Abstract
This study investigated the mechanism underlying the beneficial effects of mineralocorticoid receptor (MR) antagonists in patients with resistant hypertension and diabetic nephropathy by examining post-translational modification of the MR by O-linked-N-acetylglucosamine (O-GlcNAc), which is strongly associated with type 2 diabetes. Coimmunoprecipitation assays in HEK293T cells showed that MR is a target of O-GlcNAc modification (O-GlcNAcylation). The expression levels and transcriptional activities of the receptor increased in parallel with its O-GlcNAcylation under high-glucose conditions. Liquid chromatography–tandem mass spectrometry revealed O-GlcNAcylation of the MR at amino acids 295–307. Point mutations in those residues decreased O-GlcNAcylation, and both the protein levels and transcriptional activities of MR. In db/db mouse kidneys, MR protein levels increased in parallel with overall O-GlcNAc levels of the tissue, accompanied by increased SGK1 mRNA levels. The administration of 6-diazo-5-oxo-L-norleucin, an inhibitor of O-GlcNAcylation, reduced tissue O-GlcNAc levels and MR protein levels in db/db mice. Thus, our study showed that O-GlcNAcylation of the MR directly increases protein levels and transcriptional activities of the receptor under high-glucose conditions in vitro and in vivo. These findings provide a novel mechanism of MR as a target for prevention of complications associated with diabetes mellitus.

Role of O-linked-N-acetylglucosamine modification (O-GlcNAcylation) in mineralocorticoid receptor (MR) expression and transcriptional activity. Aldosterone binding promotes the proteasomal degradation of MR. O-GlcNAcylation of MR inhibits ubiquitin attachment to the MR and interferes with the receptor’s aldosterone-induced proteasomal degradation. As a result, O-GlcNAcylation increases the sensitivity of the MR to aldosterone and exacerbates aldosterone-associated complications (resistant hypertension, diabetic nephropathy and cardiovascular diseases).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
13 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41440-023-01167-4
References
Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol. 2005;19:2211–21.
Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal. 2007;5:e012.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.
Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–30.
Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25:514–23.
Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–50.
Nielsen SE, Persson F, Frandsen E, Sugaya T, Hess G, Zdunek D, et al. Spironolactone diminishes urinary albumin excretion in patients with type 1 diabetes and microalbuminuria: a randomized placebo-controlled crossover study. Diabet Med. 2012;29:e184–190.
Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Tarnow L, Rossing P, et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006;70:536–42.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–29.
Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN). Clin J Am Soc Nephrol. 2020;15:1715–27.
Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–63.
Yokota K, Shibata H, Kurihara I, Kobayashi S, Suda N, Murai-Takeda A, et al. Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J Biol Chem. 2007;282:1998–2010.
Murai-Takeda A, Shibata H, Kurihara I, Kobayashi S, Yokota K, Suda N, et al. NF-YC functions as a corepressor of agonist-bound mineralocorticoid receptor. J Biol Chem. 2010;285:8084–93.
Hayashi T, Shibata H, Kurihara I, Yokota K, Mitsuishi Y, Ohashi K, et al. High glucose stimulates mineralocorticoid receptor transcriptional activity through the protein kinase C β signaling. Int Heart J. 2017;58:794–802.
Mitsuishi Y, Shibata H, Kurihara I, Kobayashi S, Yokota K, Murai-Takeda A, et al. Epidermal growth factor receptor/extracellular signal-regulated kinase pathway enhances mineralocorticoid receptor transcriptional activity through protein stabilization. Mol Cell Endocrinol. 2018;473:89–99.
Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22.
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58.
Issad T, Masson E, Pagesy P. O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metab. 2010;36:423–35.
Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 2002;99:5313–8.
Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by o-linked N-acetylglucosamine. J Biol Chem. 2003;278:10022–7.
Jiang MS, Hart GW. A subpopulation of estrogen receptors are modified by O-linked N-acetylglucosamine. J Biol Chem. 1997;272:2421–8.
James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, et al. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB- dependent promoter activation. Diabetes. 2002;51:1146–56.
Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, et al. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–92.
