodes in unassisted water splitting
Despite the high photocurrent density of the fast-cooling CSS-Sb2Se3 photocathodes, there remain some issues prior to achieving unassisted water splitting by fabricating a tandem device in conjunction with the photoanode. After the optimisation of the electrolyte and the onset potential (Supplementary Figs. 9–11), we decided to use a Pt co-catalyst for the PEC tandem device. With the Pt co-catalyst, we inserted a CdS layer between TiO2 and the fast-cooling Sb2Se3 to further enhance the onset potential, as shown in Fig. 4a27. The thickness of the CdS and TiO2 layers were approximately 50 and 30 nm, respectively (Supplementary Fig. 12). Upon deposition of the CdS layer, the onset potential of the Sb2Se3 photocathode shifted up to 0.5 VRHE and the fill factor was also significantly enhanced (Fig. 4b). As a result, the maximum HC-STH increased from 2.33% to 3.4%, and the potential at which the maximum HC-STH was observed also shifted from 0.18 to 0.26 VRHE, as shown in Fig. 4c. Therefore, despite the slightly decreased photocurrent density at 0 VRHE, Pt/TiO2/CdS/Sb2Se3 configuration was more suitable for the PEC tandem device. The enhanced onset potential and fill factor could be attributed to the reduced band mismatch between TiO2 and Sb2Se327. Figure 4d shows the IPCE spectra for Sb2Se3 photocathodes with and without CdS layers, suggesting that the IPEC of 300–500 nm decreased with the thickness of the CdS layer. The reduced IPCE values imply that the photons absorbed by the CdS (Eg ~ 2.4 eV, ~516.6 nm) were unable to contribute to the photocurrent density. Therefore, when the thickness of the CdS layer increased, the photocurrent density decreased, whereas the onset potential remained the same (i.e. ~0.5 VRHE), indicating the necessity of careful optimisation of the CdS layer thickness (Supplementary Fig. 13).
a Cross-sectional SEM image of TiO2/CdS/Sb2Se3 on Au/FTO substrate. b J–V curves for Sb2Se3 photocathodes with/without a CdS layer in pH 1 H2SO4 under simulated 1 sun air mass 1.5 G chopped illumination at a scan speed of 5 mV s−1 from positive to negative potential and c corresponding HC-STH efficiencies. d Wavelength-dependent IPCE at 0 VRHE of Sb2Se3 photocathodes with/without CdS layer. Source data used to generate this figure can be found in the Source Data file.
D4 tandem cell for unbiased solar overall water splitting
Since the band gap of Sb2Se3 is 1.2 eV, which is suitable for realising a D4 tandem cell as a bottom electrode with a different top light absorber39, we fabricated tandem cells coupled with BiVO4-based photoanodes (Eg ~ 2.4 eV), which have been widely used for high-performance photocathode–photoanode tandem cells10 (Fig. 5a). The H,Mo:BiVO4 photoanode used here revealed good transparency (Supplementary Fig. 14) and performance (~4.7 mA cm−2 at 1.23 VRHE and onset potential of 0.25–0.3 VRHE) as a top light absorber. To achieve high efficiency, further optimisation of the electrolyte was conducted for the tandem cell comprising two photoelectrodes involving opposite reactions (Supplementary Figs. 15–18).
a Scheme of the NiFeOx/H,Mo:BiVO4/FTO–Pt/TiO2/CdS/Sb2Se3/Au/FTO tandem cell operating in pH 7.0 phosphate buffer. b J–V curve for the photocathode and photoanode; the operating point is marked for the tandem cell (photoelectrode active area: 0.32 cm2). c Two-electrode measurements for photoanode–counter electrode (C. E.) and photoanode–photocathode tandem cells (active area: 0.28 cm2). d IPCE at 0.4 VRHE measured for the photoanode and photocathodes. e Photocurrent generation under short circuit conditions (0 against counter electrode) of the photoanode–photocathode tandem cell. All analyses were conducted in 0.5 M phosphate buffer + 0.01 M V2O5 (pH 7.0) at a scan rate of 20 mV s−1. Distance between the photoanode and photocathode was ~0.5 cm. Source data used to generate this figure can be found in the Source Data file.
