Abstract
Objective
To evaluate the effectiveness, treatment patterns and long-term safety of ranibizumab 0.5 mg in treatment-naïve patients with central retinal vein occlusion (CRVO) in a real-world setting.
Methods
LUMINOUS, a 5-year, global, prospective, multicentre, multi-indication, observational, open-label study, recruited treatment naïve or prior treated patients who were treated as per the local ranibizumab label. Here, we report the mean change in visual acuity (VA; Early Treatment Diabetic Retinopathy Study [ETDRS] letters), treatment exposure over year (Y) 1 and 5-year safety in treatment-naïve CRVO patients.
Results
At baseline, the mean age of treatment-naïve CRVO patients (n = 327) was 68.9 years, with a mean (Standard deviation [SD]) VA of 40.6 (23.9) letters. At Y1, patients (n = 144) had a mean (SD) VA gain from baseline of 10.8 (19.66) letters, with a mean (SD) of 5.4 (2.65) ranibizumab injections. Patients demonstrated mean (SD) VA gains of 2.7 (19.35), 11.6 (20.56), 13.9 (18.08), 11.1 (18.46) and 8.2 (24.86) letters with 1, 2–3, 4–5, 6–8 and >8 ranibizumab injections, respectively. Mean (SD) VA gains at Y1 in patients receiving loading (67.4%) and no loading dose (32.6%) was 11.9 (20.42) and 8.4 (17.99) letters, respectively. Over five years, the incidence of ocular/non-ocular adverse events (AEs) and serious AEs was 11.3%/8.6% and 1.2%/6.7%, respectively.
Conclusions
These results demonstrate the effectiveness of ranibizumab in treatment-naïve CRVO patients at Y1 with clinically meaningful VA gains and no new safety findings over five years. These findings may help inform routine practice and enable better clinical management to achieve optimal visual outcomes.
Similar content being viewed by others
Introduction
The global prevalence of central retinal vein occlusion (CRVO) is 0.08% in patients aged ≥ 30 years and does not significantly vary with regard to race or gender [1,2,3]. Anti-vascular endothelial growth factor (anti-VEGF) therapy is the current standard of care for the treatment of CRVO patients [4,5,6,7,8].
Ranibizumab (Lucentis®; Novartis Pharma AG, Basel, Switzerland, and Genentech Inc., South San Francisco, CA, USA) was the first anti-VEGF agent to be approved for the treatment of patients with visual impairment due to macular oedema secondary to retinal vein occlusion (branch and central) and is currently approved in many countries globally for this indication [9,10,11]. The efficacy and safety profile of ranibizumab in patients with CRVO is well established based on several randomised controlled trials [9, 10, 12]. However, real-world evidence is limited to specific regions, countries or small patient populations [13,14,15,16,17].
Hence, the LUMINOUS (NCT01318941) study is, to our knowledge, the largest, prospective, observational, global trial in the field of medical retina designed to evaluate the long-term effectiveness, safety and treatment patterns associated with ranibizumab 0.5 mg in routine clinical practice across five approved indications: (i) neovascular age-related macular degeneration, (ii) diabetic macular oedema, (iii) branch retinal vein occlusion, (iv) CRVO and (v) myopic choroidal neovascularisation. The effectiveness of ranibizumab at one year and safety over five years for treatment-naïve patients with CRVO enroled in this study are reported here.
Materials and methods
Study design
LUMINOUS was a 5-year, open-label, single-arm, global, observational study conducted from March 2011 to April 2016 at 488 clinical sites across 42 countries. Patients with any of the approved indications as per the ranibizumab label at the time of the study were enroled and treated at outpatient or private ophthalmology clinics with intravitreal ranibizumab 0.5 mg according to the local ranibizumab label [18]. As patients were recruited over time and the date of study completion was pre-set, the follow-up time varied between patients based on their study entry date. The minimum potential follow-up for each patient was defined as one year in the protocol. The frequency of patient visits was determined by the investigator. It was recommended to capture data at every visit or at a minimum of every three months. Investigators were encouraged to follow-up with patients who did not visit the clinic for at least six months since their last visit. Patients who were not seen at least once per year or those who switched to another anti-VEGF therapy were discontinued from the study.