Anthonisen EH, Berven L, Holm S, Nygard M, Nebb HI, Gronning-Wang LM. Nuclear receptor liver X receptor is O-GlcNAc-modified in response to glucose. J Biol Chem. 2010;285:1607–15.
Kurihara I, Shibata H, Kobayashi S, Suda N, Ikeda Y, Yokota K, et al. Ubc9 and protein inhibitor of activated STAT 1 activate chicken ovalbumin upstream promoter-transcription factor I-mediated human CYP11B2 gene transcription. J Biol Chem. 2005;280:6721–30.
Kobayashi S, Shibata H, Yokota K, Suda N, Murai A, Kurihara I, et al. FHL2, UBC9, and PIAS1 are novel estrogen receptor alpha-interacting proteins. Endocr Res. 2004;30:617–21.
Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–89.
Yokota K, Shibata H, Kurihara I, Kobayashi S, Murai-Takeda A, Itoh H. CASZ1b is a novel transcriptional corepressor of mineralocorticoid receptor. Hypertens Res. 2021;44:407–16.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9.
Zennaro MC, Souque A, Viengchareun S, Poisson E, Lombes M. A new human MR splice variant is a ligand-independent transactivator modulating corticosteroid action. Mol Endocrinol. 2001;15:1586–98.
Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–7.
Faresse N, Ruffieux-Daidie D, Salamin M, Gomez-Sanchez CE, Staub O. Mineralocorticoid receptor degradation is promoted by Hsp90 inhibition and the ubiquitin-protein ligase CHIP. Am J Physiol Ren Physiol. 2010;299:F1462–1472.
Han I, Oh ES, Kudlow JE. Responsiveness of the state of O-linked N-acetylglucosamine modification of nuclear pore protein p62 to the extracellular glucose concentration. Biochem J. 2000;350(Pt 1):109–14.
Fuse H, Kitagawa H, Kato S. Characterization of transactivational property and coactivator mediation of rat mineralocorticoid receptor activation function-1 (AF-1). Mol Endocrinol. 2000;14:889–99.
Pascual-Le Tallec L, Kirsh O, Lecomte MC, Viengchareun S, Zennaro MC, Dejean A, et al. Protein inhibitor of activated signal transducer and activator of transcription 1 interacts with the N-terminal domain of mineralocorticoid receptor and represses its transcriptional activity: implication of small ubiquitin-related modifier 1 modification. Mol Endocrinol. 2003;17:2529–42.
Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5.
Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–8.
Ovejera AA, Houchens DP, Catane R, Sheridan MA, Muggia FM.Efficacy of 6-diazo-5-oxo-L-norleucine and N-[N-gamma-glutamyl-6-diazo-5-oxo-norleucinyl]-6-diazo-5-oxo-norleucine against experimental tumors in conventional and nude mice.Cancer Res.1979;39:3220–4.
Shelton LM, Huysentruyt LC, Seyfried TN. Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. International journal of cancer. J Int Cancer. 2010;127:2478–85.
Tzuman YC, Sapoznik S, Granot D, Nevo N, Neeman M. Peritoneal adhesion and angiogenesis in ovarian carcinoma are inversely regulated by hyaluronan: the role of gonadotropins. Neoplasia. 2010;12:51–60.
Park SY, Kim HS, Kim NH, Ji S, Cha SY, Kang JG, et al. Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. EMBO J. 2010;29:3787–96.
Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–83.
Ruan H-B, Nie Y, Yang X. Regulation of protein degradation by O-GlcNAcylation: crosstalk with ubiquitination. Mol Cell Proteom. 2013;12:3489–97.
Hirschberg D, Jagerbrink T, Samskog J, Gustafsson M, Stahlberg M, Alvelius G, et al. Detection of phosphorylated peptides in proteomic analyses using microfluidic compact disk technology. Anal Chem. 2004;76:5864–71.
Martinez F, Mansego ML, Escudero JC, Redon J, Chaves FJ. Association of a mineralocorticoid receptor gene polymorphism with hypertension in a Spanish population. Am J Hypertens. 2009;22:649–55.