As shown in Fig. 5b, the operating point of the two photoelectrodes, as estimated by the intersection of two J–V curves, was approximately 1.2 mA cm−2 at 0.4 VRHE, which corresponded to a STH efficiency of 1.5%. It should be noted that photocurrent density of BiVO4 at 0.4 VRHE varies from 1.2 to 0.8 mA cm−2, thus the overall STH efficiencies of the tandem cell range from 1.48% to 0.98%. In addition, the polarisation curves of BiVO4–Pt (as the counter electrode) and BiVO4–Sb2Se3 (as the counter electrode) demonstrated a ~0.5 V anodic shift induced by the Sb2Se3 photocathode, indicating that the Sb2Se3 photocathode provided a photovoltage of 0.5 V (Fig. 5c). The BiVO4–Sb2Se3 tandem cell exhibited 1.2 mA cm−2 at 0 V against the counter electrode (i.e. unbiased conditions), which was in agreement with the estimated value according to the overlapped J–V curves shown in Fig. 5b. This observation verified that the total photovoltage of 1.45 V (0.52 V from the Sb2Se3 photocathode and 0.93 V from the BiVO4 photoanode) was sufficient to implement unbiased overall water splitting, resulting in evolution of both H2 and O2, by overcoming the thermodynamic potential requirement (1.23 V). In comparison to previously reported photoelectrodes, the total photovoltage of 1.45 V was a moderate value considering the band gap energies40. Thus it is noteworthy that a photovoltage increment of ~0.1 V and the enhanced fill factor obtained using CdS played a critical role in driving the overall water splitting.
Photon utilisation capability of the BiVO4–Sb2Se3 tandem cell was confirmed by IPCE measurements. For BiVO4, photons up to ~510 nm (Eg of 2.45 eV) could be converted and the absorption current density, which represents the maximum photocurrent density under the absorbance of BiVO4, was calculated to be 5.5 mA cm−2 (Supplementary Fig. 14b). Sum of the photon flux before and after passing through BiVO4 up to the wavelength of 1033 nm (~1.2 eV, Eg of Sb2Se3) was calculated to be 39.9 and 24.4 mA cm−2, respectively, which are much higher than the photocurrent of BiVO4 at the top of the tandem cell configuration.
We compared BiVO4 and Sb2Se3 in two different bias conditions: high bias near short circuit of the individual photoelectrode (1.0 VRHE for photoanode and 0.1 VRHE for photocathode; Supplementary Fig. 19) and operating bias at ‘actual’ operating point (0.4 VRHE for both the photocathode and photoanode; Fig. 5d). In both cases, a broad range of photons could be effectively utilised by the synergetic effect of BiVO4 (to 510 nm) and Sb2Se3 (from 450 to 1000 nm). In case of the high bias condition, both the photocathode and photoanode showed a quite high IPCE (~70% at 450 nm for BiVO4 and ~60% for Sb2Se3). The photocurrent calculated from the IPCE was 3.2 mA cm−2 for the BiVO4 photoanode. In addition, the photocurrent of the Sb2Se3 photocathodes was 18.5 and 10.3 mA cm−2 with or without BiVO4, respectively. These values are in a good agreement with photocurrents shown in the J–V curves (Supplementary Fig. 18). On the other hand, as expected, a lower IPCE was observed under the operating bias (0.4 VRHE): ~25% at 450 nm for BiVO4 and ~15% for Sb2Se3, as shown in Fig. 5d. The photocurrent density calculated using the IPCE spectra of BiVO4 at 0.4 VRHE was about 1.1 mA cm−2, which is similar to the values shown by the J–V curve (Supplementary Fig. 18). However, the photocurrent density of Sb2Se3 photocathodes at 0.4 VRHE with and without BiVO4 were 5.0 and 2.2 mA cm−2, respectively, which are higher than the photocurrents shown by the J–V curve. This discrepancy between the photocurrent density at 0.4 VRHE demonstrated by the IPCE and J–V curve can be attributed to the light-intensity-dependent carrier mobility of Sb2Se3. As the carrier mobility of Sb2Se3 is much higher under low-intensity light32, Sb2Se3 photocathodes showed a better fill factor in IPCE measurement conditions (~100 μW cm−2) compared with the 1 sun conditions (100 mW cm−2), thereby resulting in a higher photocurrent according to the IPCE spectra at 0.4 VRHE. The D4 tandem cell consisting of Sb2Se3 and BiVO4 operated stably for over 10 h without any noticeable degradation in the photocurrent density (Fig. 5e). The efficiency and stability of our Sb2Se3-based tandem cells are comparable to those of the best-performing tandem cells containing other types of photocathode materials (vide infra). Finally, a larger tandem cell was constructed using a BiVO4 photoanode (0.81 cm−2) and a Sb2Se3 photocathode (0.35 cm−2) under simulated ~3 sun, which clearly demonstrated a constant generation of O2 and H2 gas evolution (Supplementary video 1, Supplementary Fig. 20).