The study protocol was reviewed and approved by an Independent Ethics Committee or Institutional Review Board for each centre; a complete list of these by study centre is provided in the Supplementary Table 1. The study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices issued by the International Society for Pharmacoepidemiology [19] and any applicable national guidelines and ethical principles laid down in the Declaration of Helsinki. Patients provided written informed consent. The study is registered with ClinicalTrials.gov as NCT01318941 [20].
Key eligibility criteria
The key inclusion criteria for the LUMINOUS study have been described previously [21,22,23,24]. Patients were excluded if they were simultaneously participating in a study that included administration of any investigational drug or procedure, had undergone systemic treatment with any VEGF inhibitor in the 90 days prior to study enrolment or had received ocular treatment with any anti-VEGF other than ranibizumab in the month prior to study enrolment.
Assessments
Effectiveness assessments included visual acuity (preferably best-corrected visual acuity [VA]) evaluation by each participating physician as a part of routine care practice using Early Treatment Diabetic Retinopathy Study (ETDRS) letters, Snellen charts or equivalent. To facilitate data analysis, Snellen fractions and decimals were converted to the ETDRS equivalent letter scores. It was recommended that the same method of visual acuity assessment be used throughout the study wherever possible.
Other assessments, such as optical coherence tomography (i.e. for central retinal thickness evaluation) and ocular examinations (pre-injection intraocular pressure measurements), were optional but included if the data were available. The number of ranibizumab injections administered overall and over time; the average time interval (in weeks) between consecutive injections; visit frequency; and treatment patterns (unilateral [involving single eye]/bilateral [involving both eyes]) were recorded. All adverse events (AEs), including serious AEs (SAEs), that occurred during the study were recorded irrespective of suspected causal association.
Statistical analysis
All effectiveness and safety data were summarised descriptively. Owing to the design of the study, one year data were potentially available for all patients, while the availability of data for subsequent years depended on the patient’s study entry date. The effectiveness data are therefore presented here for up to one year. Safety data are presented over the entire 5-year period to provide a comprehensive safety profile.
The enroled set included all patients who signed the informed consent and had at least a baseline assessment. The safety set comprised patients in the enroled set, who, in case of treatment naïve patients, were treated with at least one dose of ranibizumab during the study and had at least one safety assessment after the first injection.
The primary treated eye set included all primary treated eyes (i.e. the first eye treated during the study) in patients from the safety set. Treatment exposure up to one year was analysed for patients in the safety set who stayed in the study for at least 365 days. For year 1 effectiveness, patients from the primary treated eye set who had both baseline and year 1 data and remained in the study for at least 365 days are presented.
The primary effectiveness end point was the mean change in VA ETDRS letter score from baseline to year 1. Additional effectiveness analyses not prespecified in the protocol but included in the statistical analysis plan are: (1) the mean change in VA from baseline at year 1 by (a) injection frequency during year 1 (1, 2–3, 4–5, 6–8 and >8 injections); (b) patients who received a loading dose (the initial three ranibizumab injections administered up to day 100) versus those who did not; (c) baseline VA category (<23, 23 to <39, 39 to <60, 60 to <74 and ≥74 letters); (d) baseline VA < 73 letters or ≥73 letters (good starting vision or Snellen equivalent 20/40) at year 1; (2) proportion of patients with VA loss (defined as ≤0-letter change from baseline) or gain (defined as >0-letter change from baseline) of >0 to <5 letters, 5 to <10 letters, 10 to <15 letters and ≥15 letters at year 1.
Safety was assessed based on the incidence, proportion, relationship and severity of treatment-emergent ocular and non-ocular AEs. Ocular AEs were assessed for the primary treated eye set, and non-ocular AEs were assessed for the safety set.
Results
A total of 30,138 patients were enroled across all five approved indications in the overall LUMINOUS trial. Of the total population, 1048 (3.5%) patients had CRVO, of whom 327 (31.2%) were treatment naïve. The following countries enroled the majority of treatment-naïve patients with CRVO: the United Kingdom (24.5%), Canada (13.1%), Russia (11.0%), Germany (9.5%) and Poland (9.4%).
Of the 327 treatment-naïve patients, 249 (76.14%) remained in the study until the end of year 1, and 171 (52.3%) completed the study. The most common reason for study discontinuation was ‘loss to follow-up’ at year 1 (n = 27, 8.3%) and year 5 (n = 64, 19.6%; Fig. 1). The year 1 effectiveness analysis in the primary treated eye set included 144 patients for whom both baseline and 1-year VA data were available (owing to the flexible scheduling of visits, not all patients who completed year 1 in the study had a year 1 visit).