Kuningas M, de Rijk RH, Westendorp RG, Jolles J, Slagboom PE, van Heemst D. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology. 2007;32:1295–301.
Cai T, Ke Q, Fang Y, Wen P, Chen H, Yuan Q, et al. Sodium–glucose cotransporter 2 inhibition suppresses HIF-1α-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death Dis. 2020;11:390.
Li J, Liu H, Takagi S, Nitta K, Kitada M, Srivastava SP, et al. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight. 2020;5:e129034.
Drumm K, Kress TR, Gassner B, Krug AW, Gekle M. Aldosterone stimulates activity and surface expression of NHE3 in human primary proximal tubule epithelial cells (RPTEC). Cell Physiol Biochem. 2006;17:21–28.
Pergher PS, Leite-Dellova D. Mello-Aires Md. Direct action of aldosterone on bicarbonate reabsorption in in vivo cortical proximal tubule. Am J Physiol-Ren Physiol. 2009;296:F1185–F1193.
Leite-Dellova DCA, Malnic G, Mello-Aires M. Genomic and nongenomic stimulatory effect of aldosterone on H+-ATPase in proximal S3 segments. Am J Physiol-Ren Physiol. 2011;300:F682–F691.
Salyer SA, Parks J, Barati MT, Lederer ED, Clark BJ, Klein JD, et al. Aldosterone regulates Na+, K+ ATPase activity in human renal proximal tubule cells through mineralocorticoid receptor. Biochimica et Biophysica Acta (BBA) - Mol Cell Res. 2013;1833:2143–52.
Degrell P, Cseh J, Mohas M, Molnar GA, Pajor L, Chatham JC, et al. Evidence of O-linked N-acetylglucosamine in diabetic nephropathy. Life Sci. 2009;84:389–93.
Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, et al. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology. 2006;147:5363–73.
Lee SH, Yoo TH, Nam BY, Kim DK, Li JJ, Jung DS, et al. Activation of local aldosterone system within podocytes is involved in apoptosis under diabetic conditions. Am J Physiol Ren Physiol. 2009;297:F1381–1390.
Andrali SS, Qian Q, Ozcan S. Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J Biol Chem. 2007;282:15589–96.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (to HS, IK, and RJ), Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research (to HS and IK), and grant from the Smoking Research Foundation (to HI and HS). We are grateful to Dr. Bert W.O’Malley, Dr. Christie P. Thomas and Dr. Shigeaki Kato for generously providing plasmid DNAs, Ryoji Fujiki (Kazusa DNA Research Institute) for MS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HS has an honorarium from Daiichi-Sankyo Company, Mochida Pharmaceuticals, Astrazeneca, Novartis Pharma, Bayer, and Astellas. HS also received scholarships from Chugai and Bayer. Other authors have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
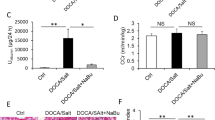
The original online version of this article was revised: After publication, I found a mistake in the description about the dosage of 6-diazo-5-oxo-L-norleucine (DON) in animal studies. I made a mistake in writing the unit of the reagent. I wrote it as 3 μg/kg, but it should have been 3 μg/g. There are three errors regarding units in the methods, results and caption of Fig. 5E. Please refer to the corrigenda on the next page.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jo, R., Shibata, H., Kurihara, I. et al. Mechanisms of mineralocorticoid receptor-associated hypertension in diabetes mellitus: the role of O-GlcNAc modification. Hypertens Res 46, 19–31 (2023). https://doi.org/10.1038/s41440-022-01036-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01036-6
Keywords
This article is cited by
-
Winners for the 14th Hypertension Research Awards and outstanding papers in Hypertension Research
Hypertension Research (2024)
-
2023 update and perspectives
Hypertension Research (2024)
-
Fat mass: the most sensitive predictor of persistent hypertension in unilateral primary aldosteronism
Hypertension Research (2023)
-
Nutrient-derived modification of mineral corticoid receptors is relevant to diabetic kidney disease progression
Hypertension Research (2023)
-
Therapieoptionen bei diabetischer Nephropathie
Die Diabetologie (2023)