Discussion
Even though we report herein the first demonstration of unbiased water splitting using a Sb2Se3 photocathode in a single reactor, better tandem cell systems are likely achievable by using two different electrolytes that are separately optimised for each photoelectrode (e.g. pH 1 for Sb2Se3 and pH 9 borate for BiVO4) along with a bipolar membrane41 or stabilising both the photocathode and photoanode using additives (e.g. chelating agent to deter poisoning) in the electrolyte42. Bearing in mind its short history and potential for further enhancement, the performance of the Sb2Se3-based tandem cell (STH efficiency of 1.5% and stability of over 10 h) is quite remarkable particularly in comparison with previously reported results for photocathode–photoanode tandem cells4 (Fig. 6a, Table 1 in Supplementary Information). We categorised the tandem cells based on the bottom light absorbers (silicone based, III–V, Cu-chalcogenides, metal oxides, and perovskites). Most of them used BiVO4, which is currently the most suitable photoanode material and ensures good transparency (~80% beyond photon utilisation threshold (~520 nm))10. It was found that the efficiency of the BiVO4–Sb2Se3 tandem cell (1.5% STH) was not the highest when compared to that of BiVO4–CIGS (3.7%)43, BiVO4–Cu2O (3.0%)44, and BiVO4–c-Si (2.1%)41. Considering the cost of materials (much lower than that of CIGS), small band gap (compared to that of Cu2O, Eg ~ 2.0 eV), simple preparation, and low material usage (compared to that of Czochralski-grown Si wafer and complicated Si microwires), the >1% STH can be considered a significant milestone for PEC tandem cells. Moreover, 10 h of stability is also the best result reported for photoanode–photocathode tandem cells except for the 20 h stability achieved by the expensive CIGS-based photocathodes45 (Fig. 6b). The exceptional performance and stability of the Sb2Se3 photocathode presented here, as well as several desirable material characteristics of Sb2Se3 such as its high α, optimal band gap of ~1.2 eV, and comparable photovoltage to that of c-Si (~0.5 V), clearly present a promising pathway towards realising a competitive overall water splitting PEC cell system, which could potentially result in an STH of approximately 10% using BiVO4 photoanodes whose Eg is 2.4 eV (Supplementary Fig. 21a). In order to demonstrate a higher STH efficiency, the areas of focus should include enhancing both the photovoltage and fill factor through interface engineering, such as the recently reported dipole engineering at the buried junction46. In addition, the development of an ideal photoanode material with an Eg close to 1.8 eV could potentially enable an STH efficiency of >20% on using the Sb2Se3 photocathode (Supplementary Fig. 21b). Although the value of STH 1.5% is not superior in comparison to that of other systems, such as photovoltaic–electrolytic systems (average 10–20%), buried-junction photoelectrodes (10–15%), and photovoltaic–PEC systems (3–8%), because of the simplicity of the photoanode–photocathode system, the price of hydrogen produced from such a system will be lower. Therefore, not only the STH efficiency but also the cost should be considered when comparing such systems. The reported STH efficiencies of the photoanode–photocathode tandem system are increasing very rapidly (Fig. 6), which suggests increasing research interest in reducing the complexity of overall water splitting devices4.
a Solar-to-hydrogen efficiencies reported in recent years and b their operation duration. All reports are summarised in Supplementary Table 1.
In summary, we have demonstrated a high-performance photocathode using a low-cost Sb2Se3 semiconductor, which has a small Eg (~1.2 eV), good optoelectronic properties, and no secondary phase. By suppressing the growth kinetics during the CSS deposition, a compact and pinhole-free Sb2Se3 thin film was obtained, which enabled a highest photocurrent density of up to 30 mA cm−2 by avoiding recombination and accelerating charge separation through well-aligned (Sb4Se6)n ribbons. Inserting a CdS layer between Sb2Se3 and TiO2 increased the onset potential and consequently improved the HC-STH efficiency up to 3.4%. Finally, by combining the material with a BiVO4 photoanode, unbiased overall water splitting was achieved with impressive efficiency (~1.5%) and high stability of 10 h. These performances and stability significantly surpass those of previously reported Sb2Se3 photocathodes and are comparable to those of other expensive thin film photocathodes. Given the relatively short history of photoelectric materials, it is expected that rapid progress of Sb2Se3 photocathodes will lead to better efficiency in the near future and our findings represent an important demonstration of a photocathode–photoanode-based PEC device. We believe that the emerging Sb2Se3 substrate can be an attractive breakthrough material for practical solar fuel production.