At baseline, the mean (standard deviation [SD]) age of the patients was 68.9 (13.0) years, 56.9% were male, and the majority (76.8%) were White. Most patients (n = 323, 98.78%) were treated in one eye, and four (1.22%) patients were treated bilaterally. No eminent differences in baseline characteristics were noted when comparing the overall treatment-naïve CRVO population, patients included in the year 1 effectiveness analysis and those not included due to unavailable data or participation in the study for <365 days (Table 1).
At baseline, the mean age, mean VA and median time from diagnosis to treatment varied across the top five recruiting countries (Supplementary Fig. 1). The median time from diagnosis to first treatment was one day in Canada and Poland, eight days in Germany, 30 days in the United Kingdom and 48.5 days in Russia.
Treatment exposure and visits
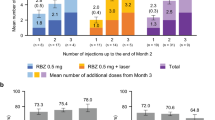
The mean (SD) number of ranibizumab injections administered up to year 1 was 5.4 (2.65), and the mean (SD) number of visits was 8.7 (2.98). In the first year, 50.1% of patients received six or more injections (Fig. 2).
Effectiveness outcomes
At year 1, a mean (SD) VA gain of 10.8 (19.7) letters from a baseline of 40.7 (22.17) letters was observed (Fig. 3A). The mean VA gain and the mean number of ranibizumab injections at year 1 varied across the enroling countries (Fig. 3A).
A For global and the top five enroling countries. B By injection frequency. C By baseline VA category. The year 1 primary treated eye set included patients from the primary treated eye set who had both baseline and year 1 data and had remained in the study for at least 365 days. ETDRS early treatment diabetic retinopathy study, n number of patients, VA visual acuity.
By injection category, the highest mean VA gains from baseline to year 1 were observed in patients receiving 4–5 injections. Across different injection categories, the mean (SD) VA gains at year 1 ranged from 2.7 (19.35) to 13.9 (18.08) (Fig. 3B). By baseline VA category, patients with a lower VA (<23 letters) at baseline had higher VA gains at year 1 (n = 32; baseline VA: 9.4 [8.35]; VA gain at year 1: 22.0 [23.02] letters); however, the actual VA at year 1 was higher in patients with a higher baseline VA. Eight patients (baseline VA: 78.4 [4.41] letters) with baseline VA ≥ 74 letters showed a mean (SD) change in their vision at year 1 by −5.0 (7.52) letters. The mean number of injections by the end of year 1 ranged from 4.1 to 6.0 across different baseline VA categories (Fig. 3C).
VA gains in patients who received the loading dose of three initial consecutive monthly ranibizumab injections (n = 97 of 144; 67.4%) was [11.9 (20.42)] letters and in those who did not was 8.4 (17.99) letters; 95% CI (confidence interval) for the difference (─3.14, 10.14) (Supplementary Fig. 2).
At month 6 and year 1, 46.5% (n = 60, N = 129) and 44.4% (n = 64, N = 144) of patients treated with ranibizumab had VA gains of ≥15 letters, respectively. At year 1, VA was maintained at baseline levels in 7.6% (n = 11) of patients; a VA loss of ≥15 letters was seen in 11.8% (n = 17) of patients (Supplementary Fig. 3).
Safety outcomes
At the end of year 1, the incidence of ocular/non-ocular AEs in the 144 patients analysed was 8.3% (n = 12)/5.6% (n = 8), and that of the SAEs was 0.7% (n = 1)/4.2% (n = 6). Over the 5-year period, ocular AEs in the safety set (n = 327) were reported in 11.3% (n = 37) of patients. Glaucoma (n = 5, 1.5%), ocular hypertension (n = 4, 1.2%) and cataract (n = 4, 1.2%) were the most commonly reported ocular AEs (Table 2). Non-ocular AEs were reported in 8.6% (n = 28) of patients; hypertension, pneumonia and confusional state were the most common, occurring in two patients each (n = 2, 0.6%). Overall, 3.4% (n = 11) of ocular AEs were suspected to be related to the study treatment or ocular injections by the investigator (Supplementary Table 2). Non-ocular treatment-related AEs suspected to be related to treatment were not observed. One ocular AE (0.3%, retinal injury) and six non-ocular AEs (all SAEs) led to treatment discontinuation.
