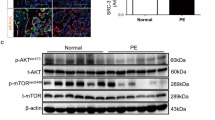
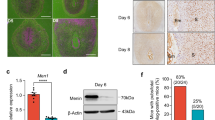
Abstract
Defective decidual function contributes to the pathogenesis of preeclampsia. However, the precise mechanism of defective decidua during preeclampsia has not been characterized. During decidualization, endometrial stromal cells undergo phenotypic changes that are consistent with mesenchymal-epithelial transition (MET). cGMP-dependent kinase protein I (PKGI)/VASP signaling is important in cell motility proliferation, differentiation and cell adhesion. To investigate this aim, we analyzed PKGI levels, phosphorylated VASP protein levels, and eNOS and sGC protein expression levels during pregnancy complicated by preeclampsia, which indicated that PKGI/VASP signaling function is decreased by the condition. Moreover, we evaluated the differential expression of genes that regulate MET in the decidua resulting from preeclampsia and healthy pregnancies. We discovered that vimentin mRNA levels are decreased in the decidua of preeclampsia, which indicates that excessive MET occurs in the decidua of preeclampsia pregnancies. A fundamental developmental MET program occurred in response to signaling pathways. These results suggest the important role of decreased PKGI/VASP signaling during excessive MET in the pathogenesis of preeclampsia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Knight M, Tuffnell D. A view from the UK: the UK and Ireland confidential enquiry into maternal deaths and morbidity. Clin Obstet Gynecol. 2018;61:347–58.
Juan L, Xiaohong L, Chuyun K, Yanping W, Rachel KX, CM M, et al. Maternal mortality ratios in 2852 Chinese counties, 1996–2015, and achievement of Millennium Development Goal 5 in China: a subnational analysis of the Global Burden of Disease Study 2016. Lancet. 2019;393:241–52.
Haram K, Mortensen JH, Myking O, Roald B, Magann EF, Morrison JC. Early development of the human placenta and pregnancy complications. J Maternal Fetal Neonatal Med. 2020;33:3538–45.
Garrido-Gomez T, Dominguez F, Quinonero A, Diaz-Gimeno P, Kapidzic M, Gormley M, et al. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc Natl Acad Sci USA. 2017;114:E8468–77.
Tong J, Zhao W, Lv H, Li W-P, Chen Z, Zhang C. Transcriptomic profiling in human decidua of severe preeclampsia detected by RNA sequencing. J Cell Biochem. 2017;119:607–15.
Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol. 2016;38:635–49.
Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK. Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells Dev. 2013;22:964–74.
Rutherford RA, McCarthy A, Sullivan MH, Elder MG, Polak JM, Wharton J. Nitric oxide synthase in human placenta and umbilical cord from normal, intrauterine growth-retarded and pre-eclamptic pregnancies. Br J Pharmacol. 1995;116:3099–109.
Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–63.
Bolnick JM, Kilburn BA, Bolnick AD, Diamond MP, Singh M, Hertz M, et al. Sildenafil stimulates human trophoblast invasion through nitric oxide and guanosine 3’,5’-cyclic monophosphate signaling. Fertil Steril. 2015;103:1587–95. e1581–82.
Dordea AC, Sweeney M, Taggart J, Lartey J, Wessel H, Robson SC, et al. Differential vasodilation of human placental and myometrial arteries related to myofilament Ca(2+)-desensitization and the expression of Hsp20 but not MYPT1. Mol Hum Reprod. 2013;19:727–36.
Lund N, Henrion D, Tiede P, Ziche M, Schunkert H, Ito WD. Vimentin expression influences flow dependent VASP phosphorylation and regulates cell migration and proliferation. Biochemical biophysical Res Commun. 2010;395:401–6.
Kayisli UA, Demir R, Erguler G, Arici A. Vasodilator-stimulated phosphoprotein expression and its cytokine-mediated regulation in vasculogenesis during human placental development. Mol Hum Reprod. 2002;8:1023–30.
Chu S, Zhang X, Sun Y, Liang Y, Sun J, Lu M, et al. Atrial natriuretic peptide inhibits epithelial-mesenchymal transition (EMT) of bronchial epithelial cells through cGMP/PKG signaling by targeting Smad3 in a murine model of allergic asthma. Exp lung Res. 2019;45:245–54.
Vyas-Read S, Shaul PW, Yuhanna IS, Willis BC. Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L212–21.
Duran-Reyes G, Gomez-Melendez MR, Morali-de la Brena G, Mercado-Pichardo E, Medina-Navarro R, Hicks-Gomez JJ. Nitric oxide synthesis inhibition suppresses implantation and decreases cGMP concentration and protein peroxidation. Life Sci. 1999;65:2259–68.
ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–25.
Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PloS ONE. 2010;5:e10258.
Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381.
McCauley SD, Gilchrist M, Befus AD. Nitric oxide: a major determinant of mast cell phenotype and function. Mem Inst Oswaldo Cruz. 2005;100:11–14.
Cokic VP, Schechter AN. Effects of nitric oxide on red blood cell development and phenotype. Curr Top Dev Biol. 2008;82:169–215.
Wolfertstetter S, Huettner JP, Schlossmann J. cGMP-dependent protein kinase inhibitors in health and disease. Pharmaceuticals. 2013;6:269–86.
Brennecke SP, Gude NM, Di Iulio JL, King RG. Reduction of placental nitric oxide synthase activity in pre-eclampsia. Clin Sci. 1997;93:51–55.
Kayisli UA, Selam B, Demir R, Arici A. Expression of vasodilator-stimulated phosphoprotein in human placenta: possible implications in trophoblast invasion. Mol Hum Reprod. 2002;8:88–94.
Ali M, Rogers LK, Heyob KM, Buhimschi CS, Buhimschi IA. Changes in vasodilator-stimulated phosphoprotein phosphorylation, Profilin-1, and Cofilin-1 in accreta and protection by DHA. Reprod Sci. 2019;26:757–65.
TML TAWyatt, Pryzwansky KB. Vimentin is transiently co-localized with and phosphorylated by cyclic GMP-dependent protein kinase in formylpeptide-stimulated neutrophils. J Biol Chem. 1991;266:21274–80.
Owusu-Akyaw A, Krishnamoorthy K, Goldsmith LT, Morelli SS. The role of mesenchymal-epithelial transition in endometrial function. Hum Reprod Update. 2019;25:114–33.
Zhang XH, Liang X, Liang XH, Wang TS, Qi QR, Deng WB, et al. The mesenchymal-epithelial transition during in vitro decidualization. Reprod Sci. 2013;20:354–60.
Patterson AL, Pirochta J, Tufano SY, Teixeira JM. Gain-of-function beta-catenin in the uterine mesenchyme leads to impaired implantation and decidualization. J Endocrinol. 2017;233:119–30.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2017YFC1001402 and 2018YFC10029002), the National Natural Science Foundation (No. 81830045, 81671533 and 82071652), and the General Program of Guangdong Province Natural Science Foundation (No. 2020A1515010273).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Ren, W., Lin, L. et al. Abnormal cGMP-dependent protein kinase I-mediated decidualization in preeclampsia. Hypertens Res 44, 318–324 (2021). https://doi.org/10.1038/s41440-020-00561-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-00561-6