Methods
Preparation of Sb2Se3 thin films
Sb2Se3 films were deposited via a two-step CSS process31 using a custom-made CSS system. First, a compact layer was grown from stoichiometric Sb2Se3 (Alfa Aesar, 99.999% metals basis) using a source temperature of 340 °C and a substrate temperature of 390 °C for 2 min at a pressure of 0.05 mbar. Subsequently, deposition was completed using a source temperature of 460 °C for 15 min and a pressure of 13 mbar. These films were then cooled either slowly or fast, by turning the heater off and flowing N2 over the sample at a rate of either 0 or 5 L min−1, respectively.
Deposition of overlayers and catalysts for photocathodes
CdS layers were deposited by the chemical bath deposition (CBD) method. Prior to CBD, the sample was pre-treated in a bath containing a solution of CdSO4 (Sigma Aldrich, 99.99%) and NH4OH (Duksan, 28 wt%) at 60 °C for 10 min. CBD of CdS was performed by immersing the pre-treated sample in a solution containing CdSO4, thiourea (99%, Sigma Aldrich), deionised (DE) water, and NH4OH for 5 min at 60 °C. TiO2 layers were deposited using an ALD system (Lucida D100, NCD Inc.). The ALD process was performed at 150 °C, with tetrakis(dimethylamido)titanium (TDMAT) and H2O as the Ti and O sources, respectively. A total of 600 ALD cycles were carried out, each of which comprised a TDMAT pulse of 0.3 s followed by 15 s of N2 purging and a H2O pulse of 0.2 s followed by 15 s of N2 purging. The approximate growth rate of TiO2 was 0.55 Å per cycle, as estimated using an ellipsometer. The Pt catalyst was sputtered onto the TiO2-coated Sb2Se3 electrode using an Auto Sputter Coater (Ted Pella, Redding, CA, USA) under an applied current of 10 mA for 120 s. A galvanostatic photo-deposition technique was used for RuOx deposition. The prepared photoelectrodes were immersed in a 1.3 mM solution of KRuO4 at a current density of −28.3 μA cm−2 for 15 min under simulated 1 sun illumination.
Preparation of BiVO4 films
All chemicals used in this study were of analytical grade and used without further purification. BiVO4 film was prepared by a modified metal–organic decomposition method according to our previously reported procedure47 with slight modifications. In brief, 291 mg of Bi(NO3)·5H2O (99.8%; Kanto Chemicals) and 163 mg of VO(acac)2 (98.0%; Sigma Aldrich) were dissolved in 15 mL of acetyl acetone (>99.0%; Kanto Chemicals). As the dopant solution, 0.03 M MoO2(acac)2 (98.0%; Sigma Aldrich) in acetyl acetone (>99.0%; Kanto Chemicals) was prepared. A 0.2 M Bi(NO3)·5H2O (99.8%; Kanto Chemicals) solution dissolved in acetic acid (>99.0%; Kanto Chemicals) was used to compensate the Mo precursor-added solution. For Mo doping, a Bi:(V+Mo) = 1:1 atomic ratio was used for preparing the 1% Mo:BiVO4 films. To fabricate the BiVO4 film, 35 µL of the solution was dropped on a FTO glass sample (2 cm × 2.5 cm) and dried for 10 min in Ar atmosphere. The FTO glass (TEC 8; Pilkington) was cleaned using ethanol +35 wt% H2O2 (Samchun Chemical) used in a ratio of 5:1 under sonication (~20 min) and washed with copious amounts of ethanol and finally stored in 2-propanol before use. The greenish transparent precursor film was calcined at 550 °C for 30 min to form a yellow BiVO4 film. After the annealing process, the 2 cm × 2.5 cm BiVO4/FTO was split to obtain photoanodes with a net irradiation area of 0.36 cm2 connected by silver paste and copper wire and sealed with epoxy resin.