Ocular SAEs were reported in four (1.2%) patients: glaucoma, n = 2 (0.6%) and amaurosis fugax, blindness and cataract trauma, n = 1 each (0.3%) (Supplementary Table 3). No cases of endophthalmitis or retinal break/detachment were reported. The incidence of non-ocular SAEs was 6.7% (n = 22); pneumonia and confusional state (0.6%; n = 2 each) were the most frequently reported non-ocular SAEs (Supplementary Table 3). In total, there were six deaths, with reasons including breast cancer, pneumonia, sepsis and unknown cause.
Discussion
The results from the global, prospective LUMINOUS study support the safety of ranibizumab 0.5 mg treatment over 5 years in treatment-naïve patients with CRVO in a broad real-world setting. Ranibizumab treatment resulted in improved VA outcomes at the end of 1 year in these patients. Across all enroling countries we identified a similar pattern, i.e. that patients with a low baseline VA or those receiving higher numbers of ranibizumab injections or those treated with an adequate loading dose achieved better VA outcomes. Overall, there were no new safety findings, and very few AEs, SAEs or AEs leading to treatment discontinuation and treatment-related AEs were reported. The safety results observed in the LUMINOUS study are consistent with those observed in other CRVO studies with ranibizumab [10, 11, 25, 26]. These findings may help inform routine practice and enable better clinical management to achieve optimal visual outcomes in treatment-naïve CRVO patients. Although efficacy could not be assessed on the basis of anatomical response, the observed influences of consistent upload/start of therapy highlight the risk of limited visual acuity gain under hesitant therapy as also found by Sagkriotis et al. [27].
The mean age (years) of patients in this study (68.9) was similar to that of patients included in the CRUISE (67.6) [10, 11], CRYSTAL (65.5) [9] and OCEAN (70.3) [28, 29] studies. Furthermore, the majority of treatment-naïve patients with CRVO included in LUMINOUS were White (77%), consistent with other CRVO studies with ranibizumab (83.1–94.4%) [9,10,11, 28]. Patients in the LUMINOUS study had a lower baseline VA (40.7 letters), similar to the CRVO treatment arm of the OCEAN study (43.7 letters) [28, 29], when compared with the CRUISE (48.3 letters) and CRYSTAL (53.0 letters) studies. A lower baseline VA is expected to result in higher VA gains. However, the overall VA gains were lower in LUMINOUS (10.8 letters) than in the CRYSTAL (12.3 letters) and CRUISE (13.9 letters) studies. These lower VA gains were in line with the real-world OCEAN study [29]. While this difference could possibly be due to the lower mean number of injections in the LUMINOUS (5.4) and OCEAN (5.11) [29] studies compared with the CRUISE (8.9) [10, 11] and CRYSTAL (8.1) [9] studies, between-trial comparisons have significant limitations when considering the differences in their study designs.
Gains in VA with treatment were dependent on the patient’s baseline VA as well as on the early initiation of treatment after diagnosis of the disease in studies [10, 16, 30,31,32,33,34,35,36,37,38]. In the LUMINOUS study, results by baseline VA categories support the observations made in phase 3 studies of ranibizumab in patients with CRVO (CRUISE and CRYSTAL) [9,10,11], suggesting that patients with a poor baseline VA achieve higher VA gains. However, the actual VA at year 1 in the LUMINOUS study as well as in the phase 3 studies, was higher in patients with a better baseline VA, stressing the need for early diagnosis and prompt treatment of the disease.
Very few real-world studies have investigated ranibizumab in treatment-naïve patients with CRVO. In a retrospective, observational, multicentre study carried out in Portugal, 76 treatment-naïve patients with CRVO were included and treated with ranibizumab or bevacizumab. The median age of patients and distribution of gender were comparable to those in the LUMINOUS study, but the baseline VA and VA at month 12 in patients treated with ranibizumab were higher compared with those in the LUMINOUS study (median VA at baseline: 0.7 logarithm of the minimum angle of resolution [~65 ETDRS letters] and median VA at 12 months: 0.5 logarithm of the minimum angle of resolution [~75 letters]); the median number of injections up to month 12 was four. Consistent with the findings in LUMINOUS, patients with a lower baseline VA had better gains at 12 months [16].