Hydrogen treatment of metal oxide films
Hydrogen treatment was conducted using the borohydride decomposition method reported by Hao et al.48. First, 16 mmol of NaBH4 (>98%; Sigma Aldrich) was introduced in a 200-mL alumina crucible and another smaller alumina bottle (15 mL) was introduced to the NaBH4 powder. In this smaller bottle, an as-prepared metal oxide film (2 cm × 2.5 cm) was placed and finally the 200-mL alumina crucible was covered with an alumina cover. This reactor was placed in an already heated furnace at 500 °C for 30 min. Then the crucible was taken out from the furnace and cooled naturally.
NiFeOx co-catalyst deposition on BiVO4 film
The NiFeOx co-catalysts were deposited by photo-assisted electrodeposition under air mass (AM) 1.5 G illumination according to a reported procedure49. In all, 60 mg of Fe(SO4)2⋅7 H2O (≥99%; Sigma Aldrich) and 20 mg of Ni(SO4)2⋅6H2O (99%; Sigma Aldrich) were put in a glass bottle and 200 mL of 0.5 M KHCO3 (pH 8.3, purged with Ar gas for 30 min before use) was introduced, resulting in a transparent and yellow solution. For deposition, under illumination (AM 1.5 G, 100 mW cm−2), linear sweep voltammetry was conducted with a bias of −0.3 to 0.5 V against the reference electrode (Ag/AgCl) for 12–15 times with pre-treatment of −0.3 V for 5 s. Sequential linear sweep voltammetry resulted in reduced current density and over-deposition of NiFeOx. After deposition, the photoelectrode was taken out and washed with copious amounts of DI water. Right after being taken out, the photoelectrode showed a slightly darkened colour (Ni(OH)2 species) but gradually changes to a colour identical to that of the photoelectrode before NiFeOx deposition. The photoelectrodes were stored in an Ar gas-filled bottle before use.
Characterisation
The surface morphology of Sb2Se3 thin films were analysed via field-emission scanning electron microscope (JSM-7001F, JEOL Ltd, Tokyo, Japan). The crystallinity of the samples was investigated using XRD (MiniFlex 600, Rigaku, Tokyo, Japan) with Cu Kα radiation (λ = 1.54178 Å). The surface reflectance of the Sb2Se3 thin films was measured using an ultraviolet–visible (UV-vis) spectrophotometer (V-670, JASCO, Easton, MD, USA). UV-Vis absorbance of BiVO4 was recorded with a UV/Vis spectrometer (UV-2401PC, Shimadzu). As a reference, BaSO4 powder was used. In addition, KPFM (SPA 400, Seiko Instrument Inc., Chiba, Japan) measurements were performed using a gold-coated cantilever (SI-DF3-A).
Determination of PEC performance
PEC measurements for Sb2Se3 photocathodes were performed in a typical three-electrode system with a Ag/AgCl/KCl (4 M) reference electrode and a Pt wire counter electrode. The Sb2Se3 photocathodes were submerged in an acidic (H2SO4, pH ~ 1) or a neutral (phosphate buffer, pH ~ 6.25) electrolyte and simulated solar light illumination. Calibration of the 1-sun level was performed using a standard Si reference cell certified by the Newport Corporation, consisting of a readout device and a 2 × 2 cm2 calibrated solar cell made of monocrystalline silicon. During calibration, the Si reference cell was located at the same position of the sample for PEC measurement. The scan rate for the J–V curves was 5 mV s−1. For Sb2Se3 photocathodes, the applied potentials were recorded against the RHE to allow comparison with previously reported results, employing the relationship ERHE = EAg/AgCl + 0.059 pH + 0.197. IPCE for Sb2Se3 photocathodes was measured with an electrochemical workstation (Zennium, Zahner, Germany) combined with a potentiostat (PP211, Zahner, Germany) under monochromatic light.
PEC performance of the BiVO4 photoanode was measured with a photoanode as the working electrode, Pt mesh as the counter electrode, and Ag/AgCl (3 M NaCl) as the reference electrode. The scan rate for the J–V curves was 20 mV s−1. For electrolyte, 0.5 M potassium phosphate (K2HPO4 or KPi) buffer + 0.01 M V2O5 (pH 7.0) was used as a standard electrolyte as reported previously for the borate buffer (pH 9.5). To accelerate dissolution of V2O5, it was placed in oven at 80 °C for 3 h. The pH of the KPi buffer barely changed (<0.01) while the pH of 8 or 9 showed a noticeable change that could be compensated by adding a small amount of 0.5 M KOH. Potentials were recorded with correction according to the Nernst relationship ERHE = EAg/AgCl + 0.0591 pH + 0.209, in which EAg/AgCl is the applied bias potential and 0.209 is a conversion factor from the Ag/AgCl electrode to the RHE scale. The electrochemical data for BiVO4 photoanodes were recorded by using a potentiostat (IviumStat, Ivium Technologies). A 300 W Xenon lamp was used to produce simulated 1 sun light irradiation conditions (AM 1.5 G, 100 mW cm−2) employing a solar simulator (Oriel 91160) with an AM 1.5 G filter calibrated with a reference cell certified by the National Renewable Energy Laboratories, USA.
For the BiVO4–Sb2Se3 tandem cell, black masked (0.32–0.36 cm2) photoelectrodes were aligned with a spacing of ~0.5 cm in a single electrolyte bed reactor. Photocurrent of Sb2Se3 was recorded with or without the BiVO4 photoelectrode (fully modified, NiFeOx/H,Mo:BiVO4) under backward scan (~0.5–0 VRHE). The BiVO4 photoelectrode was scanned in the forward scan (0.2–1.4 VRHE). For the BiVO4–Sb2Se3 two-electrode full cell, BiVO4 was set as the working electrode and Sb2Se3 was set as the counter electrode. Bias was applied towards the counter electrode and thus 0 V against the counter electrode indicated a 0 V potential applied to the full cell. For linear sweep, forward scan was applied for the BiVO4–Pt rod (0.3–1.3 V) or BiVO4–Sb2Se3 (−0.3 to 0.5 V). All scans were performed with 10 s of pre-treatment at the initial potential at a speed of 20 mV s−1.
IPCE measurements for BiVO4 and Sb2Se3 behind BiVO4 were conducted using a 300 W Xe lamp as the light source with a liquid infrared filter and a monochromator (Oriel Cornerstone 130 1/8 m monochromator) with a bandwidth limit of 5 nm. The intensity of light was measured before IPCE measurements by a photodiode detector (Oriel 70260). Calculation of IPCE was carried out according to the equation:
where J = photocurrent density (mA cm−2), P = light power density (mW cm−2) at λ, and λ = wavelength of incident light (nm). J was gathered under the condition that the PEC cell individually installed and the constant potential applied for a photoelectrode and wavelength of illuminated beam (1 cm × 1 cm) was periodically changed. The active area of the characterised photoelectrode was preferably smaller than this beam source (0.32–0.36 cm2). The scanning wavelength range was 300–1100 nm with an interval of 10 nm/7 s.
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request. The source data underlying Figs. 2, 3e, f, 4b–d, 5b–e, and 6 and Supplementary Figs. 2, 3, 4, 5c, 7, 8, 9, 10, 11a, 13, 14, 15, 16, 17, 18b–d, 19, and 21a are provided as a Source Data file.
References
Cheng, W.-H. et al. Monolithic photoelectrochemical device for direct water splitting with 19% efficiency. ACS Energy Lett. 3, 1795–1800 (2018).
Turner, J. A. Shining a light on solar water splitting—response. Science 344, 469–470 (2014).
Sivula, K. & Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 1, 15010 (2016).
Kim, J. H., Hansora, D., Sharma, P., Jang, J.-W. & Lee, J. S. Toward practical solar hydrogen production–an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
Yao, T., An, X., Han, H., Chen, J. Q. & Li, C. Photoelectrocatalytic materials for solar water splitting. Adv. Energy Mater. 8, 1800210 (2018).
Hu, S., Xiang, C., Haussener, S., Berger, A. D. & Lewis, N. S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems. Energy Environ. Sci. 6, 2984–2993 (2013).
Pinaud, B. A. et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002 (2013).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Tamirat, A. G., Rick, J., Dubale, A. A., Su, W.-N. & Hwang, B.-J. Using hematite for photoelectrochemical water splitting: a review of current progress and challenges. Nanoscale Horiz. 1, 243–267 (2016).
Kim, J. H. & Lee, J. S. Elaborately modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 31, 1806938 (2019).
Paracchino, A., Laporte, V., Sivula, K., Grätzel, M. & Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 10, 456 (2011).
Yang, W. et al. Molecular chemistry-controlled hybrid ink-derived efficient Cu2ZnSnS4 photocathodes for photoelectrochemical water splitting. ACS Energy Lett. 1, 1127–1136 (2016).
Yu, Y.-X. et al. Solution-processed Cu2S photocathodes for photoelectrochemical water splitting. ACS Energy Lett. 3, 760–766 (2018).