The strengths of the LUMINOUS study are that it is the first large-scale, global, multicentre, multi-indication, post-market, observational study of an anti-VEGF agent, with flexible inclusion criteria. LUMINOUS included a broader patient population than other randomised controlled trials and provides information on patients with multiple geographic, demographic and baseline characteristics, with varying degrees of healthcare access. It allowed evaluating the hypotheses of positive or negative outcomes in the different care systems (i.e. time to first treatment, application of the complete loading dose, etc.). Limitations include a potential selection bias by analysing patients for effectiveness who were ongoing in the study at one year and had a one-year visit. The restriction was implemented to be in a position to compare these results with other studies which showed one-year results. It is difficult to assess the direction of the bias. Information bias was expected to be minimal owing to systematic site training, the use of standardised case report forms and other guidance documentation to ensure consistent data collection. Along with manual data reviews, programmable data edit checks for missing, illogical or out-of-range values were built into the electronic data capture system to ensure data quality at all sites. Potential bias was taken into account for the interpretation of study results. Other limitations include variable treatment schedules across regions, host country healthcare systems, physician’s discretion of inclusion, access to treatment, cost and reimbursement criteria, all of which can lead to under-treatment, suboptimal therapeutic effectiveness and poor patient follow-up potentially affecting outcomes. In addition, the imaging data were not collected uniformly and analysed. The study also lacked a comparator arm; options for comparator arms were very limited at the start of the study. Other intravitreal VEGF-inhibitors were not yet available, and design options for comparing the treatment patterns of ranibizumab 0.5 mg were not in scope as the newly emerging treatment regimens like ‘treat-and-extend’ were not very widespread at the time of the study.
To conclude, the results from the LUMINOUS study support the robust real-world effectiveness of ranibizumab over a 1-year treatment period and safety over a 5-year period in treatment-naïve patients with CRVO. There were no new safety signals identified, and the safety profile was consistent with the known safety profile of ranibizumab.
Summary
What was known before
-
Ranibizumab, the first anti-VEGF agent approved for the treatment of patients with visual impairment due to macular oedema secondary to retinal vein occlusion (branch and central) is currently approved in many countries globally for this indication.
-
Though the efficacy and safety profile of anti-VEGF treatment in patients with CRVO is well established based on several randomised controlled trials, real-world evidence is limited to specific regions, countries or small patient populations.
What this study adds
-
LUMINOUS is the largest, prospective, observational study in medical retina and evaluated the long-term effectiveness, treatment patterns, and safety of ranibizumab 0.5 mg across all approved indications in a real-world scenario over 5 years.
-
The present article reports the one year effectiveness and five years safety of ranibizumab for treatment-naive patients with CRVO.
References
Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–9.e1.
Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: review of the literature. Eye(Lond). 2011;25:981–8.
Rhoades W, Dickson D, Nguyen QD, Do DV. Management of macular edema due to central retinal vein occlusion—the role of aflibercept. Taiwan J Ophthalmol. 2017;7:70–6.
Braithwaite T, Nanji AA, Lindsley K, Greenberg PB. Anti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion. Cochrane database Syst Rev. 2014;5:Cd007325.
Campochiaro PA. Anti-vascular endothelial growth factor treatment for retinal vein occlusions. Ophthalmologica.2012;227:30–5.
Gerding H, Monés J, Tadayoni R, Boscia F, Pearce I, Priglinger S. Ranibizumab in retinal vein occlusion: treatment recommendations by an expert panel. Br J Ophthalmol. 2015;99:297–304.
Adelman RA, Parnes AJ, Bopp S, Saad Othman I, Ducournau D. Strategy for the management of macular edema in retinal vein occlusion: the European VitreoRetinal Society macular edema study. Biomed Res Int. 2015;2015:870987.
Channa R, Smith M, Campochiaro PA. Treatment of macular edema due to retinal vein occlusions. Clin Ophthalmol. 2011;5:705–13.
Larsen M, Waldstein SM, Boscia F, Gerding H, Monés J, Tadayoni R, et al. Individualized ranibizumab regimen driven by stabilization criteria for central retinal vein occlusion: twelve-month results of the CRYSTAL study. Ophthalmology. 2016;123:1101–11.
Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124–33.e1.
Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–9.
Scott IU, Figueroa MJ, Oden NL, Ip MS, Blodi BA, VanVeldhuisen PC. SCORE2 report 5: vision-related function in patients with macular edema secondary to central retinal or hemiretinal vein occlusion. Am J Ophthalmol. 2017;184:147–56.
Babiuch AS, Han M, Conti FF, Wai K, Silva FQ, Singh RP. Association of disorganization of retinal inner layers with visual acuity response to anti-vascular endothelial growth factor therapy for macular edema secondary to retinal vein occlusion. JAMA Ophthalmol. 2019;137:38–46.
Madanagopalan VG, Kumari B. Predictive value of baseline biochemical parameters for clinical response of macular edema to bevacizumab in eyes with central retinal vein occlusion: a retrospective analysis. Asia Pac J Ophthalmol (Phila). 2018;7:321–30.
Nghiem-Buffet S, Baillif S, Regnier S, Skelly A, Yu N, Sodi A. Treatment patterns of ranibizumab intravitreal injection and dexamethasone intravitreal implant for retinal vein occlusion in the USA. Eye (Lond). 2017;31:551–9.
Vaz-Pereira S, Marques IP, Matias J, Mira F, Ribeiro L, Flores R. Real-world outcomes of anti-VEGF treatment for retinal vein occlusion in Portugal. Eur J Ophthalmol. 2017;27:756–61.
Winterhalter S, Eckert A, Vom Brocke GA, Schneider A, Pohlmann D, Pilger D, et al. Real-life clinical data for dexamethasone and ranibizumab in the treatment of branch or central retinal vein occlusion over a period of six months. Graefes Arch Clin Exp Ophthalmol. 2018;256:267–79.
European Medicines Agency. Lucentis® summary of product characteristics. Novartis Pharma AG, Basel, Switzerland. 2016. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000715/WC500043546.pdf. Accessed on 26 July, 2021.
Epstein M. Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf. 2005;14:589–95.
ClinicalTrials.gov. Observe the effectiveness and safety of Ranibizumab in real life setting (LUMINOUS). Available at: https://clinicaltrials.gov/ct2/show/NCT01318941. Accessed on 26 July, 2021.
Mitchell P, Sheidow TG, Farah ME, Mahmood S, Minnella AM, Eter N, et al. Effectiveness and safety of ranibizumab 0.5 mg in treatment-naïve patients with diabetic macular edema: results from the real-world global LUMINOUS study. PloS ONE. 2020;15:e0233595.
Koh A, Lai TYY, Wei WB, Mori R, Wakiyama H, Park KH, et al. Real-world effectiveness and safety of ranibizuma treatment in patients with and without polyploidal choroidal vasculopathy: twelve-month results from the LUMINOUS study. Retina (Philadelphia, Pa). 2020;40:1529–39.
Holz FG, Figueroa MS, Bandello F, Yang Y, Ohji M, Dai H, et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: results from LUMINOUS, a global real-world study. Retina (Philadelphia, Pa). 2020;40:1673–85.
Hamilton RD, Clemens A, Minnella AM, Lai TYY, Dai H, Sakamoto T, et al. Real-world effectiveness and safety of ranibizumab for the treatment of myopic choroidal neovascularization: results from the LUMINOUS study. PloS ONE. 2020;15:e0227557.
Campochiaro PA, Sophie R, Pearlman J, Brown DM, Boyer DS, Heier JS, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology. 2014;121:209–19.
Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119:802–9.
Sagkriotis A, Chakravarthy U, Griner R, Doyle O, Wintermantel T, Clemens A. Application of machine learning methods to bridge the gap between non-interventional studies and randomized controlled trials in ophthalmic patients with neovascular age-related macular degeneration. Contemp Clin Trials. 2021;104:106364.
Ziemssen F, Feltgen N, Holz FG, Guthoff R, Ringwald A, Bertelmann T, et al. Demographics of patients receiving Intravitreal anti-VEGF treatment in real-world practice: healthcare research data versus randomized controlled trials. BMC Ophthalmol. 2017;17:7.
Callizo J, Ziemssen F, Bertelmann T, Feltgen N, Vögeler J, Koch M, et al. Real-world data: ranibizumab treatment for retinal vein occlusion in the OCEAN study. Clin Ophthalmol (Auckland, NZ). 2019;13:2167–79.