Oh, Y., Yang, W., Kim, J., Jeong, S. & Moon, J. Enhanced photocurrent of transparent CuFeO2 photocathodes by self-light-harvesting architecture. ACS Appl. Mater. Interfaces 9, 14078–14087 (2017).
Song, A. et al. Cu:NiO as a hole-selective back contact to improve the photoelectrochemical performance of CuBi2O4 thin film photocathodes. J. Mater. Chem. A 7, 9183–9194 (2019).
Zhang, L. et al. Solution-processed CuSbS2 thin film: a promising earth-abundant photocathode for efficient visible-light-driven hydrogen evolution. Nano Energy 28, 135–142 (2016).
Huang, X. et al. Simple eco-friendly synthesis of the surfactant free SnS nanocrystal toward the photoelectrochemical cell application. Sci. Rep. 7, 16531 (2017).
Yang, W. & Moon, J. Rapid advances in antimony triselenide photocathodes for solar hydrogen generation. J. Mater. Chem. A 7, 20467 (2019).
Wang, L. et al. Stable 6%-efficient Sb2Se3 solar cells with a ZnO buffer layer. Nat. Energy 2, 17046 (2017).
Chen, C. et al. Characterization of basic physical properties of Sb2Se3 and its relevance for photovoltaics. Front. Optoelectron. 10, 18–30 (2017).
Ghosh, G. The Sb-Se (antimony-selenium) system. J. Phase Equilibria 14, 753–763 (1993).
Prabhakar, R. R. et al. Photocorrosion-resistant Sb2Se3 photocathodes with earth abundant MoSx hydrogen evolution catalyst. J. Mater. Chem. A 5, 23139–23145 (2017).
Kim, J. et al. Self-oriented Sb2Se3 nanoneedle photocathodes for water splitting obtained by a simple spin-coating method. J. Mater. Chem. A 5, 2180–2187 (2017).
Yang, W. et al. Adjusting the anisotropy of 1D Sb2Se3 nanostructures for highly efficient photoelectrochemical water splitting. Adv. Energy Mater. 8, 1702888 (2018).
Tan, J. et al. Fullerene as a photoelectron transfer promoter enabling stable TiO2‐protected Sb2Se3 photocathodes for photo‐electrochemical water splitting. Adv. Energy Mater. 9, 1900179 (2019).
Lee, H. et al. Cu-doped NiOx as effective hole-selective layer for high-performance Sb2Se3 photocathode for photoelectrochemical water splitting. ACS Energy Lett. 4, 995–1003 (2019).
Park, J. et al. Efficient solar to hydrogen conversion from neutral electrolytes using morphology-controlled Sb2Se3 light absorber. ACS Energy Lett. 4, 517–526 (2019).
Phillips, L. J. et al. Current enhancement via a TiO2 window layer for CSS Sb2Se3 solar cells: performance limits and high Voc. IEEE J. Photovolt. 9, 544–551 (2019).
Major, J. D. et al. A low-cost non-toxic post-growth activation step for CdTe solar cells. Nature 511, 334–337 (2014).
Guo, Q. et al. The growth of a CH3NH3PbI3 thin film using simplified close space sublimation for efficient and large dimensional perovskite solar cells. Energy Environ. Sci. 9, 1486–1494 (2016).
Hutter, O. S., Phillips, L. J., Durose, K. & Major, J. D. 6.6% efficient antimony selenide solar cells using grain structure control and an organic contact layer. Sol. Energy Mater. Sol. Cells 188, 177–181 (2018).
Yang, W. et al. Time-resolved observations of photo-generated charge-carrier dynamics in Sb2Se3 photocathodes for photoelectrochemical water splitting. ACS Nano 12, 11088–11097 (2018).
Zhang, J. et al. Alternative back contacts for Sb2Se3 solar cells. Sol. Energy 182, 96–101 (2019).
Tilley, S. D., Schreier, M., Azevedo, J., Stefik, M. & Graetzel, M. Ruthenium oxide hydrogen evolution catalysis on composite cuprous oxide water‐splitting photocathodes. Adv. Funct. Mater. 24, 303–311 (2014).
Chen, C. et al. Efficiency improvement of Sb2Se3 solar cells via grain boundary inversion. ACS Energy Lett. 3, 2335–2341 (2018).
Vishwakarma, M. et al. A direct measurement of higher photovoltage at grain boundaries in CdS/CZTSe solar cells using KPFM technique. Sol. Energy Mater. Sol. Cells 183, 43–40 (2018).
Kim, G. Y. et al. Nanoscale investigation of surface potential distribution of Cu2ZnSn(S,Se)4 thin films grown with additional NaF layers. Nano Convergence 1, 27 (2014).
Luo, J. et al. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett. 16, 1848–1857 (2016).
Prévot, M. S. & Sivula, K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C 117, 17879–17893 (2013).
Mayer, M. T. Photovoltage at semiconductor–electrolyte junctions. Curr. Opin. Electrochem. 2, 104–110 (2017).
Vijselaar, W. et al. Spatial decoupling of light absorption and catalytic activity of Ni–Mo-loaded high-aspect-ratio silicon microwire photocathodes. Nat. Energy 3, 185 (2018).
Kaneko, H., Minegishi, T., Kobayashi, H., Kuang, Y. & Domen, K. Suppression of poisoning of photocathode catalysts in photoelectrochemical cells for highly stable sunlight-driven overall water splitting. J. Chem. Phys. 150, 041713 (2019).
Kobayashi, H. et al. Development of highly efficient CuIn0.5Ga0.5Se2-based photocathode and application to overall solar driven water splitting. Energy Environ. Sci. 11, 3003–3009 (2018).
Pan, L. et al. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catalysis 1, 412 (2018).
Higashi, T. et al. Overall water splitting by photoelectrochemical cells consisting of (ZnSe)0.85(CuIn0.7Ga0.3Se2)0.15 photocathodes and BiVO4 photoanodes. Chem. Commun. 53, 11674–11677 (2017).
Wick-Jaliat., R. et al. Stable and tunable phosphonic acid dipole layer for band edge engineering of photoelectrochemical and photovoltaic heterojunction devices. Energy Environ. Sci. 12, 1901–1909 (2019).
Kim, J. H. et al. Wireless solar water splitting device with robust cobalt-catalyzed, dual-doped BiVO4 photoanode and perovskite solar cell in tandem: a dual absorber artificial leaf. ACS Nano 9, 11820–11829 (2015).
Hao, Y., Deng, J., Zhou, L., Sun, X. & Zhong, J. Depth-reduction induced low onset potential of hematite photoanodes for solar water oxidation. RSC Adv. 5, 31086–31090 (2015).
Li, F. et al. An iron-based thin film as a highly efficient catalyst for electrochemical water oxidation in a carbonate electrolyte. Chem. Commun. 52, 5753–5756 (2016).
Acknowledgements
This work was supported by a National Research Foundation (NRF) of Korea grant (No. 2012R1A3A2026417); a Creative Materials Discovery Program (NRF-2018M3D1A1058793) funded by the Ministry of Science and ICT; the Korea Center for Artificial Photosynthesis (KCAP, No. 2009–0093880), funded by the MSIT; Project No. 10050509 funded by the MOTIE of Republic of Korea; and the UK EPSRC project number EP/N014057/1.
Author information
Authors and Affiliations
Contributions
W.Y. conceived the idea, organised the collaboration, conducted experiments, analysed the data, and wrote the manuscript. J.H.K. prepared the BiVO4 photoanodes, tested the tandem device, and co-wrote the manuscript. O.S.H. and L.J.P. prepared and optimised the Sb2Se3 thin films via the CSS method. J.T., J.P. and H.L. assisted in device optimisation and data analysis for the Sb2Se3 photocathodes. J.D.M. helped to the idea and manuscript preparation. J.S.L. directed the research and contributed to the writing of the manuscript. J.M. designed and supervised the project, directed the research, and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, W., Kim, J.H., Hutter, O.S. et al. Benchmark performance of low-cost Sb2Se3 photocathodes for unassisted solar overall water splitting. Nat Commun 11, 861 (2020). https://doi.org/10.1038/s41467-020-14704-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-14704-3
This article is cited by
-
Improved performances in Sb2Se3 solar cells based on CdS buffered TiO2 electron transport layer
Journal of Sol-Gel Science and Technology (2024)
-
Green lithium: photoelectrochemical extraction
PhotoniX (2023)
-
Recent Advancements in Photoelectrochemical Water Splitting for Hydrogen Production
Electrochemical Energy Reviews (2023)
-
Polyimide stabilized Cu2O photocathode for efficient PEC water reduction
Ionics (2023)
-
Hydrogel protection strategy to stabilize water-splitting photoelectrodes
Nature Energy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.