Kim SJ, Yoon YH, Kim HK, Yoon HS, Kang SW, Kim JG, et al. Baseline predictors of visual acuity and retinal thickness in patients with retinal vein occlusion. J Korean Med Sci. 2015;30:475–82.
Arias L, Armadá F, Donate J, García-Arumí J, Giralt J, Pazos B, et al. Delay in treating age-related macular degeneration in Spain is associated with progressive vision loss. Eye (Lond). 2009;23:326–33.
Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130:1153–61.
Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22.
Williams TA, Blyth CP. Outcome of ranibizumab treatment in neovascular age related macula degeneration in eyes with baseline visual acuity better than 6/12. Eye (Lond). 2011;25:1617–21.
Oliver-Fernandez A, Bakal J, Segal S, Shah GK, Dugar A, Sharma S. Progression of visual loss and time between initial assessment and treatment of wet age-related macular degeneration. Can J Ophthalmol. 2005;40:313–9.
Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–7.
Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–52.
Thach AB, Yau L, Hoang C, Tuomi L. Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion: BRAVO and CRUISE trials. Ophthalmology. 2014;121:1059–66.
Acknowledgements
The authors thank all the investigators (Supplementary Table 4) for their valuable contribution towards this study and also thank Jafar Hyder Ali Shaik, Lakshmi Venkatraman and Swapna Ganduri (Medical and Clinical Solutions/NBS CONEXTS, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for medical writing and editorial assistance in the development of this article.
Funding
This study was funded and sponsored by Novartis Pharma AG, Basel, Switzerland and is registered with https://clinicaltrials.gov/(NCT01318941). The sponsor had a role in the study design, study conduction, data collection, data analysis, data interpretation and manuscript preparation. In addition, Novartis Pharma AG was responsible for the conduct of the study and oversight of the collection and management of data. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Consortia
Contributions
AC, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design AL, AC, RT, XX, MS, MN, FZ, C D-B, RT. Acquisition, analysis, or interpretation of data AC, AL, FZ. Drafting of the manuscript AL, AC, RT, XX, MS, MN, FZ, C D-B, RT. Critical revision of the manuscript for important intellectual content AL, AC, RT, XX, MS,MN, FZ, C D-B, RT. Statistical analysis C D-B. Obtained funding Novartis Pharma AG, Basel, Switzerland. Administrative, technical, or material support NBS CONEXTS. Supervision AC (as the corresponding author).
Corresponding author
Ethics declarations
Competing interests
AL: Consultant for Novartis and Allergan. AC: Shareholder and full-time employee of Novartis Pharma AG. RT: Consultant for Roche; has received grants from Novartis, Roche and Apellis. XX: Nothing to disclose. MS: Nothing to disclose. MN: Consultant for Alcon and Santen; has lectured for Allergan; has performed trials or received grants from Novartis, Bayer, Roche, Sensimed, Ivantis, Formycon and Coronis GmbH. FZ: Consultant for Alimera, Allergan, Bayer, Boheringer Ingelheim, MSD, Novartis, Novonordisk, Oxurion, Optos and Roche; has lectured for Allergan, Bayer, CME Health, Novartis and ODOS. CD-B: Shareholder and part-time employee of Novartis Pharma AG. RT: Consultant for Novartis, Roche, Genentech, Bayer, Allergan, Oculis, Thea, Alcon, B + L, Zeiss, Moria and Cutting Edge. The aforementioned disclosures for all authors do not alter our adherence to journal policies on sharing data and materials.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A full list of members and their affiliations appears in the Supplementary Information.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lotery, A., Clemens, A., Tuli, R. et al. Effectiveness and safety of ranibizumab in patients with central retinal vein occlusion: results from the real-world, global, LUMINOUS study. Eye 36, 1656–1661 (2022). https://doi.org/10.1038/s41433-021-01702-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01702-y
This article is cited by
-
Real-world outcomes of intravitreal bevacizumab treat-and-extend for cystoid macular oedema secondary to central retinal vein occlusion
International Ophthalmology (2023)
-
The impact of laboratory findings and optical coherence tomography biomarkers on response to intravitreal anti-VEGF treatment in patients with retinal vein occlusion
International Ophthalmology (2022)